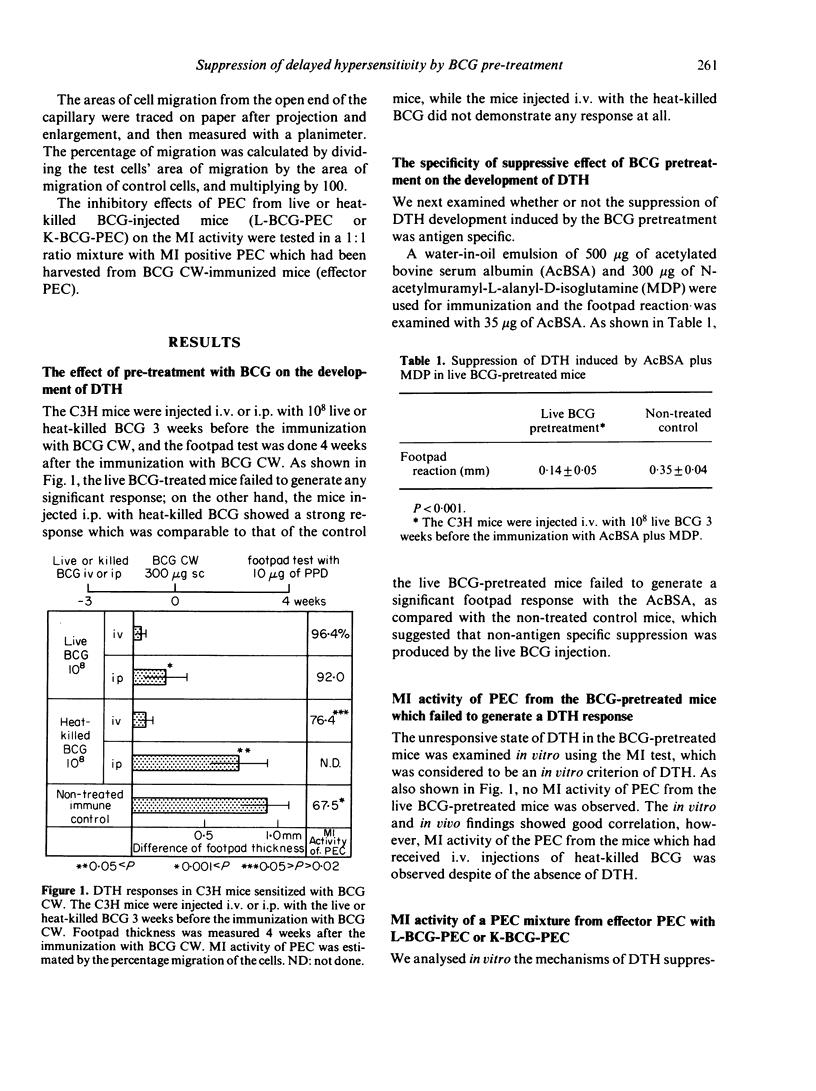
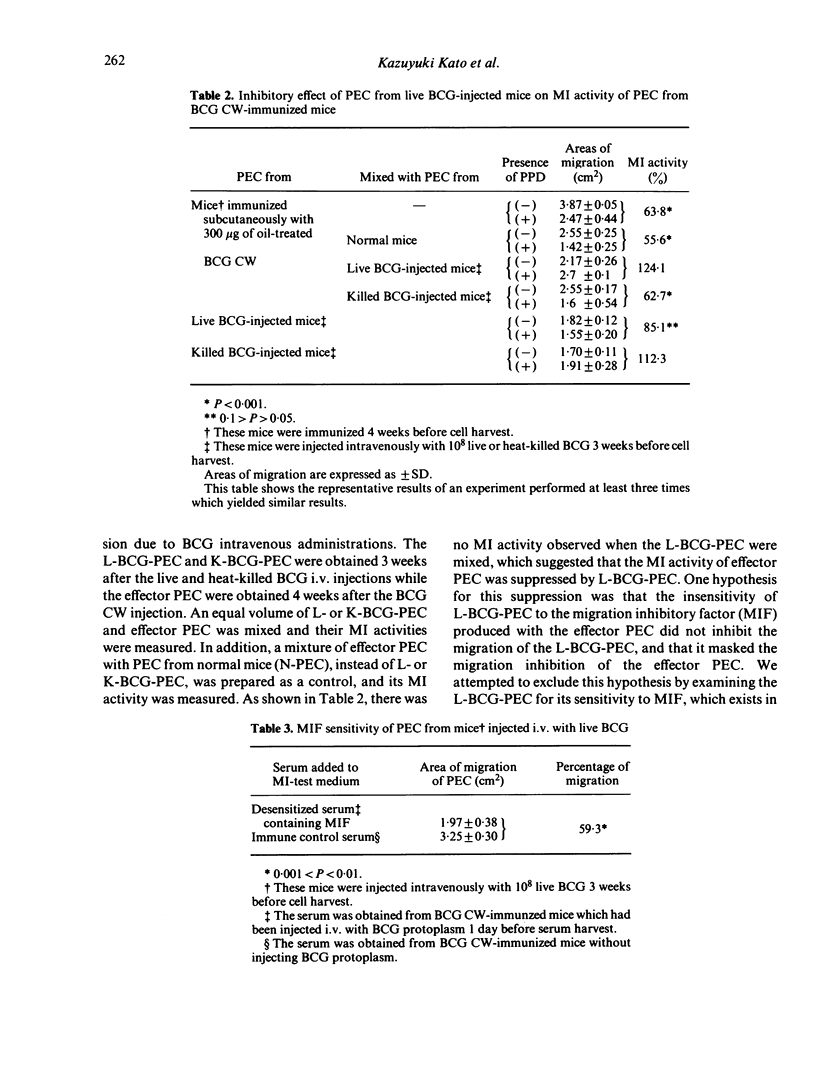
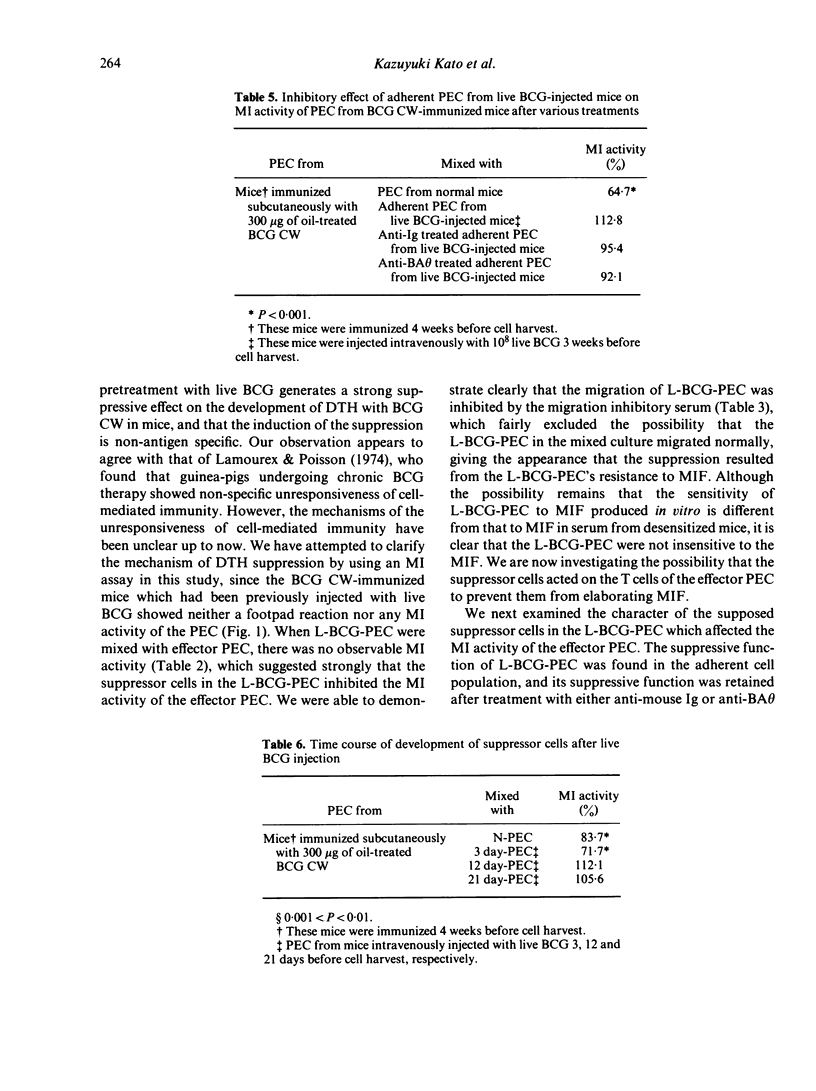
Abstract
Previous injections of live Bacillus Calmette-Guérin (BCG) in mice produced a suppression of delayed-type hypersensitivity (DTH) induced by oil-treated BCG cell walls (CW). This phenomenon was analysed by the macrophage migration inhibition (MI) test in which peritoneal exudate cells (PEC) from live BCG-injected mice were mixed with PEC from BCG CW-immunized mice, with the result that the former cells suppressed the MI activity in the latter. We considered the Mi test to be a reliable method for demonstrating the existence of suppressor cells induced by the injection of live BCG. Moreover, we found that the adherent cells of PEC possessed a suppressive effect which was retained even after treatment with either anti-mouse Ig or anti-brain associated theta (BA theta) antigen; that the PEC from mice injected with live BCG on at least the 12th day before cell harvesting showed the suppression; and that the suppression operated across the H-2 barrier.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brehmer W., Anacker R. L., Ribi E. Immunogenicity of cell walls from various mycobacteria against airborne tuberculosis in mice. J Bacteriol. 1968 Jun;95(6):2000–2004. doi: 10.1128/jb.95.6.2000-2004.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Ellner J. J. Suppressor adherent cells in human tuberculosis. J Immunol. 1978 Dec;121(6):2573–2579. [PubMed] [Google Scholar]
- Golub E. S. Brain-associated theta antigen: reactivity of rabbit anti-mouse brain with mouse lymphoid cells. Cell Immunol. 1971 Aug;2(4):353–361. doi: 10.1016/0008-8749(71)90070-0. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Holden H. T., Herberman Splenic suppressor macrophages induced in mice by injection of Corynebacterium parvum. J Immunol. 1975 Nov;115(5):1212–1216. [PubMed] [Google Scholar]
- Klimpel G. R., Henney C. S. BCG-induced suppressor cells. I. Demonstration of a macrophage-like suppressor cell that inhibits cytotoxic T cell generation in vitro. J Immunol. 1978 Feb;120(2):563–569. [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B. A stable form of delayed-type hypersensitivity. J Exp Med. 1975 Jan 1;141(1):82–96. doi: 10.1084/jem.141.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoureux G., Poisson R. Letter: B.C.G. and immunological anergy. Lancet. 1974 May 18;1(7864):989–990. doi: 10.1016/s0140-6736(74)91296-3. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Ishibashi T. The modifying effect of BCG on the immunological induction of T cells. J Exp Med. 1974 Jun 1;139(6):1540–1552. doi: 10.1084/jem.139.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markenson J. A., Morgan J. W., Lockshin M. D., Joachim C., Winfield J. B. Responses of fractionated cells from patients with systemic lupus erythematosus and normals to plant mitogen: evidence for a suppressor population of monocytes. Proc Soc Exp Biol Med. 1978 May;158(1):5–9. doi: 10.3181/00379727-158-40127. [DOI] [PubMed] [Google Scholar]
- Scott M. T. Biological effects of the adjuvant Corynebacterium parvum. II. Evidence for macrophage-T-cell interaction. Cell Immunol. 1972 Nov;5(3):469–479. doi: 10.1016/0008-8749(72)90073-1. [DOI] [PubMed] [Google Scholar]
- Spring S. B., Nisonoff A. Allotypic markers on Fab fragments of mouse immunoglobulins. J Immunol. 1974 Aug;113(2):470–478. [PubMed] [Google Scholar]
- Stobo J. D. Immunosuppression in man: suppression by macrophages can be mediated by interactions with regulatory T cells. J Immunol. 1977 Sep;119(3):918–924. [PubMed] [Google Scholar]
- Turcotte R., Lafleur L., Labrèche M. Opposite effects of BCG on spleen and lymph node cells: lymphocyte proliferation and immunoglobulin synthesis. Infect Immun. 1978 Sep;21(3):696–704. doi: 10.1128/iai.21.3.696-704.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey J. J., Laughter A. H., Farrow S., Douglass C. C. Hodgkin's disease. An immunodepleting and immunosuppressive disorder. J Clin Invest. 1975 Aug;56(2):467–475. doi: 10.1172/JCI108113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Anacker R. L., Ribi E. Macrophage Migration Inhibition Studies with Cells from Mice Vaccinated with Cell Walls of Mycobacterium bovis BCG: Relationship Between Inhibitory Activity of Lung Cells and Resistance to Airborne Challenge with Mycobacterium tuberculosis H37Rv. Infect Immun. 1970 Jun;1(6):595–599. doi: 10.1128/iai.1.6.595-599.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Karinuma M. Genetic control of granuloma response to oil-associated BCG cell wall vaccine in mice. Microbiol Immunol. 1978;22(6):335–348. doi: 10.1111/j.1348-0421.1978.tb00378.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Takahashi Y. Macrophage migration inhibition by serum from desensitized animals previously sensitized with tubercle bacilli. Nat New Biol. 1971 Oct 27;233(43):261–263. doi: 10.1038/newbio233261a0. [DOI] [PubMed] [Google Scholar]


