Abstract
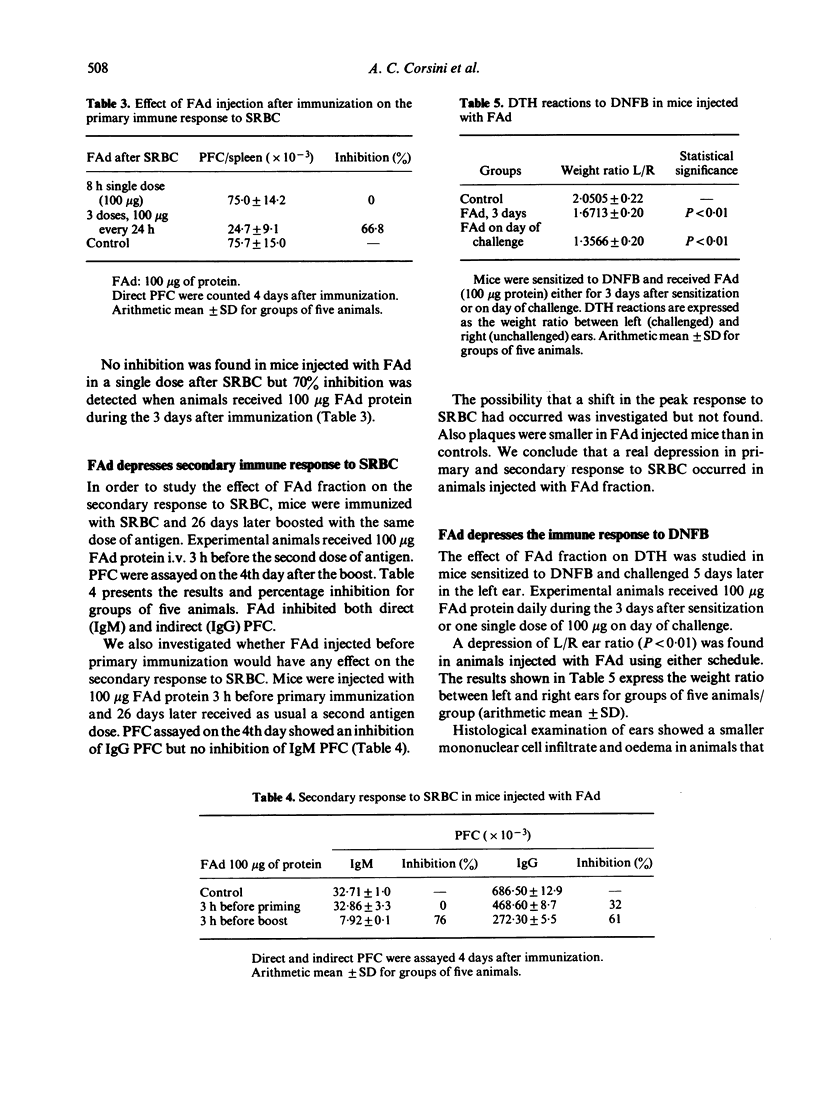
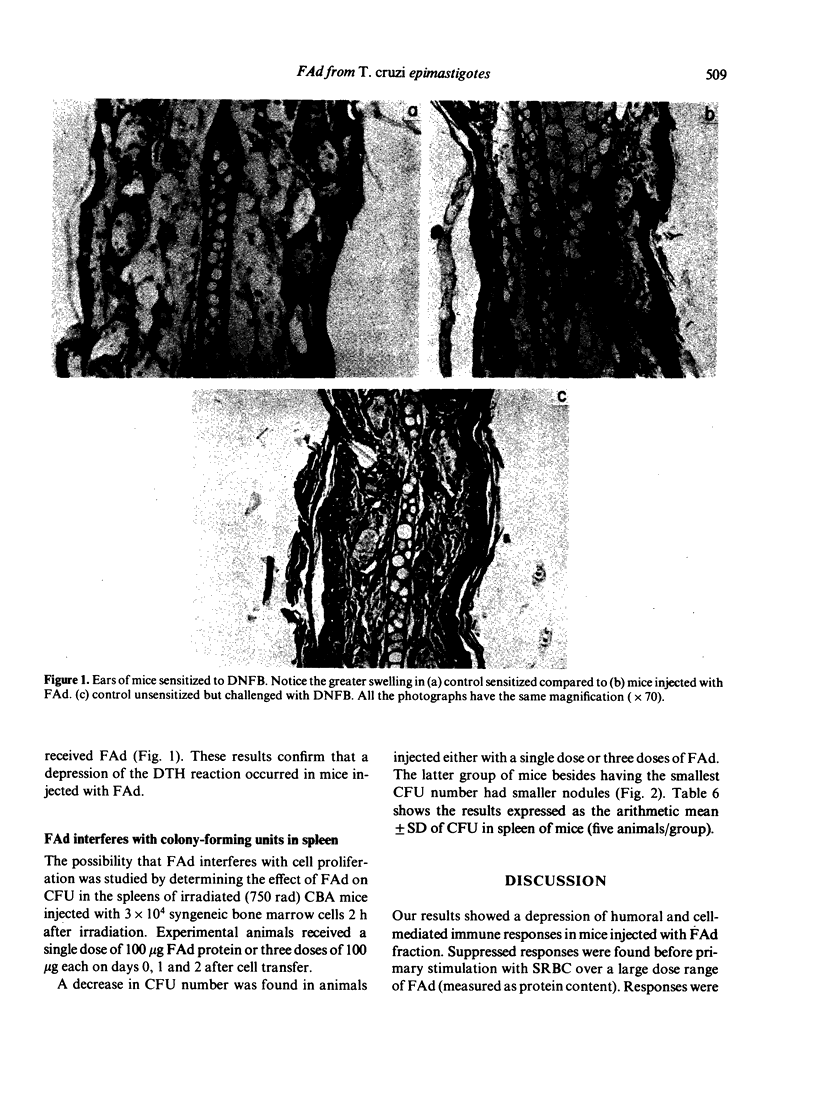
The primary immune response to SRBC in BALB/c mice was depressed when they were injected with a fraction (FAd) obtained from Trypanosoma cruzi epimastigotes grown in LIT medium. Plaque-forming cell (PFC) number was 50% less than controls when FAd was injected i.v. 15 min before antigen in doses ranging from 70 microgram up to 400 microgram of protein. Similar depression was observed when 100 microgram FAd was injected up to 6 h before antigen. There was no shift in the peak response to SRBC, neither was depression detected, when a total of 100 microgram FAd protein was given in 20 microgram amounts twice a day before immunization. Mice injected with FAd fraction only showed no increase in background PFC. Both secondary IgM and secondary IgG PFC were depressed when FAd was given before the boosting injection. However, only IgG PFC were depressed when FAd was injected before the priming dose. The delayed-type hypersensitivity reaction to DNFB was depressed when animals were injected either during the 3 days after sensitization or with a single dose of 100 microgram of protein of FAd on day of challenge. Bone marrow colony-forming units in spleens of mice injected with FAd were depressed and nodules in the treated animals were smaller than in controls. We conclude that FAd affects humoral and cell-mediated immune responses by interfering with cell division at some stage of the cell cycle.
Full text
PDF




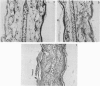
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askonas B. A., Corsini A. C., Clayton C. E., Ogilvie B. M. Functional depletion of T- and B-memory cells and other lymphoid cell subpopulations-during trypanosomiasis. Immunology. 1979 Feb;36(2):313–321. [PMC free article] [PubMed] [Google Scholar]
- Askonas B. A., North J. R. The life style of B cells--cellular proliferation and the invariancy of IgG. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):749–759. doi: 10.1101/sqb.1977.041.01.086. [DOI] [PubMed] [Google Scholar]
- Clinton B. A., Ortiz-Ortiz L., Garcia W., Martinez T., Capin R. Trypanosoma cruzi: early immune responses in infected mice. Exp Parasitol. 1975 Jun;37(3):417–425. doi: 10.1016/0014-4894(75)90012-0. [DOI] [PubMed] [Google Scholar]
- Corsini A. C., Bellucci S. B., Costa M. G. A simple method of evaluating delayed type hypersensitivity in mice. J Immunol Methods. 1979;30(2):195–200. doi: 10.1016/0022-1759(79)90093-0. [DOI] [PubMed] [Google Scholar]
- Corsini A. C., Clayton C., Askonas B. A., Ogilvie B. M. Suppressor cells and loss of B-cell potential in mice infected with Trypanosoma brucei. Clin Exp Immunol. 1977 Jul;29(1):122–131. [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Separate signals for the initiation of proliferation and differentiation in the b cell response to antigen. Transplant Rev. 1975;23:66–77. doi: 10.1111/j.1600-065x.1975.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H., Eardley D. D. Activation of distinct helper and suppressor T cells in experimental trypanosomiasis. J Immunol. 1978 Aug;121(2):622–628. [PubMed] [Google Scholar]
- Kemshead J. T., North J. R., Askonas B. A. IgG anti-hapten antibody secretion in vitro commences after extensive precursor proliferation. Immunology. 1977 Oct;33(4):485–490. [PMC free article] [PubMed] [Google Scholar]
- Mansfield J. M. Immunobiology of African trypanosomiasis. Cell Immunol. 1978 Aug;39(1):204–210. doi: 10.1016/0008-8749(78)90094-1. [DOI] [PubMed] [Google Scholar]
- Ramos C., Lamoyi E., Feoli M., Rodriguez M., Perez M., Ortiz-Ortiz L. Trypanosoma cruzi: immunosuppressed response to different antigens in the infected mouse. Exp Parasitol. 1978 Aug;45(2):190–199. doi: 10.1016/0014-4894(78)90059-0. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Unanue E. R. The regulation of lymphocyte functions by the macrophage. Immunol Rev. 1978;40:227–255. doi: 10.1111/j.1600-065x.1978.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Vadas M. A., Miller J. F., Gamble J., Whitelaw A. A radioisotopic method to measure delayed type hypersensitivity in the mouse. I. Studies in sensitized and normal mice. Int Arch Allergy Appl Immunol. 1975;49(5):670–692. doi: 10.1159/000231449. [DOI] [PubMed] [Google Scholar]
- Zauderer M., Askonas B. A. Several proliferative phases precede maturation of IgG-secreting cells in mitogen-stimulated cultures. Nature. 1976 Apr 15;260(5552):611–613. doi: 10.1038/260611a0. [DOI] [PubMed] [Google Scholar]