Abstract
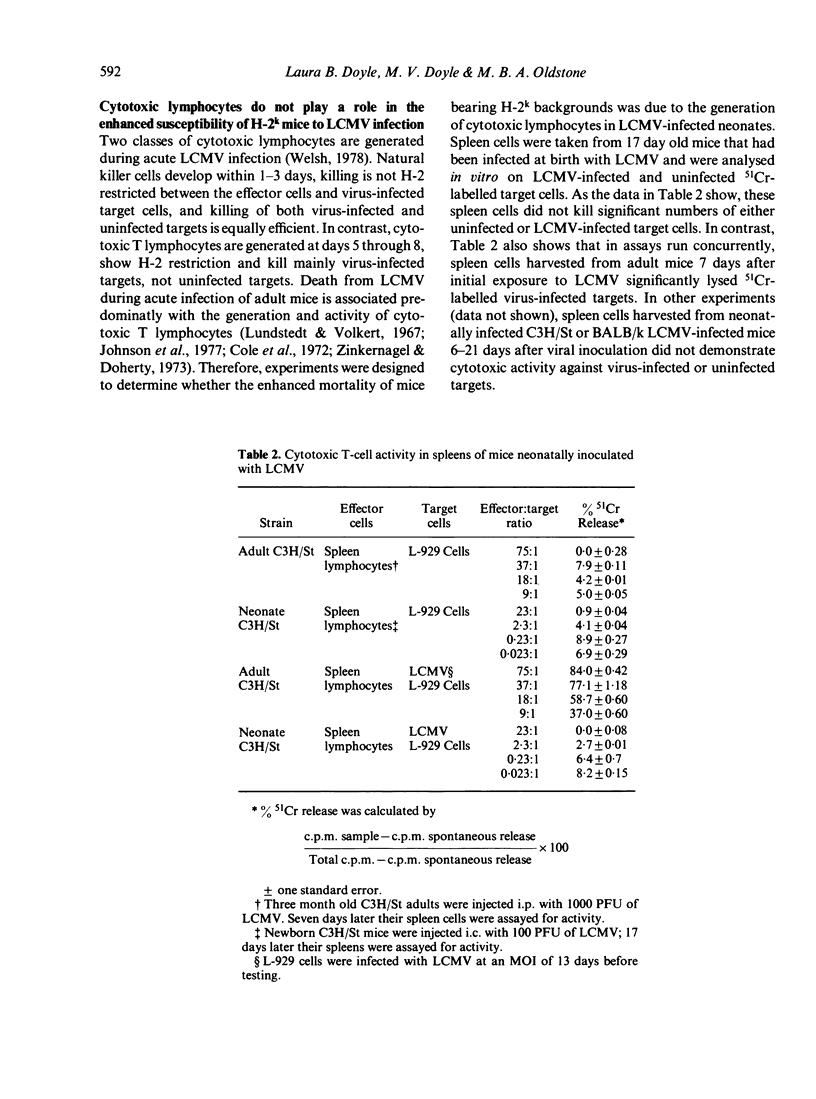
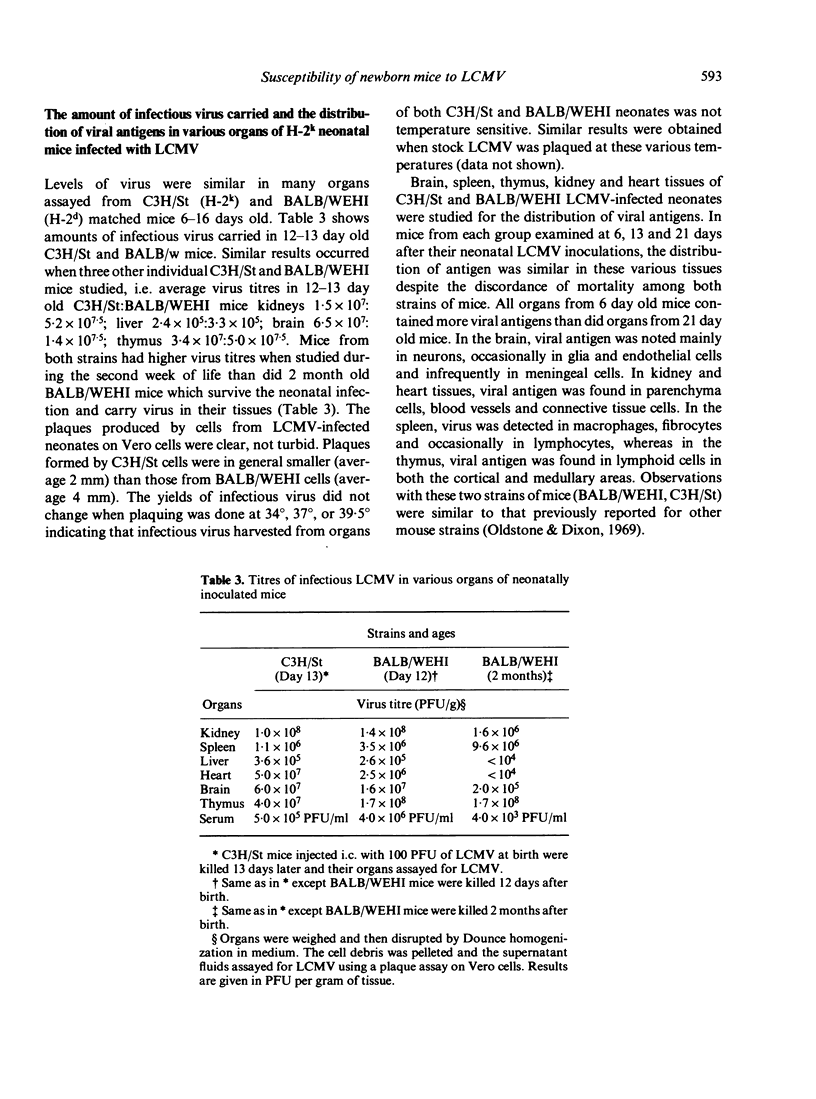
Many strains of mice, when injected at birth with an ordinarily lethal dose of lymphocytic choriomeningitis virus (LCMV), grow to adulthood despite maintaining a persistent virus infection and chronic virus-induced immune complex disease. Because the susceptibility to LCMV infection changed over several years of observation, a number of murine strains with different histocompatibiity gene loci and genetic backgrounds were compared. Neonatal mice with H-2b, H-2d, and H-2q backgrounds were relatively insensitive to the effects of LCMV infection compared to mice with H-2k backgrounds, which had a high mortality rate in this situation. Expression of the H-2k gene locus itself did not affect the rate of mortality. Use of recombinant mice indicated that susceptibility was linked to H-2k backgrounds and not H-2k gene loci. The low survival rate of newborn mice with H-2k backgrounds infected with LCMV was not caused by cytotoxic natural killer cells, cytotoxic T lymhocytes, excessive amounts of virus in the organs, a unique distribution of virus or expression of viral antigens in vivo or unusual pathology in tissues.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. II. Passive transfer of recovery mechanisms with immune lymphoid cells. J Exp Med. 1971 May 1;133(5):1074–1089. doi: 10.1084/jem.133.5.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Elder J. H., Oldstone M. B. Protein structure of lymphocytic choriomeningitis virus: identification of the virus structural and cell associated polypeptides. Virology. 1978 Aug;89(1):133–145. doi: 10.1016/0042-6822(78)90047-8. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Oldstone M. B. Virus-induced immune complex disease: identification of specific viral antigens and antibodies deposited in complexes during chronic lymphocytic choriomeningitis virus infection. J Immunol. 1978 Apr;120(4):1297–1304. [PubMed] [Google Scholar]
- Cole G. A., Gilden D. H., Monjan A. A., Nathanson N. Lymphocytic choriomeningitis virus: pathogenesis of acute central nervous system disease. Fed Proc. 1971 Nov-Dec;30(6):1831–1841. [PubMed] [Google Scholar]
- Cole G. A., Nathanson N., Prendergast R. A. Requirement for theta-bearing cells in lymphocytic choriomeningitis virus-induced central nervous system disease. Nature. 1972 Aug 11;238(5363):335–337. doi: 10.1038/238335a0. [DOI] [PubMed] [Google Scholar]
- Doyle M. V., Oldstone M. B. Interactions between viruses and lymphocytes. I. In vivo replication of lymphocytic choriomeningitis virus in mononuclear cells during both chronic and acute viral infections. J Immunol. 1978 Oct;121(4):1262–1269. [PubMed] [Google Scholar]
- Gilden D. H., Cole G. A., Nathanson N. Immunopathogenesis of acute central nervous system disease produced by lymphocytic choriomeningitis virus. II. Adoptive immunization of virus carriers. J Exp Med. 1972 Apr 1;135(4):874–889. doi: 10.1084/jem.135.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTCHIN J. E., CINITS M. Lymphocytic choriomeningitis infection of mice as a model for the study of latent virus infection. Can J Microbiol. 1958 Apr;4(2):149–163. doi: 10.1139/m58-016. [DOI] [PubMed] [Google Scholar]
- HOTCHIN J., WEIGAND H. The effects of pretreatment with x-rays on the pathogenesis of lymphocytic choriomeningitis in mice. I. Host survival, virus multiplication and leukocytosis. J Immunol. 1961 Dec;87:675–681. [PubMed] [Google Scholar]
- Hirsch M. S., Murphy F. A., Russe H. P., Hicklin M. D. Effects of anti-thymocyte serum on lymphocytic choriomeningitis (LCM) virus infection in mice. Proc Soc Exp Biol Med. 1967 Jul;125(3):980–983. doi: 10.3181/00379727-125-32254. [DOI] [PubMed] [Google Scholar]
- Lundstedt C. Interaction between antigenically different cells. Virus-induced cytotoxicity by immune lymphoid cells in vitro. Acta Pathol Microbiol Scand. 1969;75(1):139–152. [PubMed] [Google Scholar]
- Oldstone M. B., Aoki T., Dixon F. J. Activation of spontaneous murine leukemia virus-related antigen by lymphocytic choriomeningitis virus. Science. 1971 Nov 19;174(4011):843–845. doi: 10.1126/science.174.4011.843. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Acute viral infection: tissue injury mediated by anti-viral antibody through a complement effector system. J Immunol. 1971 Nov;107(5):1274–1280. [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Change in susceptibility of C3H-HeJ mice to LCM virus infection. J Immunol. 1973 Nov;111(5):1613–1615. [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Lymphocytic choriomeningitis: production of antibody by "tolerant" infected mice. Science. 1967 Dec 1;158(3805):1193–1195. doi: 10.1126/science.158.3805.1193. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. II. Relationship of the anti-lymphocytic choriomeningitis immune response to tissue injury in chronic lymphocytic choriomeningitis disease. J Exp Med. 1970 Jan 1;131(1):1–19. doi: 10.1084/jem.131.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Holmstoen J., Welsh R. M., Jr Alterations of acetylcholine enzymes in neuroblastoma cells persistently infected with lymphocytic choriomeningitis virus. J Cell Physiol. 1977 Jun;91(3):459–472. doi: 10.1002/jcp.1040910316. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med. 1978 Jul 1;148(1):163–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Oldstone M. B. Inhibition of immunologic injury of cultured cells infected with lymphocytic choriomeningitis virus: role of defective interfering virus in regulating viral antigenic expression. J Exp Med. 1977 Jun 1;145(6):1449–1468. doi: 10.1084/jem.145.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Pfau C. J. Determinants of lymphocytic choriomeningitis interference. J Gen Virol. 1972 Feb;14(2):177–187. doi: 10.1099/0022-1317-14-2-177. [DOI] [PubMed] [Google Scholar]
- Yoon J. W., Onodera T., Notkins A. L. Virus-induced diabetes mellitus. XV. Beta cell damage and insulin-dependent hyperglycemia in mice infected with coxsackie virus B4. J Exp Med. 1978 Oct 1;148(4):1068–1080. doi: 10.1084/jem.148.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Cytotoxic thymus-derived lymphocytes in cerebrospinal fluid of mice with lymphocytic choriomeningitis. J Exp Med. 1973 Nov 1;138(5):1266–1269. doi: 10.1084/jem.138.5.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Oldstone M. B. Cells that express viral antigens but lack H-2 determinants are not lysed by immune thymus-derived lymphocytes but are lysed by other antiviral immune attack mechanisms. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3666–3670. doi: 10.1073/pnas.73.10.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Welsh R. M. H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J Immunol. 1976 Nov;117(5 Pt 1):1495–1502. [PubMed] [Google Scholar]


