Abstract
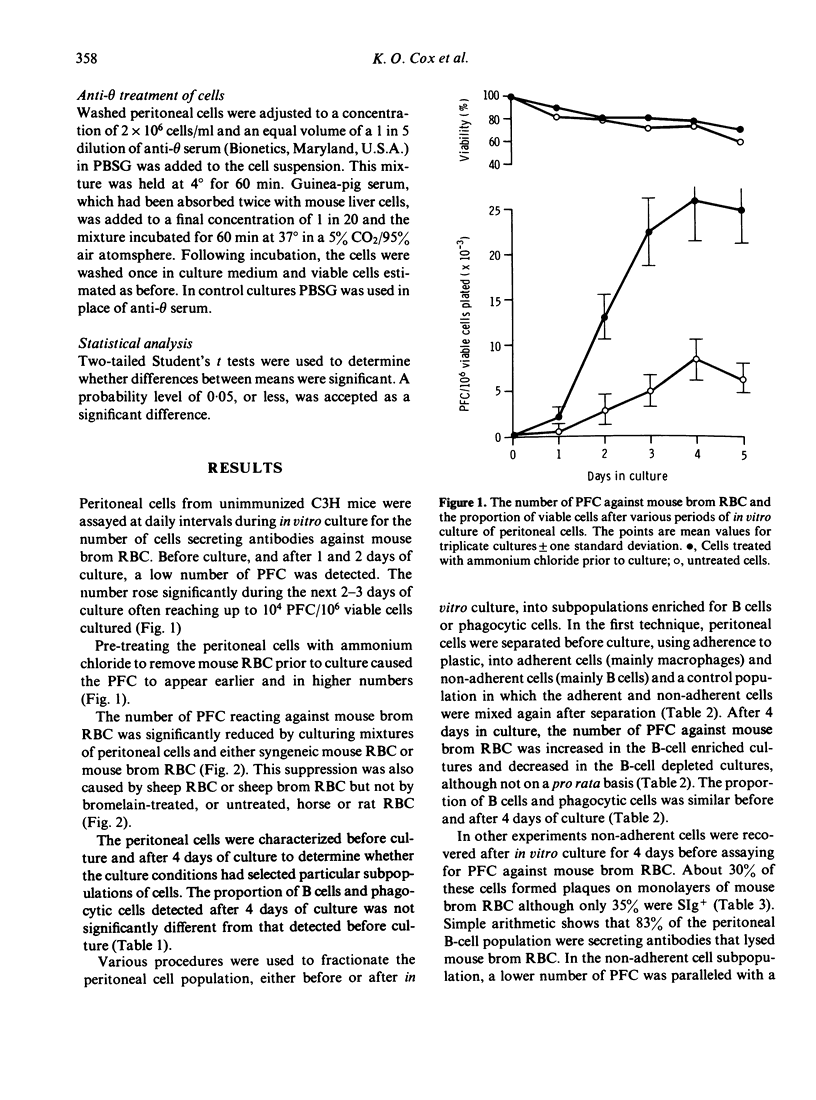
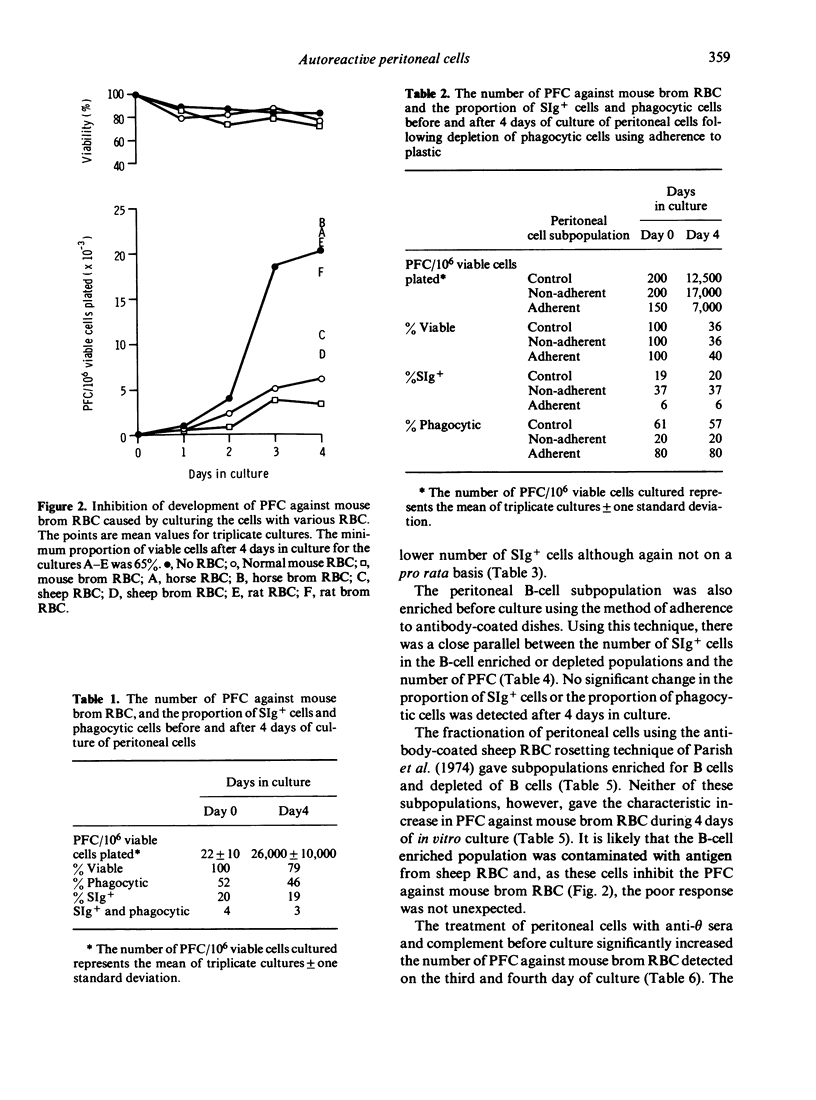
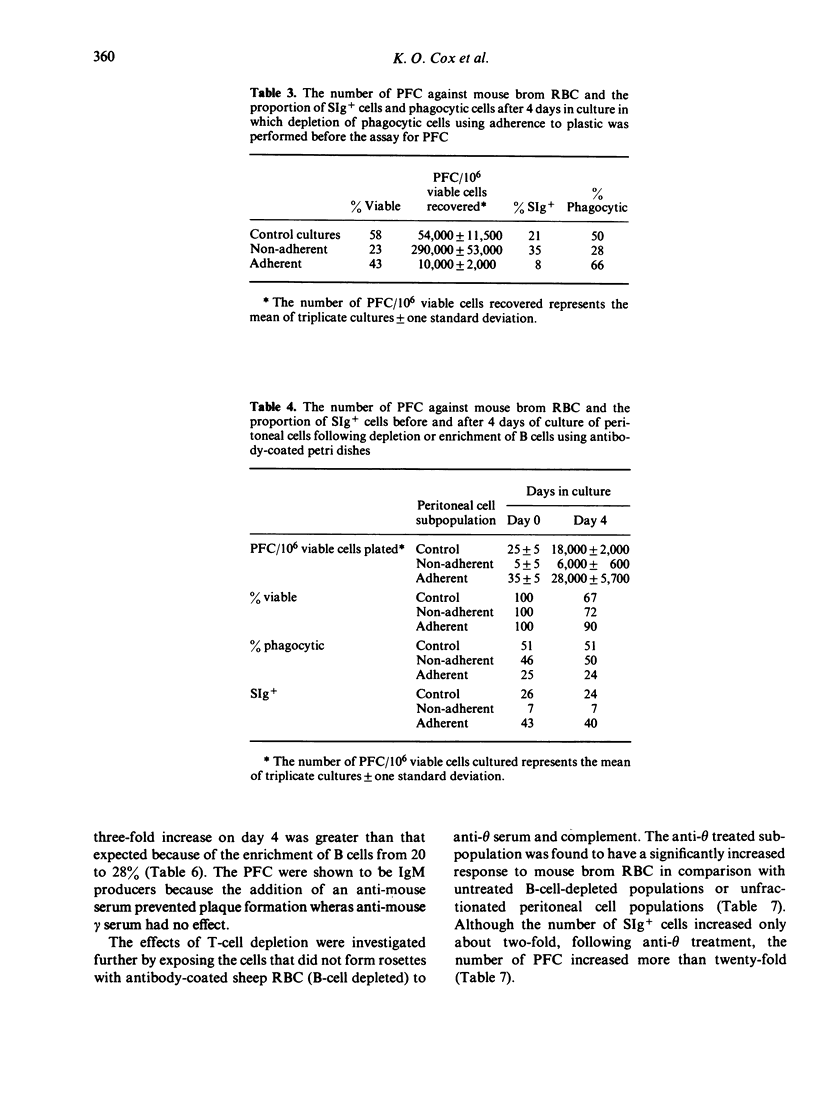
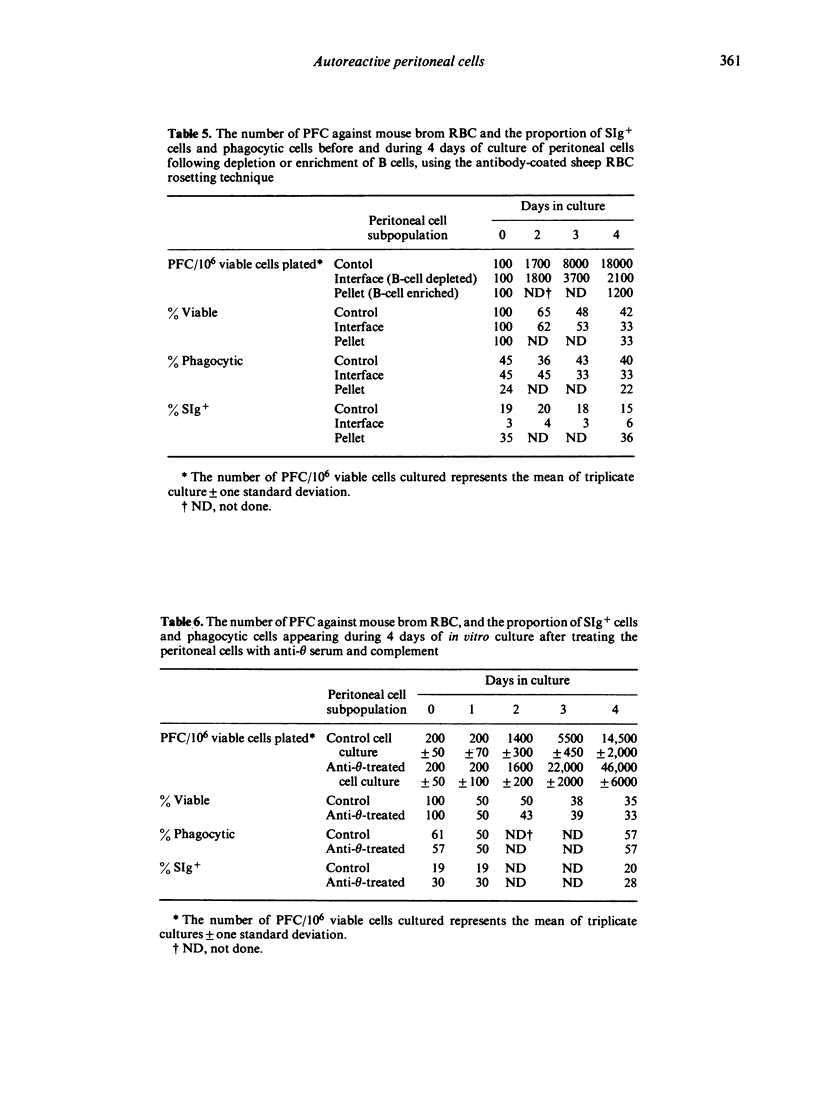
A high proportion of peritoneal cells from untreated mice, after 4--5 days in culture, develop into plaque-forming cells against bromelain-treated mouse red blood cells. The number of plaque-forming cells was increased significantly by exposing the peritoneal cells to ammonium chloride to lyse red blood cells before culture. Conversely, the increase was significantly inhibited by adding before culture untreated or bromelain-treated sheep or mouse red blood cells. Treated or untreated horse or rat red blood cells did not inhibit the increase. Treating peritoneal cells or subpopulations of peritoneal cells with anti-theta serum and complement before culture caused a significant increase in the number of plaque-forming cells against bromelain-treated red blood cells after 3--4 days of culture. Various procedures were used to fractionate peritoneal cells into B-cell enriched and B-cell depleted subpopulations before culture and after culture, to investigate whether some of the plaque-forming cells could be attributed to phagocytic cells. Generally, changes in the number of plaque-forming cells against bromelain-treated mouse red blood cells paralleled changes in B-cells. In some experiments the proportion of plaque-forming cells observed represented up to 85% of the B-cells present. The results suggest that the high level of autoreactivity is due to antibody production by B-cells and that phagocytic cells are not forming spurious plaques. Further, it appears that the autoimmunity is regulated by T-cells and can also be inhibited by mouse RBC.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bussard A. E. Antibody formation in nonimmune mouse peritoneal cells after incubation in gum containing antigen. Science. 1966 Aug 19;153(3738):887–888. doi: 10.1126/science.153.3738.887. [DOI] [PubMed] [Google Scholar]
- Calderon J., Kiely J. M., Lefko J. L., Unanue E. R. The modulation of lymphocyte functions by molecules secreted by macrophages. I. Description and partial biochemical analysis. J Exp Med. 1975 Jul 1;142(1):151–164. doi: 10.1084/jem.142.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. O., Baddams H., Evans A. Studies of the antigenicity and immunogenicity of bromelain-pretreated red blood cells. Aust J Exp Biol Med Sci. 1977 Feb;55(1):27–37. doi: 10.1038/icb.1977.3. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J. Self-tolerance maintained by active suppressor mechanisms. Transplant Rev. 1976;31:23–43. doi: 10.1111/j.1600-065x.1976.tb01451.x. [DOI] [PubMed] [Google Scholar]
- DeHeer D. H., Edgington T. S. Specific antigen-binding and antibody-secreting lymphocytes associated with the erythrocyte autoantibody responses of NZB and genetically unrelated mice. J Immunol. 1976 Apr;116(4):1051–1058. [PubMed] [Google Scholar]
- Dresser D. W. Most IgM-producing cells in the mouse secrete auto-antibodies (rheumatoid factor). Nature. 1978 Aug 3;274(5670):480–483. doi: 10.1038/274480a0. [DOI] [PubMed] [Google Scholar]
- Gisler R. H., Pagès J. M., Bussard A. E. Spontaneous development of plaque-forming cells against sheep erythrocytes by mouse peritoneal cells in culture. Ann Immunol (Paris) 1975 Feb-Mar;126(2):231–238. [PubMed] [Google Scholar]
- Lord E. M., Dutton R. W. Antigen suppression of the in vitro development of plaque-forming cells to autologous erythrocyte antigens. J Immunol. 1975 Dec;115(6):1631–1635. [PubMed] [Google Scholar]
- Lord E. M., Dutton R. W. The properties of plaque-forming cells from autoimmune and normal strains of mice with specificity for autologous erythrocyte antigens. J Immunol. 1975 Nov;115(5):1199–1205. [PubMed] [Google Scholar]
- Pages J. M., Bussard A. E. Establishment and characterization of a permanent murine hybridoma secreting monoclonal autoantibodies. Cell Immunol. 1978 Nov;41(1):188–194. doi: 10.1016/s0008-8749(78)80038-0. [DOI] [PubMed] [Google Scholar]
- Pages J., Bussard A. E. Precommitment of normal mouse peritoneal cells by erythrocyte antigens in relation to auto-antibody production. Nature. 1975 Sep 25;257(5524):316–317. doi: 10.1038/257316a0. [DOI] [PubMed] [Google Scholar]
- Ramshaw I. A., Eidinger D. T-cell mediated immunity towards antigen(s) on isologous erythrocytes. Nature. 1977 Jun 2;267(5610):441–442. doi: 10.1038/267441a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg Y. J., Parish C. R. Ontogeny of the antibody-forming cell line in mice. IV. Appearance of cells bearing Fc receptors, complement receptors, and surface immunoglobulin. J Immunol. 1977 Feb;118(2):612–617. [PubMed] [Google Scholar]
- Santoli D., Trinchieri G. Evaluation of the effect of ammonium chloride treatment on the activity of human effector cells in antibody-dependent and spontaneous cell-mediated cytotoxicity. J Immunol Methods. 1977;15(1):97–100. doi: 10.1016/0022-1759(77)90020-5. [DOI] [PubMed] [Google Scholar]
- Steele E. J., Cunningham A. J. High proportion of Ig-producing cells making autoantibody in normal mice. Nature. 1978 Aug 3;274(5670):483–484. doi: 10.1038/274483a0. [DOI] [PubMed] [Google Scholar]


