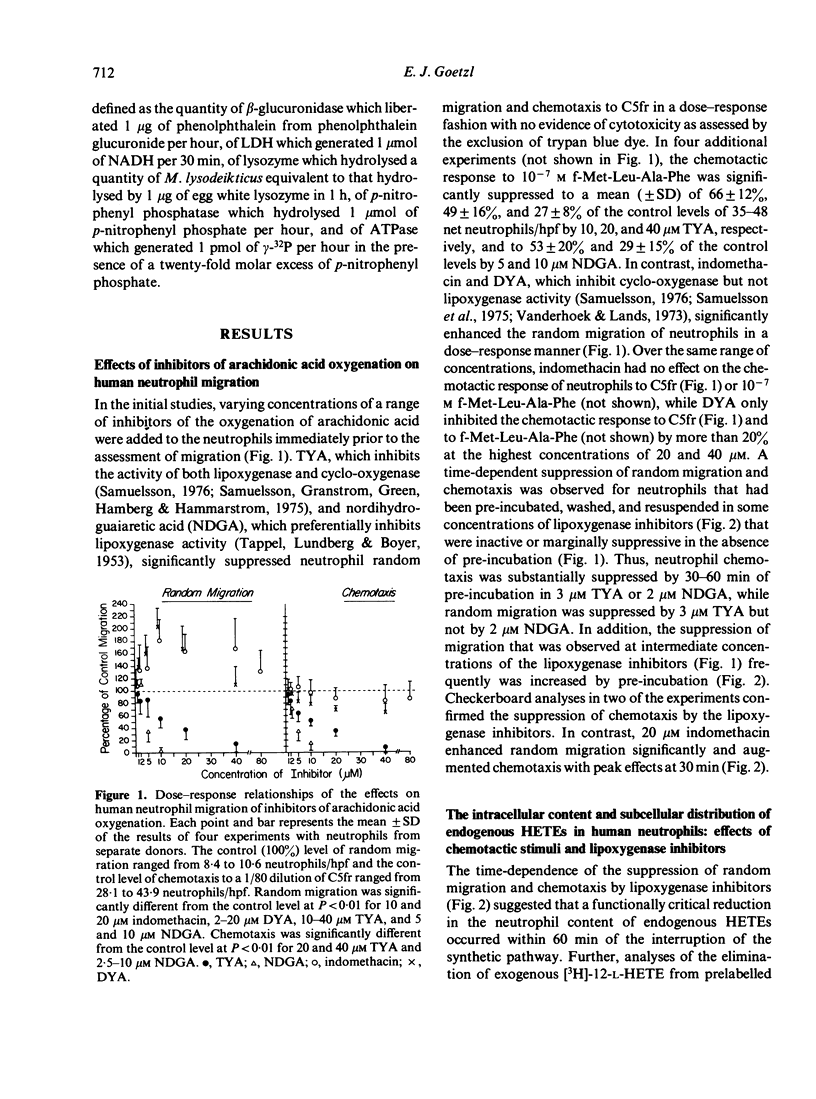
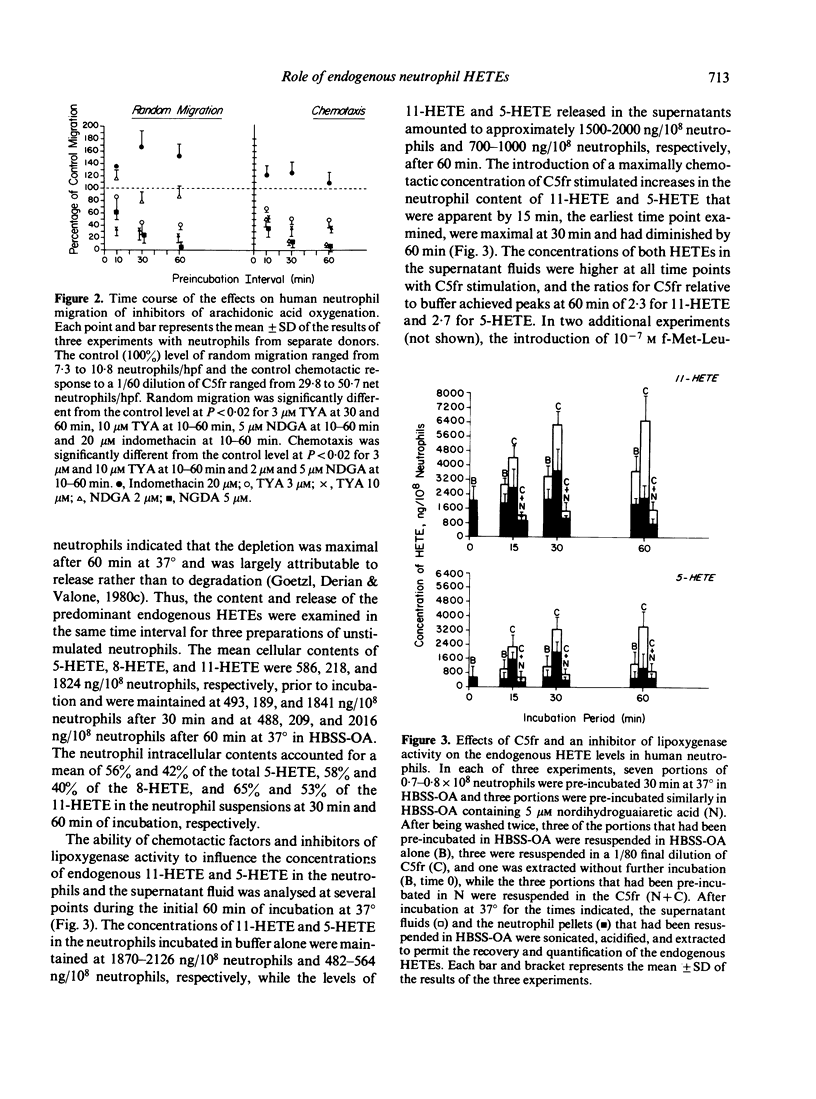
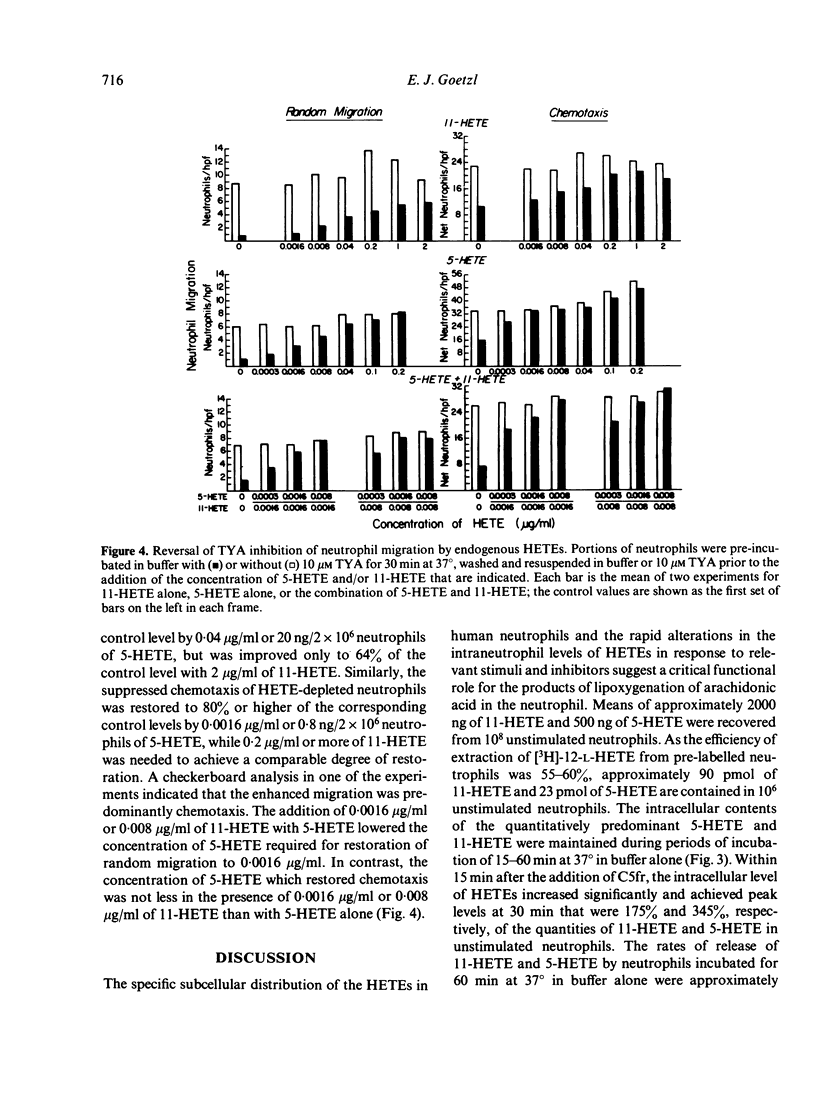
Abstract
The possibility that endogenous monohydroxy-eicosatetraenoic acids (HETEs) derived from the lipoxygenation of arachidonic acid might serve a role in human neutrophil migration was examined by studying the effects of depletion of the intracellular HETEs on random migration and chemotaxis. The intracellular contents of approximately 2000 ng of 11-HETE and 500 ng of 5-HETE per 10(8) neutrophils are distributed preferentially in the cellular membranes and are increased by specific chemotactic factors. The depletion of intracellular HETEs that resulted from pre-incubating, washing and resuspending neutrophils in 3-20 microM 5,8,11,14-eicosatetraynoic acid (TYA), an inhibitor of lipoxygenase and cyclooxygenase activity, or in 5-10 microM nordihydroguaiaretic acid (NDGA), a selective inhibitor of lipoxygenase activity, was associated with suppression of neutrophil random migration and chemotaxis to several stimuli without evidence of cytotoxicity. Maximal suppression of migration was achieved by a 30-60 min preincubation with the inhibitors, a time-course analogous to that required for optimal depletion of the endogenous HETEs. In contrast, inhibitors of cyclooxygenase activity enhanced random migration and, to a lesser extent, chemotaxis. The inhibition of migration achieved by pre-incubating and maintaining the neutrophils in TYA or NDGA was fully reversed either by washing and resuspending the neutrophils in buffer or by the addition of purified neutrophil 5-HETE in quantities as small as 20 ng/2 x 10(6) neutrophils for random migration and 0.8 ng/2 x 10(6) neutrophils for chemotaxis, while the addition of 11-HETE was less effective. The relationship of the intracellular concentrations of endogenous HETEs to neutrophil migration is consistent with a potential role of the HETEs as cellular mediators.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Hamberg M., Samuelsson B. Transformation of arachidonic acid and homo-gamma-linolenic acid by rabbit polymorphonuclear leukocytes. Monohydroxy acids from novel lipoxygenases. J Biol Chem. 1976 Dec 25;251(24):7816–7820. [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Plasma membranes of mammalian cells: a review of methods for their characterization and isolation. J Cell Biol. 1973 Feb;56(2):275–303. doi: 10.1083/jcb.56.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. A neutrophil-immobilizing factor derived from human leukocytes. I. Generation and partial characterization. J Exp Med. 1972 Dec 1;136(6):1564–1580. doi: 10.1084/jem.136.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Gorman R. R. Chemotactic and chemokinetic stimulation of human eosinophil and neutrophil polymorphonuclear leukocytes by 12-L-hydroxy-5,8,10-heptadecatrienoic acid (HHT). J Immunol. 1978 Feb;120(2):526–531. [PubMed] [Google Scholar]
- Goetzl E. J., Hoe K. Y. Chemotactic factor receptors of human PMN leucocytes. I. Effects on migration of labelling plasma membrane determinants with impermeant covalent reagents and inhibition of labelling by chemotactic factors. Immunology. 1979 Jun;37(2):407–418. [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Sun F. F. Generation of unique mono-hydroxy-eicosatetraenoic acids from arachidonic acid by human neutrophils. J Exp Med. 1979 Aug 1;150(2):406–411. doi: 10.1084/jem.150.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Weller P. F., Sun F. F. The regulation of human eosinophil function by endogenous mono-hydroxy-eicosatetraenoic acids (HETEs). J Immunol. 1980 Feb;124(2):926–933. [PubMed] [Google Scholar]
- Goetzl E. J., Woods J. M., Gorman R. R. Stimulation of human eosinophil and neutrophil polymorphonuclear leukocyte chemotaxis and random migration by 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid. J Clin Invest. 1977 Jan;59(1):179–183. doi: 10.1172/JCI108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried E. L. Lipids of human leukocytes: relation to celltype. J Lipid Res. 1967 Jul;8(4):321–327. [PubMed] [Google Scholar]
- Hall J. G., Hopkins J., Reynolds J. Studies of efferent lymph cells from nodes stimulated with oxazolone. Immunology. 1980 Feb;39(2):141–149. [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. VII. Novel transformations of arachidonic acid in guinea pig lung. Biochem Biophys Res Commun. 1974 Dec 11;61(3):942–949. doi: 10.1016/0006-291x(74)90246-0. [DOI] [PubMed] [Google Scholar]
- Harlan J., DeChatelet L. R., Iverson D. B., McCall C. E. Magnesium-dependent adenosine triphosphatase as a marker enzyme for the plasma membrane of human polymorphonuclear leukocytes. Infect Immun. 1977 Feb;15(2):436–443. doi: 10.1128/iai.15.2.436-443.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J. C., Kelly R. C., Gorman R. R., Sun F. F. Preparation and spectral properties of 12-hydroxyl eicosatetraenoic acid (HETE). Prep Biochem. 1978;8(2-3):147–153. doi: 10.1080/00327487808069056. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H. Arachidonate lipoxygenase in blood platelets. Biochim Biophys Acta. 1975 Feb 20;380(2):299–307. doi: 10.1016/0005-2760(75)90016-8. [DOI] [PubMed] [Google Scholar]
- SMOLELIS A. N., HARTSELL S. E. The determination of lysozyme. J Bacteriol. 1949 Dec;58(6):731–736. doi: 10.1128/jb.58.6.731-736.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B., Granström E., Green K., Hamberg M., Hammarström S. Prostaglandins. Annu Rev Biochem. 1975;44:669–695. doi: 10.1146/annurev.bi.44.070175.003321. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Shohet S. B. Remodeling of granulocyte membrane fatty acids during phagocytosis. J Clin Invest. 1974 Mar;53(3):726–734. doi: 10.1172/JCI107611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAPPEL A. L., LUNDBERG W. O., BOYER P. D. Effect of temperature and antioxidants upon the lipoxidase-catalyzed oxidation of sodium linoleate. Arch Biochem Biophys. 1953 Feb;42(2):293–304. doi: 10.1016/0003-9861(53)90359-2. [DOI] [PubMed] [Google Scholar]
- Turner S. R., Campbell J. A., Lynn W. S. Polymorphonulcear leukocyte chemotaxis toward oxidized lipid components of cell membranes. J Exp Med. 1975 Jun 1;141(6):1437–1441. doi: 10.1084/jem.141.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S. R., Tainer J. A., Lynn W. S. Biogenesis of chemotactic molecules by the arachidonate lipoxygenase system of platelets. Nature. 1975 Oct 23;257(5528):680–681. doi: 10.1038/257680a0. [DOI] [PubMed] [Google Scholar]
- Vanderhoek J. Y., Lands W. E. Acetylenic inhibitors of sheep vesicular gland oxygenase. Biochim Biophys Acta. 1973 Feb 14;296(2):374–381. doi: 10.1016/0005-2760(73)90095-7. [DOI] [PubMed] [Google Scholar]
- Welsh I. R., Spitznagel J. K. Distribution of lysosomal enzymes, cationic proteins, and bactericidal substances in subcellular fractions of human polymorphonuclear leukocytes. Infect Immun. 1971 Aug;4(2):97–102. doi: 10.1128/iai.4.2.97-102.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


