Abstract
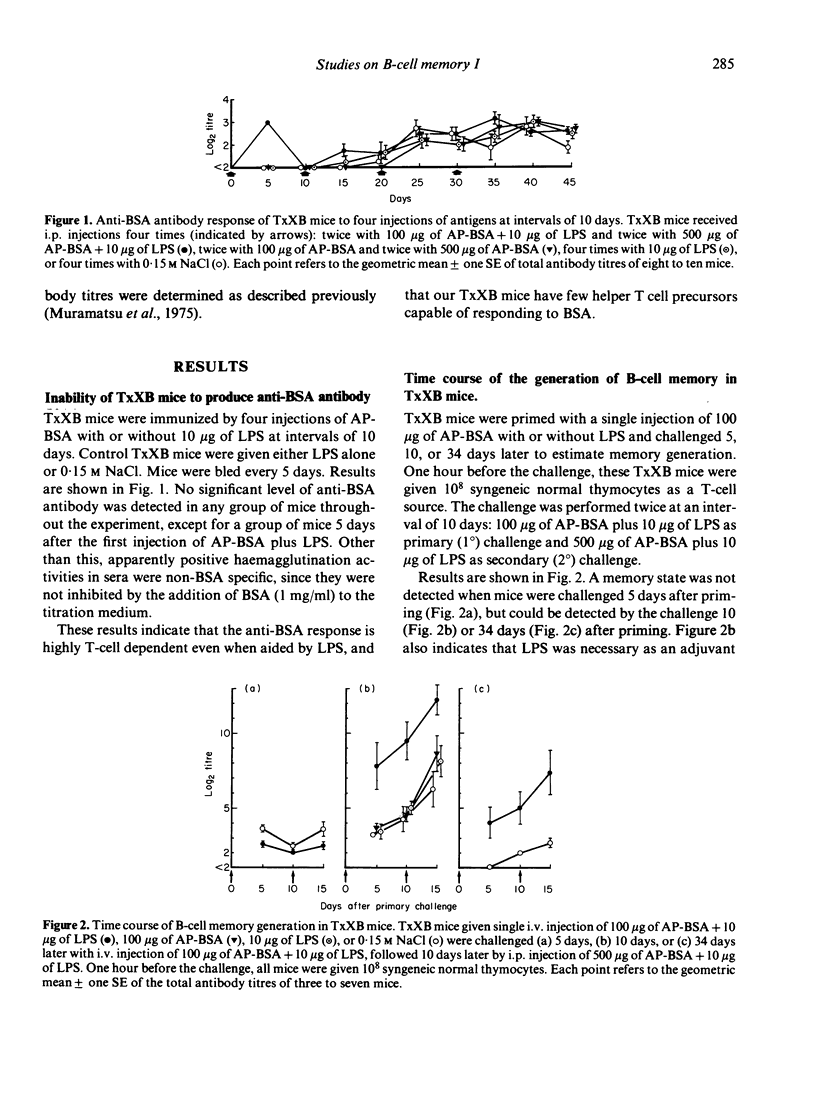
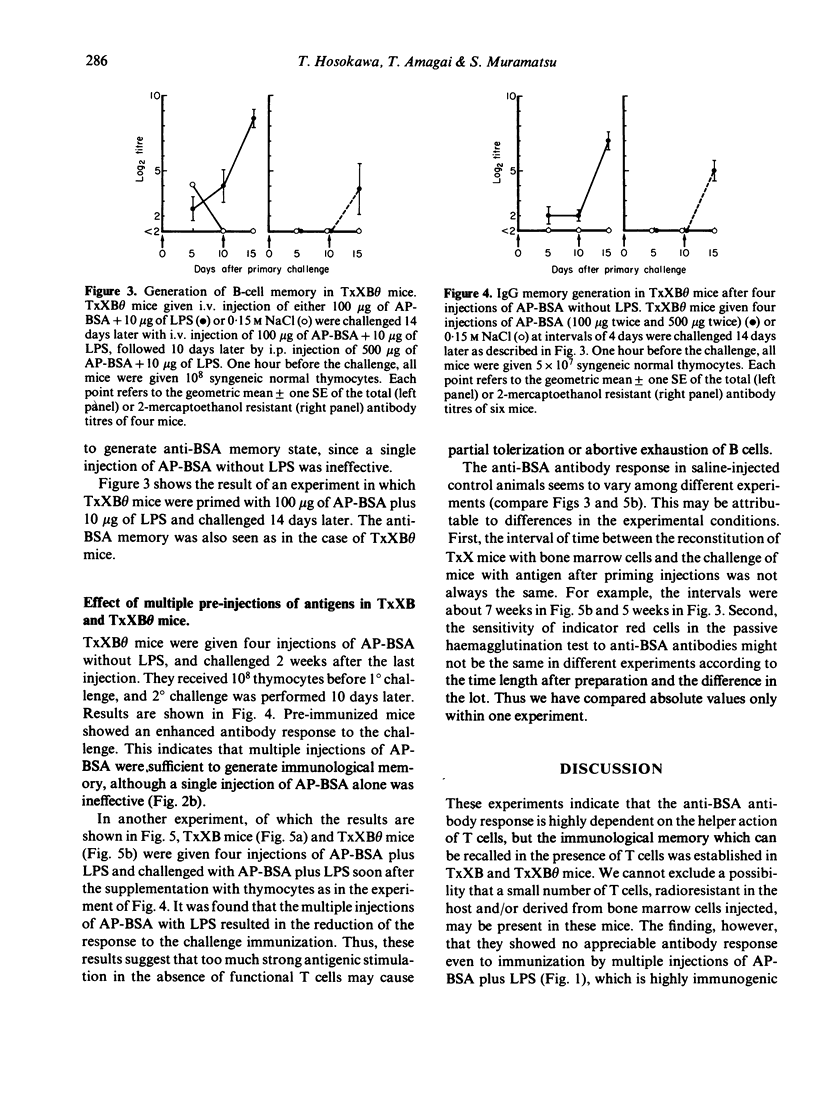
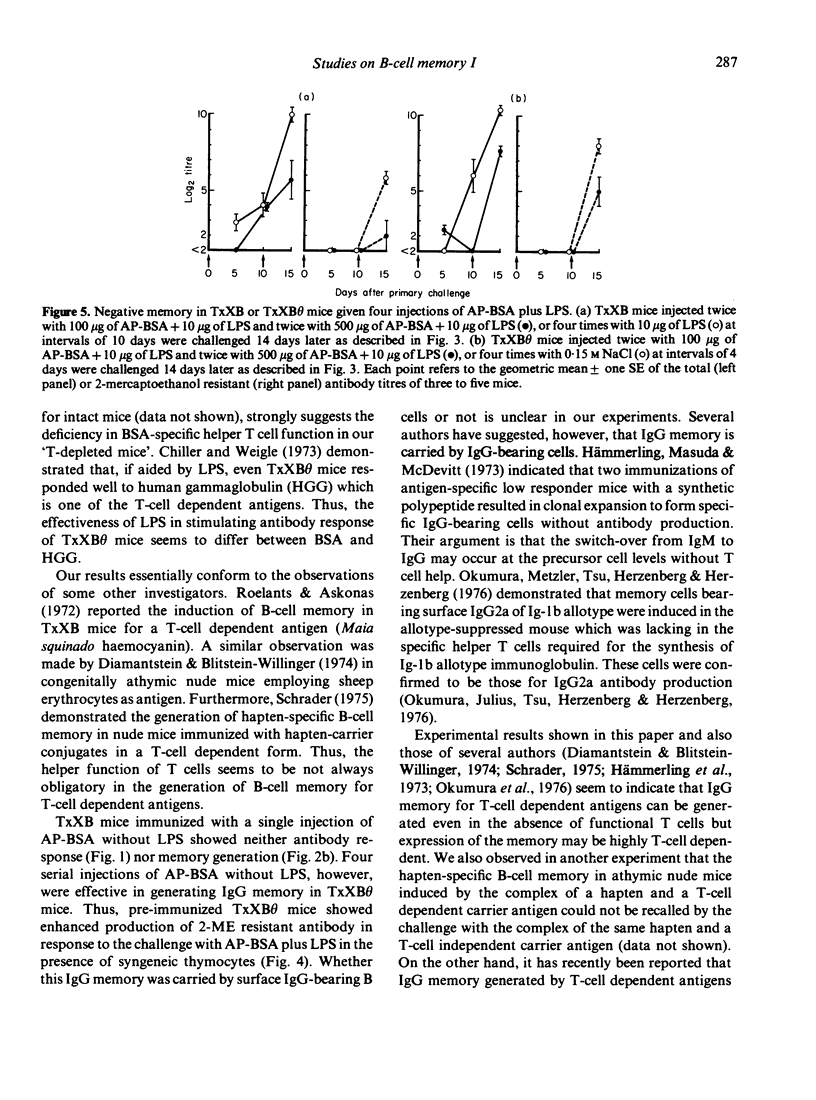
Mice depleted of T cells by adult thymectomy, X-irradiation and reconstitution with syngeneic bone marrow cells, either untreated or treated with anti-Thy-1 serum and complement, were immunized intensively with alum-precipitated bovine serum albumin (AP-BSA) along with or without bacterial lipopolysaccharide (LPS), but no significant anti-BSA antibody response was detected. Priming of the T-cell depleted mice, however, either by a single injection of AP-BSA plus LPS or by multiple injections of AP-BSA without LPS, resulted in the generation of immunological memory. A single injection of AP-BSA without LPS was ineffective. The memory required the aid of syngeneic T cells to be recalled by the challenge with AP-BSA plus LPS. On the other hand, multiple injections of AP-BSA plus LPS did not cause the generation of memory and the response of these mice to the challenge was lower than that of unprimed control mice. These results suggest that (1) the anti-BSA response is highly dependent on the helper function of T cells, (2) the degree of T-cell requirement for the memory generation is very low, and (3) priming with too much strong stimulation in the absence of functional T cells leads to the suppression or abortion of previously generated immunological memory.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braley-Mullen H. Secondary IgG responses to type III pneumococcal polysaccharide. II. Different cellular requirements for induction and elicitation. J Immunol. 1976 Apr;116(4):904–910. [PubMed] [Google Scholar]
- Chan E. L., Mishell R. I., Mitchell G. F. Cell interaction in an immune response in vitro: requirement for theta-carrying cells. Science. 1970 Dec 11;170(3963):1215–1217. doi: 10.1126/science.170.3963.1215. [DOI] [PubMed] [Google Scholar]
- Chiller J. M., Weigle W. O. Termination of tolerance to human gamma globulin in mice by antigen and bacterial lipopolysaccharide (endotoxin). J Exp Med. 1973 Mar 1;137(3):740–750. doi: 10.1084/jem.137.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Sercarz E. E. The asynchronous development of immunological memory in helper (T) and precursor (B) cell lines. Eur J Immunol. 1971 Dec;1(6):413–421. doi: 10.1002/eji.1830010602. [DOI] [PubMed] [Google Scholar]
- Hosokawa T. Studies on B-cell memory. II. T-cell independent antigen can induce B-cell memory. Immunology. 1979 Oct;38(2):291–299. [PMC free article] [PubMed] [Google Scholar]
- Hosono M., Muramatsu S. Use of 2-mercaptoethanol for distinguishing between IgM and IgG antibody-producing cells of mice immunized with bovine globulin. J Immunol. 1972 Oct;109(4):857–863. [PubMed] [Google Scholar]
- Hämmerling G. J., Masuda T., McDevitt H. O. Genetic control of the immune response. Frequency and characteristics of antigen-binding cells in high and low responder mice. J Exp Med. 1973 May 1;137(5):1180–1200. doi: 10.1084/jem.137.5.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Nakano K., Muramatsu S. Regulatory function of T lymphocytes in the immune response to polyvinyl pyrrolidone. I. Two categories of suppressor T cells. Cell Immunol. 1978 Sep;39(2):260–275. doi: 10.1016/0008-8749(78)90102-8. [DOI] [PubMed] [Google Scholar]
- Katsura Y. Studies on M and G antibody response of mice to bovine serum albumin. I. A systematic survey of immunizing conditions. Jpn J Microbiol. 1972 May;16(3):223–232. doi: 10.1111/j.1348-0421.1972.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Kimoto M., Kishimoto T., Noguchi S., Watanabe T., Yamamura Y. Regulation of antibody response in different immunoglobulin classes. II. Induction of in vitro IgE antibody response in murine spleen cells and demonstration of a possible involvement of distinct T-helper cells in IgE and IgG antibody responses. J Immunol. 1977 Mar;118(3):840–845. [PubMed] [Google Scholar]
- Miller J. F., Sprent J. Cell-to-cell interaction in the immune response. VI. Contribution of thymus-derived cells and antibody-forming cell precursors to immunological memory. J Exp Med. 1971 Jul 1;134(1):66–82. doi: 10.1084/jem.134.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S., Amagai T., Katura Y. Tolerance induction in TxXBT and TxXB mice. Immunology. 1975 May;28(5):943–957. [PMC free article] [PubMed] [Google Scholar]
- Nakashima I. Adjuvant action of capsular polysaccharide of Klebsiella pneumoniae on antibody response. I. Intensity of its action. J Immunol. 1972 Apr;108(4):1009–1016. [PubMed] [Google Scholar]
- Nakashima I., Kato N. Non-specific stimulation of immunoglobulin synthesis in mice by capsular polysaccharide of Klebsiella pneumoniae. Immunology. 1974 Aug;27(2):179–193. [PMC free article] [PubMed] [Google Scholar]
- Nakashima I., Nagase F., Yokochi T., Kojima T., Ohta M., Kato N. Comparative studies on the actions of antigen and polyclonal B-cell activator in differentiation and proliferation of B-cells and B memory cells. Immunology. 1976 Oct;31(4):649–658. [PMC free article] [PubMed] [Google Scholar]
- Okumura K., Julius M. H., Tsu T., Herzenberg L. A., Herzenberg L. A. Demonstration that IgG memory is carried by IgG-bearing cells. Eur J Immunol. 1976 Jul;6(7):467–472. doi: 10.1002/eji.1830060704. [DOI] [PubMed] [Google Scholar]
- Okumura K., Metzler C. M., Tsu T. T., Herzenberg L. A., Herzenberg L. A. Two stages of B-cell memory development with different T-cell requirements. J Exp Med. 1976 Aug 1;144(2):345–357. doi: 10.1084/jem.144.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Role of thymus-derived lymphocytes in the secondary humoral immune response in mice. Nature. 1970 Jun 27;226(5252):1257–1258. doi: 10.1038/2261257a0. [DOI] [PubMed] [Google Scholar]
- Schrader J. W. The role of T cells in IgG production; thymus-dependent antigens induce B cell memory in the absence of T cells. J Immunol. 1975 Jun;114(6):1665–1669. [PubMed] [Google Scholar]
- Takaoki M. Transition in the character of immunological memory in mice after immunization. II. Memory in T and B cell populations. Jpn J Microbiol. 1976 Dec;20(6):475–483. doi: 10.1111/j.1348-0421.1976.tb01015.x. [DOI] [PubMed] [Google Scholar]
- Tittle T. V., Rittenberg M. B. Expression of IgG memory response in vitro to thymus-dependent and thymus-independent antigens. Cell Immunol. 1978 Jan;35(1):180–190. doi: 10.1016/0008-8749(78)90138-7. [DOI] [PubMed] [Google Scholar]


