Abstract
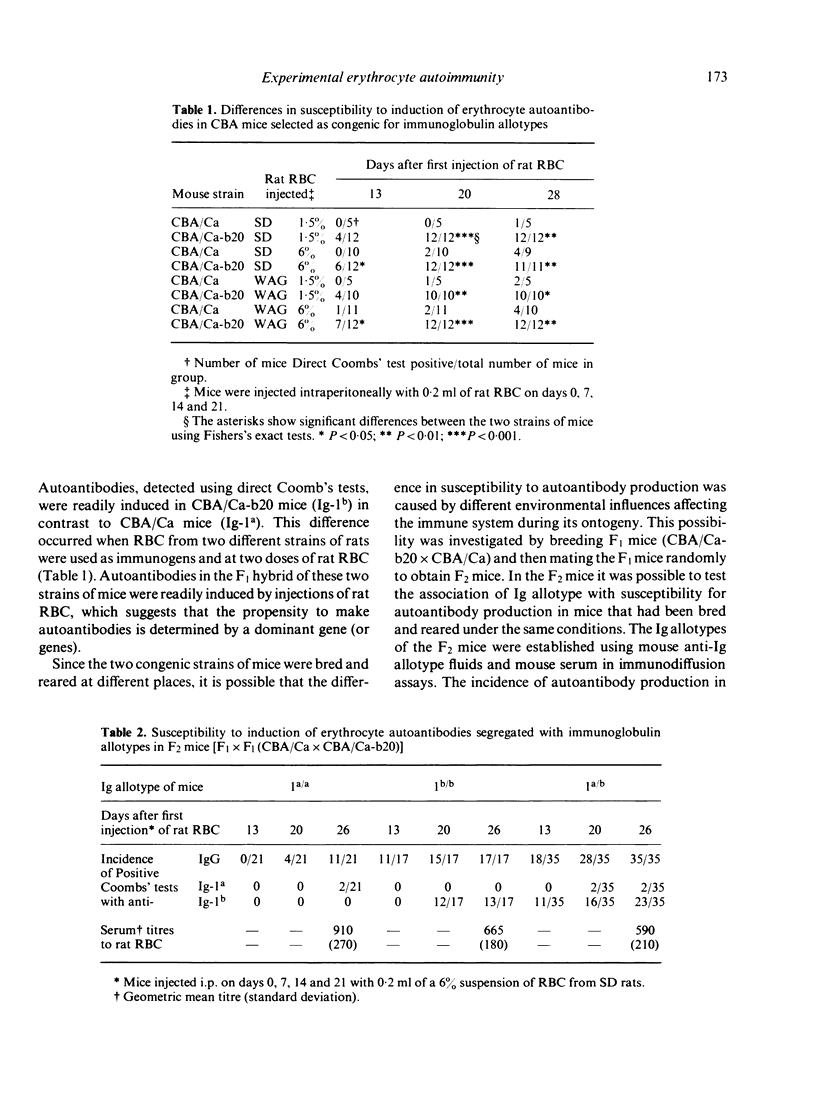
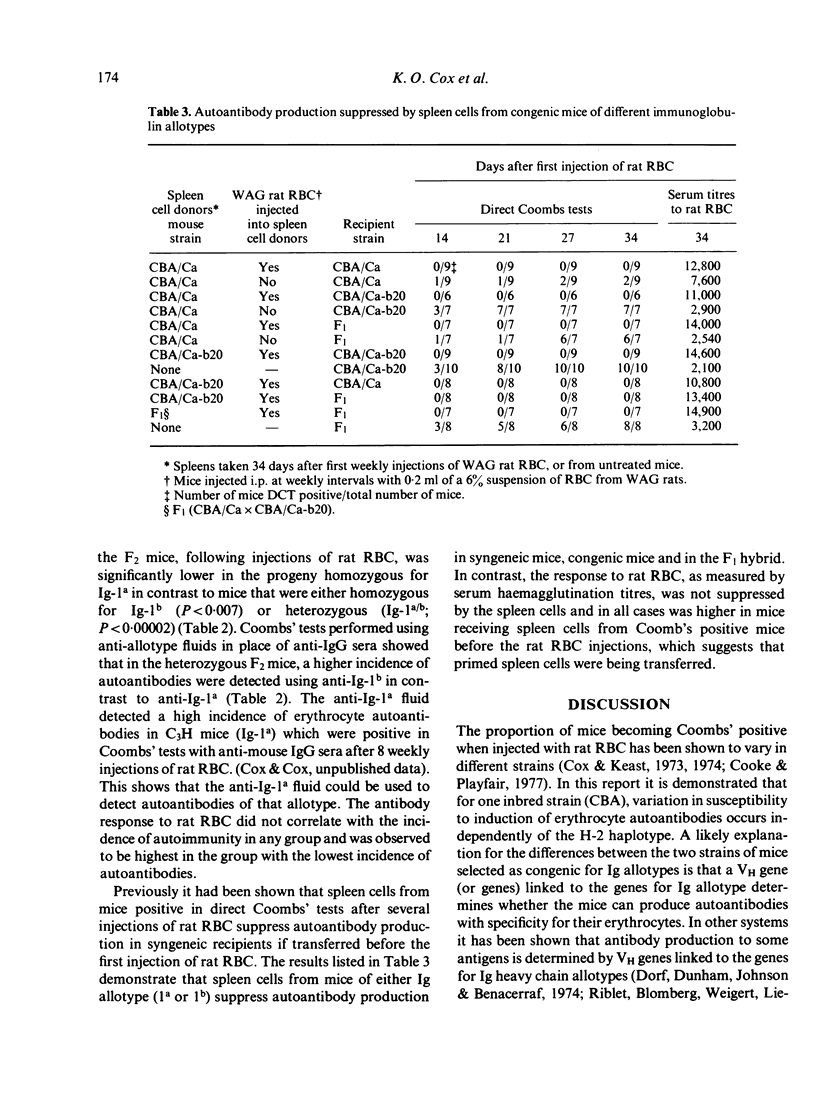
Erythrocyte autoantibodies can be elicited in mnce by injections of rat RBC which are cross-reactive with mouse RBC. This report shows that induction of autoantibodies is dependent, in part, on gene(s) outside the H-2 complex. Using CBA mice congenic for Ig allotype and the F1 and F2 hybrids, a higher incidence of autoantibody production was observed in mice bearing the Ig allotype 1b (1b/b or 1a/b) in contrast to mice homozygous for the allotype Ig-1a. Serum haemagglutination titres against rat RBC were not reduced in the groups of mice with the lower incidence of autoantibody production. A probable explanation for these observations is that the change in Ig allotype is associated with some change in the variable region determining autoimmune specificity that is governed by VH genes linked to allotype genes. The transfer of 30 x 10(6) spleen cells from Coombs' positive mice to syngeneic recipients before starting the immunization regime with rat RBC suppressed autoantibody production and enhanced antibody production against rat RBC. These suppressor cells were effective in congenic mice and in F1 hybrids, which suggest that the Ig allotype is not a crucial site for the effector stage of suppression of this autoimmune response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cebra J. J., Colberg J. E., Dray S. Rabbit lymphoid cells differentiated with respect to alpha-, gamma-, and mu- heavy polypeptide chains and to allotypic markers Aa1 and Aa2. J Exp Med. 1966 Mar 1;123(3):547–558. doi: 10.1084/jem.123.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke A., Hutchings P. R., Playfair J. H. Suppressor T cells in experimental autoimmune haemolytic anaemia. Nature. 1978 May 11;273(5658):154–155. doi: 10.1038/273154a0. [DOI] [PubMed] [Google Scholar]
- Cooke A., Playfair J. H. Hyper-responsiveness in NZB mice to the experimental induction of anti-red cell autoantibody. Clin Exp Immunol. 1977 Mar;27(3):538–544. [PMC free article] [PubMed] [Google Scholar]
- Cox K. O., Finlay-Jones J. J. Impaired regulation of erythrocyte autoantibody production after splenectomy. Br J Exp Pathol. 1979 Oct;60(5):466–470. [PMC free article] [PubMed] [Google Scholar]
- Cox K. O., Keast D. Autoimmune haemolytic anaemia induced in mice immunized with rat erythrocytes. Clin Exp Immunol. 1974 Jun;17(2):319–327. [PMC free article] [PubMed] [Google Scholar]
- Cox K. O., Keast D. Erythrocyte autoantibodies induced in mice immunized with rat erythrocytes. Immunology. 1973 Sep;25(3):531–539. [PMC free article] [PubMed] [Google Scholar]
- Cunliffe D. A., Cox K. O. Effects of bromelain and pronase on erythrocyte membranes. Mol Immunol. 1979 Jun;16(6):427–433. doi: 10.1016/0161-5890(79)90111-1. [DOI] [PubMed] [Google Scholar]
- Dorf M. E., Dunham E. K., Johnson J. P., Benacerraf B. Genetic control of the immune response: the effect of non-H-2 linked genes on antibody production. J Immunol. 1974 Apr;112(4):1329–1336. [PubMed] [Google Scholar]
- Fernandez C., Lieberman R., Möller G. The immune response to the alpha 1-6 epitope of dextran is determined by a gene linked to the IgCH locus. Scand J Immunol. 1979;10(1):77–80. doi: 10.1111/j.1365-3083.1979.tb01337.x. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Okumura K., Cantor H., Sato V. L., Shen F. W., Boyse E. A., Herzenberg L. A. T-cell regulation of antibody responses: demonstration of allotype-specific helper T cells and their specific removal by suppressor T cells. J Exp Med. 1976 Aug 1;144(2):330–344. doi: 10.1084/jem.144.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzelberger D., Eichmann K. Idiotype suppression. III. Induction of unresponsiveness to sensitization with anti-idiotypic antibody: identification of the cell types tolerized in high zone and in low zone, suppressor cell-mediated, idiotype suppression. Eur J Immunol. 1978 Dec;8(12):839–846. doi: 10.1002/eji.1830081204. [DOI] [PubMed] [Google Scholar]
- Näkelä O., Kaartinen M., Pelkonen J. L., Karjalainen K. Inheritance of antibody specificity V. Anti-2-phenyloxazolone in the mouse. J Exp Med. 1978 Dec 1;148(6):1644–1660. doi: 10.1084/jem.148.6.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., Marshall-Clarke S. Induction of red cell autoantibodies in normal mice. Nat New Biol. 1973 Jun 13;243(128):213–214. doi: 10.1038/newbio243213a0. [DOI] [PubMed] [Google Scholar]


