Abstract
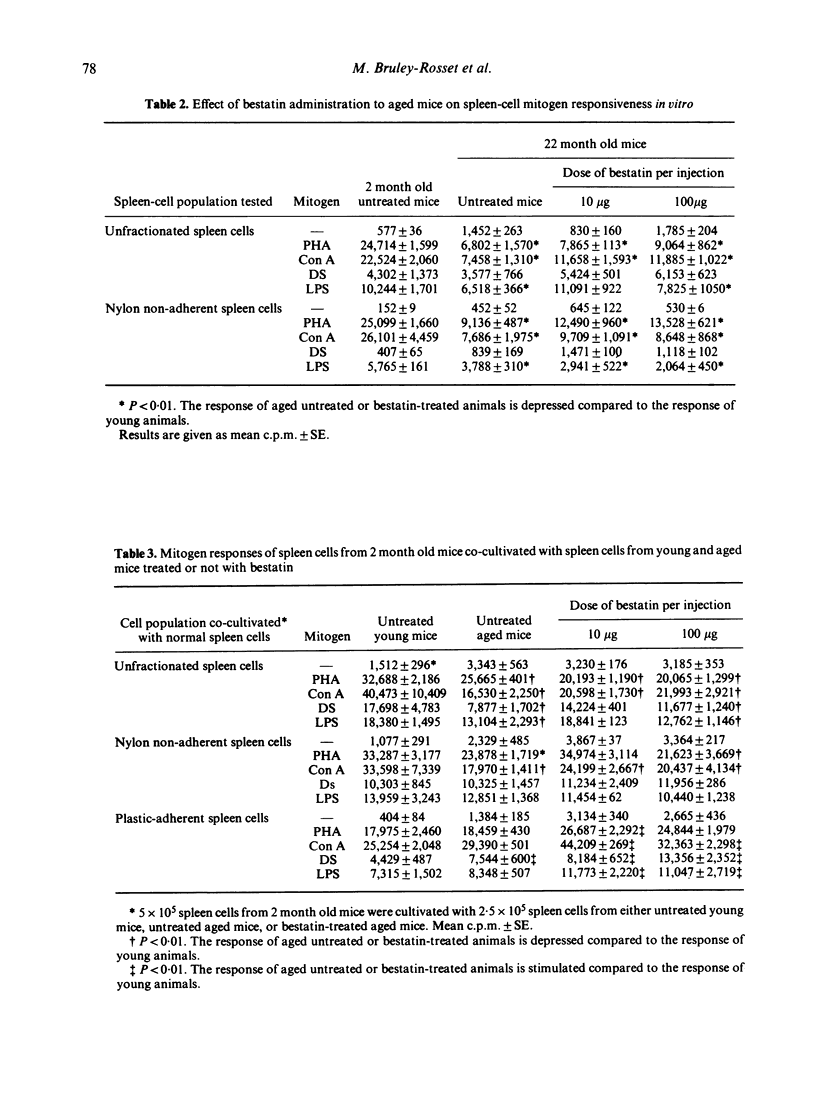
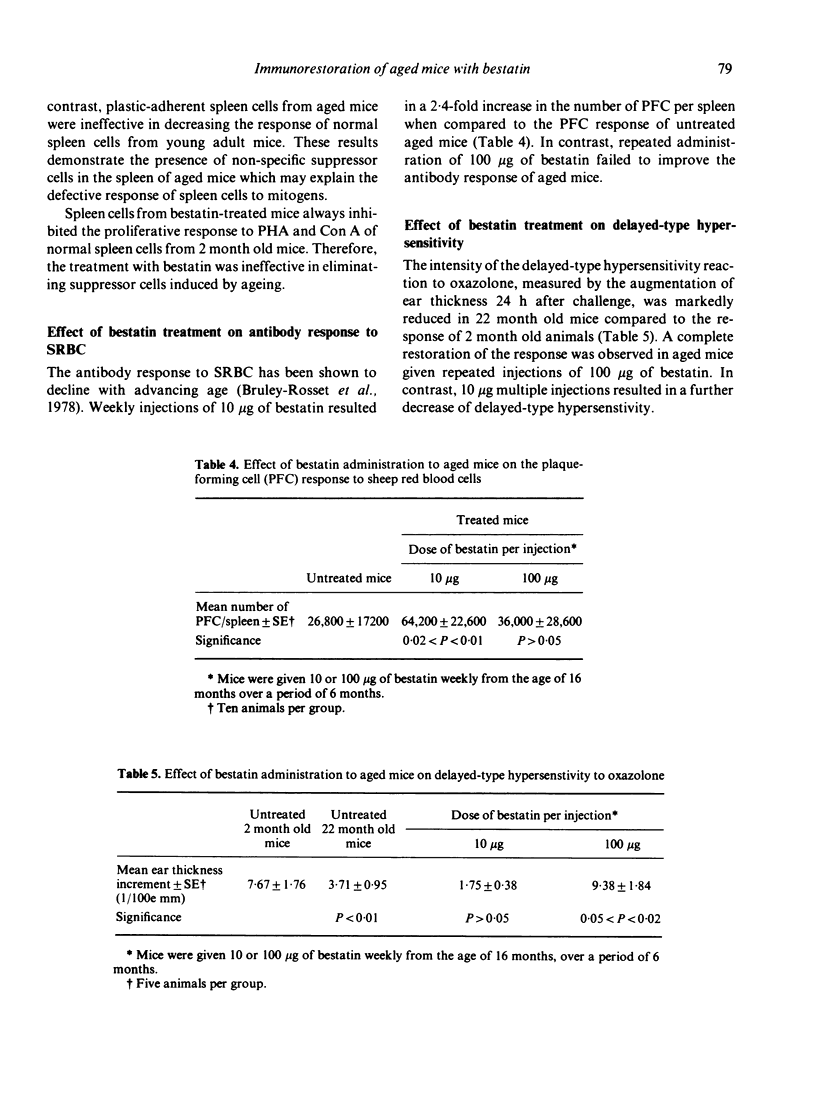
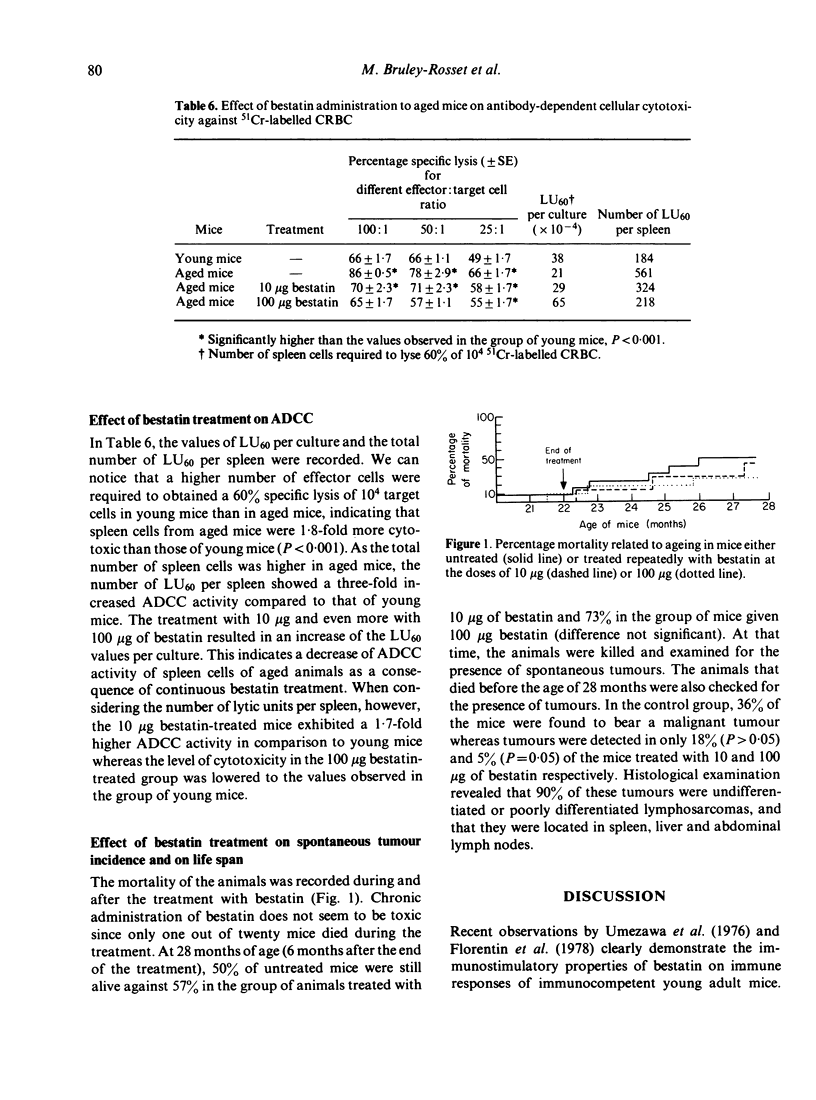
An attempt to correct the state of immunodeficiency in old age was made by repeatedly injecting a chemically defined immunostimulating agent, bestatin, to 16 month old (C57Bl/6 x BALB/c) F1 mice. Aged mice were found to have depressed T-cell and B-cell responses but increased ADCC activity. Weekly injections of bestatin over a period of 6 months resulted in varying effects depending on the dose administered. Small doses (10 microgram per injection) were more effective in restoring humoral responses to SRBC rather than delayed-type hypersensitivity reactions, whereas large doses (100 microgram per injection) acted in the opposite way. Macrophage activation was only obtained after the administration of the high doses of bestatin. Continuous treatment with bestatin did not prevent the appearance of suppressor cells induced by ageing. It led to a significant reduction of ADCC activity in aged animals near to the base line value of young animals. Animals were examined for the presence of spontaneous tumours from the end of the treatment until the age of 28 months. A significant reduction of spontaneous tumour incidence was observed in mice given repeated injections of 100 microgram bestatin when compared to untreated aged mice and to mice given the low doses of bestatin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruley-Rosset M., Florentin I., Kiger N., Davigny M., Mathé G. Effects of Bacillus Calmette-Guérin and levamisole on immune responses in young adult and age-immunodepressed mice. Cancer Treat Rep. 1978 Nov;62(11):1641–1650. [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Doria G., D'Agostaro G., Poretti A. Age-dependent variations of antibody avidity. Immunology. 1978 Oct;35(4):601–611. [PMC free article] [PubMed] [Google Scholar]
- Friedman D., Globerson A. Immune reactivity during aging. I. T-helper dependent and independent antibody responses to different antigens, in vivo and in vitro. Mech Ageing Dev. 1978 Apr;7(4):289–298. doi: 10.1016/0047-6374(78)90072-6. [DOI] [PubMed] [Google Scholar]
- Gerbase-DeLima M., Wilkinson J., Smith G. S., Walford R. L. Age-related decline in thymic-independent immune function in a long-lived mouse strain. J Gerontol. 1974 May;29(3):261–268. doi: 10.1093/geronj/29.3.261. [DOI] [PubMed] [Google Scholar]
- Ghaffar A., Calder E. A., Irvine W. J. K cell cytotoxicity against antibody-coated chicken erythrocytes in tumor-bearing mice: its development with progressively growing tumor and the effect of immunization against the tumor. J Immunol. 1976 Feb;116(2):315–318. [PubMed] [Google Scholar]
- Goidl E. A., Innes J. B., Weksler M. E. Immunological studies of aging. II. Loss of IgG and high avidity plaque-forming cells and increased suppressor cell activity in aging mice. J Exp Med. 1976 Oct 1;144(4):1037–1048. doi: 10.1084/jem.144.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Hooper J. A., Schulof R. S., Cohen G. H., Thurman G. B., McDaniel M. C., White A., Dardenne M. Thymosin and the immunopathology of aging. Fed Proc. 1974 Sep;33(9):2053–2056. [PubMed] [Google Scholar]
- Good R. A., Yunis E. Association of autoimmunity, immunodeficiency and aging in man, rabbits, and mice. Fed Proc. 1974 Sep;33(9):2040–2050. [PubMed] [Google Scholar]
- Heidrick M. L., Makinodan T. Presence of impairment of humoral immunity in nonadherent spleen cells of old mice. J Immunol. 1973 Nov;111(5):1502–1506. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Makinodan T. Immunobiology of aging: evaluation of current status. Clin Immunol Immunopathol. 1976 Nov;6(3):394–413. doi: 10.1016/0090-1229(76)90093-3. [DOI] [PubMed] [Google Scholar]
- Kishimoto S., Takahama T., Mizumachi H. In vitro immune response to the 2,4,6-trinitrophenyl determinant in aged C57BL/6J mice:changes in the humoral immune response to, avidity for the TNP determinant and responsiveness to LPS effect with aging. J Immunol. 1976 Feb;116(2):294–300. [PubMed] [Google Scholar]
- Makinodan T., Albright J. W., Good P. I., Peter C. P., Heidrick M. L. Reduced humoral immune activity in long-lived old mice: an approach to elucidating its mechanisms. Immunology. 1976 Dec;31(6):903–911. [PMC free article] [PubMed] [Google Scholar]
- Makinodan T., Perkins E. H., Chen M. G. Immunologicc activity of the aged. Adv Gerontol Res. 1971;3:171–198. [PubMed] [Google Scholar]
- Peter C. P. Possible immune origin of age-related pathological changes in long-lived mice. J Gerontol. 1973 Jul;28(3):265–275. doi: 10.1093/geronj/28.3.265. [DOI] [PubMed] [Google Scholar]
- Peterson W. J., Makinodan T. Autoimmunity in aged mice. Occurrence of autoagglutinating factors in the blood of aged mice with medium and long life-spans. Clin Exp Immunol. 1972 Oct;12(2):273–290. [PMC free article] [PubMed] [Google Scholar]
- Pollack S. B., Kraft D. S. Effector cells which mediate antibody-dependent cell-mediated cytotoxicity. II. Ontogeny of effector activity in murine spleen. Cell Immunol. 1977 Nov;34(1):1–9. doi: 10.1016/0008-8749(77)90223-4. [DOI] [PubMed] [Google Scholar]
- Segre D., Segre M. Humoral immunity in aged mice. II. Increased suppressor T cell activity in immunologically deficient old mice. J Immunol. 1976 Mar;116(3):735–738. [PubMed] [Google Scholar]
- Smith G. S., Walford R. L., Mickey M. R. Lifespan and incidence of cancer and other diseases in selected long-lived inbred mice and their F 1 hybrids. J Natl Cancer Inst. 1973 May;50(5):1195–1213. doi: 10.1093/jnci/50.5.1195. [DOI] [PubMed] [Google Scholar]
- Suda H., Takita T., Aoyagi T., Umezawa H. The chemical synthesis of bestatin. J Antibiot (Tokyo) 1976 May;29(5):600–601. doi: 10.7164/antibiotics.29.600. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Ishizuka M., Aoyagi T., Takeuchi T. Enhancement of delayed-type hypersensitivity by bestatin, an inhibitor of aminopeptidase B and leucine aminopeptidase. J Antibiot (Tokyo) 1976 Aug;29(8):857–859. doi: 10.7164/antibiotics.29.857. [DOI] [PubMed] [Google Scholar]


