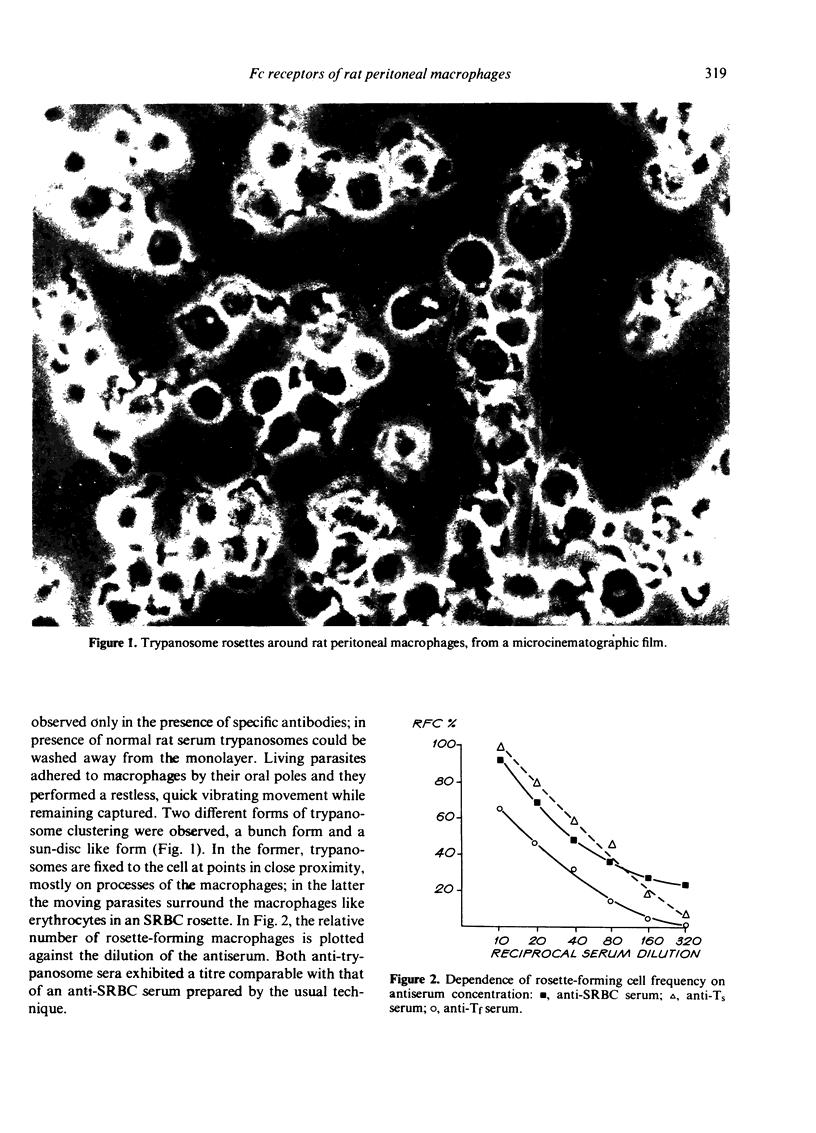
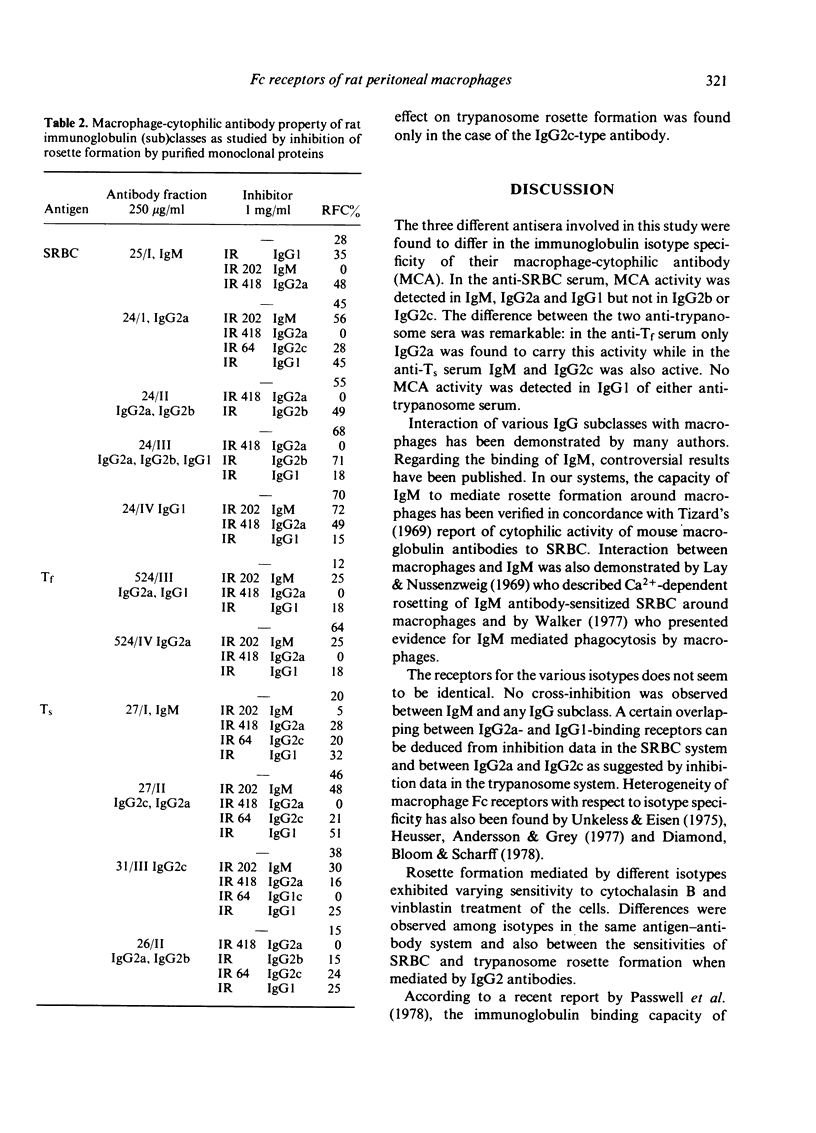
Abstract
Macrophage-cytophilic antibody activity of various immunoglobulin classes and subclasses was tested in two different rosetting systems. Cytophilic antibody activity of IgM, IgG2a and IgG1 was verified in the SRBC system, while IgM, IgG2a and IgG2c were found to be active in the trypanosome system. Sensitivity to cytochalasin B treatment of SRBC rosette formation was dependent on the class of antibody and decreased in the following order: IgM > IgG1 > IgG2a. Trypanosome rosette formation was prevented by the same drug regardless the type of antibody. Vinblastin caused an enhancement of rosette formation in the SRBC system in low concentration, except when the antibody belonged to subclass IgG1. The enhancing effect was less pronounced in the trypanosome system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson J. P., Michael J. M., Chaplin H., Jr, Parker C. W. Modulation of macrophage C3b receptor function by cytochalasin-sensitive structures. J Immunol. 1977 Apr;118(4):1292–1299. [PubMed] [Google Scholar]
- Atkinson J. P., Parker C. W. Modulation of macrophage Fc receptor function by cytochalasin-sensitive structures. Cell Immunol. 1977 Oct;33(2):353–363. doi: 10.1016/0008-8749(77)90164-2. [DOI] [PubMed] [Google Scholar]
- Bazin H., Beckers A., Querinjean P. Three classes and four (sub)classes of rat immunoglobulins: IgM, IgA, IgE and IgG1, IgG2a, IgG2b, IgG2c. Eur J Immunol. 1974 Jan;4(1):44–48. doi: 10.1002/eji.1830040112. [DOI] [PubMed] [Google Scholar]
- Diamond B., Bloom B. R., Scharff M. D. The Fc receptors of primary and cultured phagocytic cells studied with homogeneous antibodies. J Immunol. 1978 Oct;121(4):1329–1333. [PubMed] [Google Scholar]
- Heusser C. H., Anderson C. L., Grey H. M. Receptors for IgG: subclass specificity of receptors on different mouse cell types and the definition of two distinct receptors on a macrophage cell line. J Exp Med. 1977 May 1;145(5):1316–1327. doi: 10.1084/jem.145.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson D. W., Kijlstra A., Van Es L. A. Association and dissociation of aggregated IgG from rat peritoneal macrophages. J Exp Med. 1977 May 1;145(5):1368–1381. doi: 10.1084/jem.145.5.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Ca++-dependent binding of antigen-19 S antibody complexes to macrophages. J Immunol. 1969 May;102(5):1172–1178. [PubMed] [Google Scholar]
- Passwell J. H., Schneeberger E., Merler E. Cellular requirements for the formation of EA rosettes by human monocytes. Immunology. 1978 Dec;35(6):863–872. [PMC free article] [PubMed] [Google Scholar]
- Takayanagi T., Nakatake Y., Enriquez G. L. Attachment and ingestion of Trypanosoma gambiense to the rat macrophage by specific antiserum. J Parasitol. 1974 Apr;60(2):336–339. [PubMed] [Google Scholar]
- Tizard I. R. Macrophage cytophilic antibody in mice. Differentiation between antigen adherence due to these antibodies and opsoni adherence. Int Arch Allergy Appl Immunol. 1969;36(4):332–346. [PubMed] [Google Scholar]
- Unkeless J. C., Eisen H. N. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J Exp Med. 1975 Dec 1;142(6):1520–1533. doi: 10.1084/jem.142.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. S. Mediation of macrophage cytolytic and phagocytic activities by antibodies of different classes and class-specific Fc-receptors. J Immunol. 1977 Aug;119(2):367–373. [PubMed] [Google Scholar]