Abstract
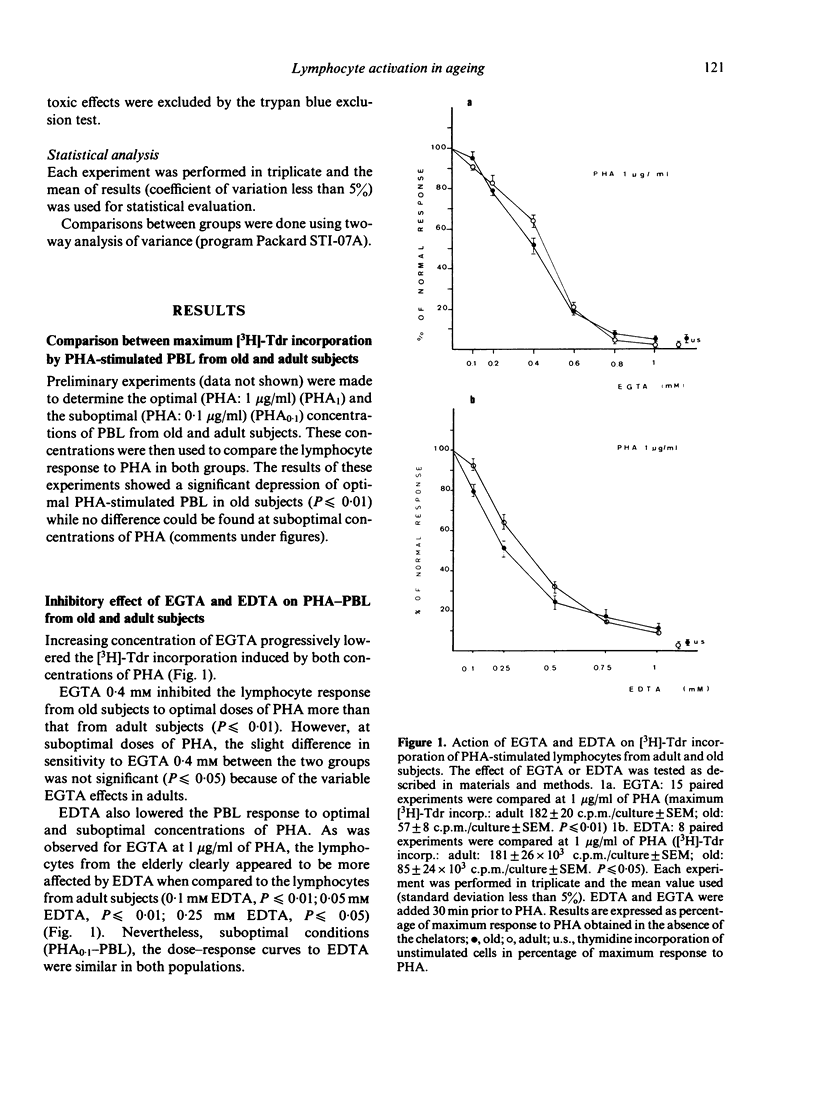
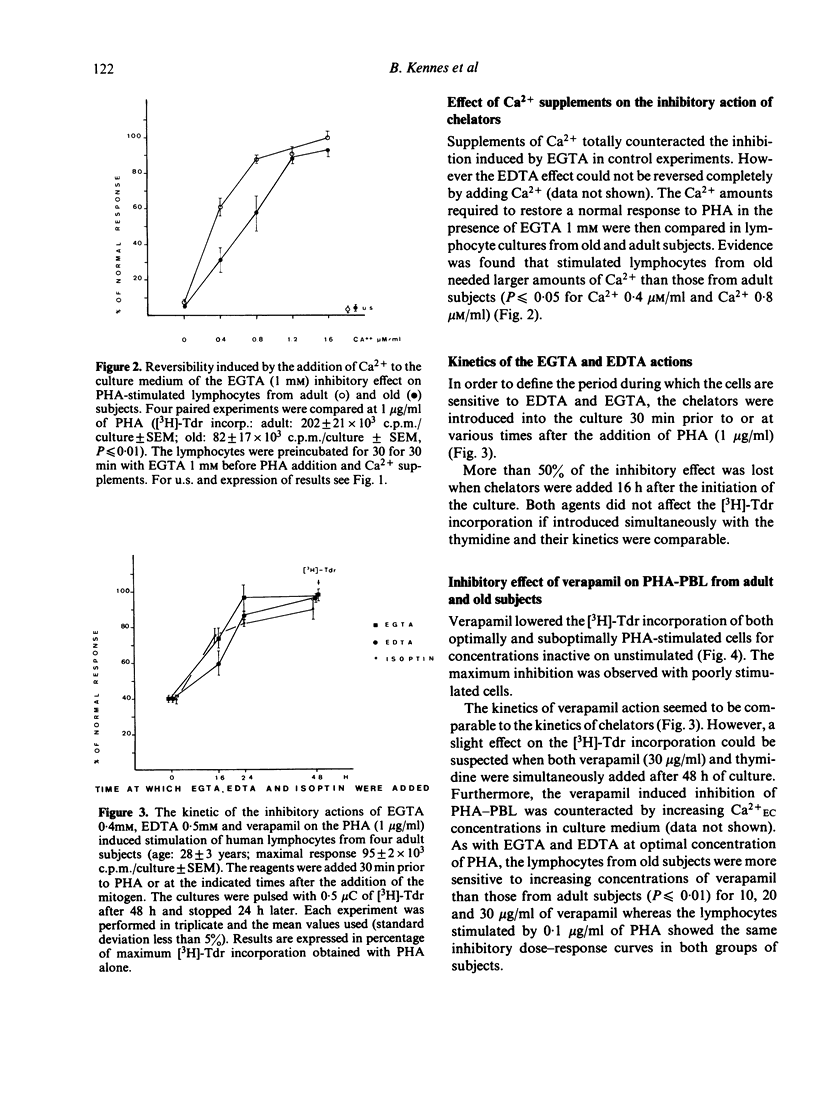
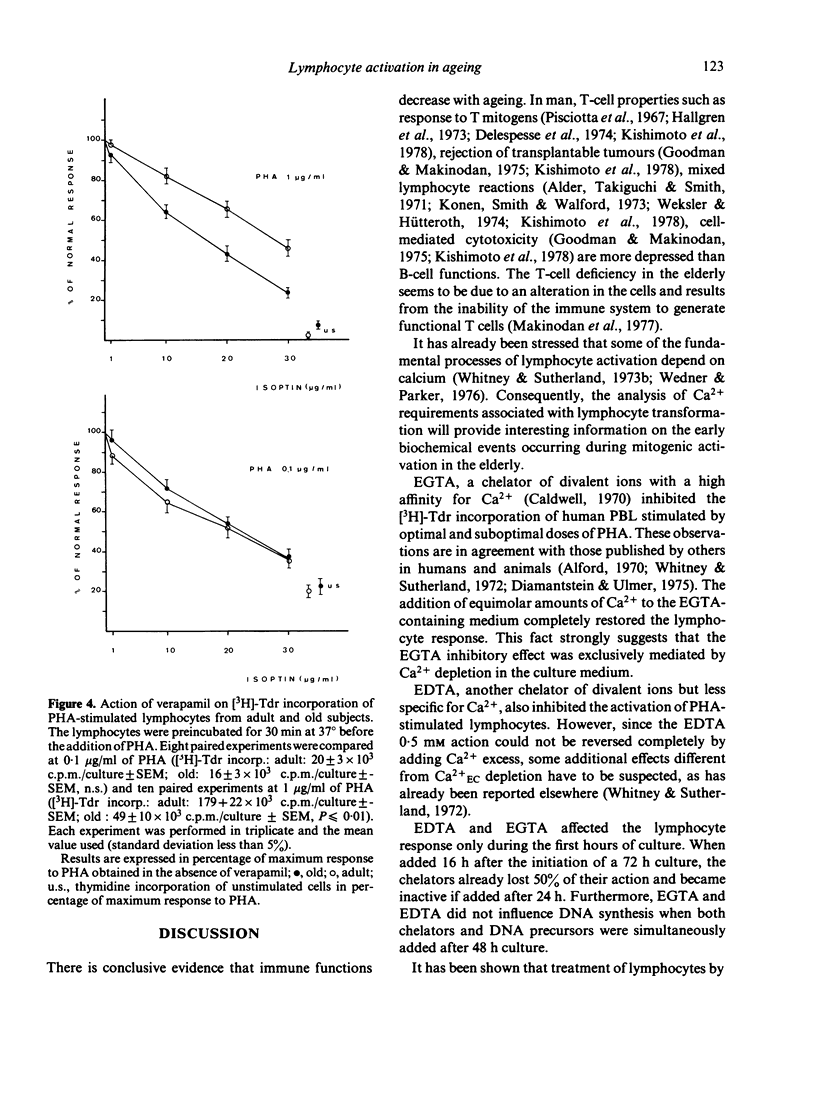
The requirement for Ca2+, a divalent ion which plays a fundamental role in cell activation, has been analysed in cultures of PHA-stimulated human peripheral blood lymphocytes (PHA-PBL) from adult (range: 20-35 years) and old (over 70 years) subjects. For this purpose, increasing concentrations of Ca2+ chelators (EGTA and EDTA) were added to cultures in order to compare the effect of progressive extracellular Ca2+ (Ca2+EC) depletion on [3H]-Tdr incorporation by PHA-PBL. Kinetic analysis showed that Ca2+EC requirement was restricted to the first 24 h after culture initiation. At optimal doses of PHA, the PHA-PBL from old subjects were more sensitive than those from adult subjects to increasing concentrations of both chelators. They also required larger amounts of Ca2+ supplements to restore their normal response after total inhibition by EGTA. Furthermore, the PHA-PBL from the elderly were hypersensitive to verapamil (Isoptin), a drug which instigates a reversible inhibition of Ca2+-dependent processes associated with lymphocyte transformation, by a quite similar reaction to that induced by chelators. We conclude that the Ca2+-dependent processes in lymphocyte activation are impaired with ageing. Following further experiments and recent work suggesting that lymphocytes need more than one signal to proliferate, the authors speculate on a deficiency of a late activation signal requiring cell-cell interactions in the elderly.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler W. H., Takiguchi T., Smith R. T. Effect of age upon primary alloantigen recognition by mouse spleen cells. J Immunol. 1971 Nov;107(5):1357–1367. [PubMed] [Google Scholar]
- Alexopoulos C., Babitis P. Letter: Age dependence of T lymphocytes. Lancet. 1976 Feb 21;1(7956):426–426. doi: 10.1016/s0140-6736(76)90259-2. [DOI] [PubMed] [Google Scholar]
- Alford R. H. Metal cation requirements for phytohemagglutinin-induced transformation of human peripheral blood lymphocytes. J Immunol. 1970 Mar;104(3):698–703. [PubMed] [Google Scholar]
- Allwood G., Asherson G. L., Davey M. J., Goodford P. J. The early uptake of radioactive calcium by human lymphocytes treated with phytohaemagglutinin. Immunology. 1971 Sep;21(3):509–516. [PMC free article] [PubMed] [Google Scholar]
- Augener W., Cohnen G., Reuter A., Brittinger G. Letter: Decrease of T lymphocytes during ageing. Lancet. 1974 Jun 8;1(7867):1164–1164. doi: 10.1016/s0140-6736(74)90647-3. [DOI] [PubMed] [Google Scholar]
- Blitstein-Willinger E., Diamantstein T. Inhibition by isoptin (a calcium antagonist) of the mitogenic stimulation of lymphocytes prior to the S-phase. Immunology. 1978 Feb;34(2):303–307. [PMC free article] [PubMed] [Google Scholar]
- Carosella E. D., Mochanko K., Braun M. Rosette-forming T cells in human peripheral blood at different ages. Cell Immunol. 1974 May;12(2):323–325. doi: 10.1016/0008-8749(74)90085-9. [DOI] [PubMed] [Google Scholar]
- Del Pozo Perez M. A., Prieto Valtueña J., Gonzalez Guilabert M. I., Velasco Alonso R. Effects of age and sex on T and B lymphocyte populations in man. Biomedicine. 1973 Aug 10;19(8):340–344. [PubMed] [Google Scholar]
- Delespesse G., Duchateau J., Bastenie P. A., Lauvaux J. P., Collet H., Govaerts A. Cell-mediated immunity in diabetes mellitus. Clin Exp Immunol. 1974 Dec;18(4):461–467. [PMC free article] [PubMed] [Google Scholar]
- Delespesse G., Vrijens R., de Maubeuge J., Hudson D., Kennes B., Govaerts A. Influence of levamisole on the immune response of old people. Int Arch Allergy Appl Immunol. 1977;54(2):151–157. doi: 10.1159/000231818. [DOI] [PubMed] [Google Scholar]
- Diamantstein T., Ulmer A. The control of immune response in vitro by Ca2+. II. The Ca2+-dependent period during mitogenic stimulation. Immunology. 1975 Jan;28(1):121–125. [PMC free article] [PubMed] [Google Scholar]
- Diaz-Jouanen E., Williams R. C., Jr, Strickland R. G. Letter: Age-related changes in T and B cells. Lancet. 1975 Mar 22;1(7908):688–689. doi: 10.1016/s0140-6736(75)91794-8. [DOI] [PubMed] [Google Scholar]
- Freedman M. H. Early biochemical events in lymphocyte activation. I. Investigations on the nature and significance of early calcium fluxes observed in mitogen-induced T and B lymphocytes. Cell Immunol. 1979 May;44(2):290–313. doi: 10.1016/0008-8749(79)90007-8. [DOI] [PubMed] [Google Scholar]
- Freedman M. H., Raff M. C. Induction of increased calcium uptake in mouse T lymphocytes by concanavalin A and its modulation by cyclic nucleotides. Nature. 1975 May 29;255(5507):378–382. doi: 10.1038/255378a0. [DOI] [PubMed] [Google Scholar]
- Goodman S. A., Makinodan T. Effect of age on cell-mediated immunity in long-lived mice. Clin Exp Immunol. 1975 Mar;19(3):533–542. [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P. Sensitivity of lymphocytes to prostaglandin E2 increases in subjects over age 70. J Clin Invest. 1979 Aug;64(2):434–439. doi: 10.1172/JCI109480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren H. M., Buckley C. E., 3rd, Gilbertsen V. A., Yunis E. J. Lymphocyte phytohemagglutinin responsiveness, immunoglobulins and autoantibodies in aging humans. J Immunol. 1973 Oct;111(4):1101–1107. [PubMed] [Google Scholar]
- Hung C. Y., Perkins E. H., Yang W. K. Age-related refractoriness of PHA-induced lymphocyte transformation. II. 125-I-PHA binding to spleen cells from young and old mice. Mech Ageing Dev. 1975 Mar-Apr;4(2):103–112. doi: 10.1016/0047-6374(75)90012-3. [DOI] [PubMed] [Google Scholar]
- Inkeles B., Innes J. B., Kuntz M. M., Kadish A. S., Weksler M. E. Immunological studies of Aging. III. Cytokinetic basis for the impaired response of lymphocytes from aged humans to plant lectins. J Exp Med. 1977 May 1;145(5):1176–1187. doi: 10.1084/jem.145.5.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasińska J., Steffen J. A., Michalowski A. Studies on in vitro lymphocyte proliferation in cultures synchronized by the inhibition of DNA synthesis. II. Kinetics of the initiation of the proliferative response. Exp Cell Res. 1970 Aug;61(2):333–341. doi: 10.1016/0014-4827(70)90455-6. [DOI] [PubMed] [Google Scholar]
- Katz S. P., Kierszenbaum F., Waksman B. H. Mechanisms of action of "lymphocyte-activating factor" (LAF). III. Evidence that LAF acts on stimulated lymphocytes by raising cyclic GMP in G1. J Immunol. 1978 Dec;121(6):2386–2391. [PubMed] [Google Scholar]
- Kishimoto S., Tomino S., Inomata K., Kotegawa S., Saito T., Kuroki M., Mitsuya H., Hisamitsu S. Age-related changes in the subsets and functions of human T lymphocytes. J Immunol. 1978 Nov;121(5):1773–1780. [PubMed] [Google Scholar]
- Konen T. G., Smith G. S., Walford R. L. Decline in mixed lymphocyte reactivity of spleen cells from aged mice of a long-lived strain. J Immunol. 1973 May;110(5):1216–1221. [PubMed] [Google Scholar]
- Pisciotta A. V., Westring D. W., DePrey C., Walsh B. Mitogenic effect of phytohaemagglutinin at different ages. Nature. 1967 Jul 8;215(5097):193–194. doi: 10.1038/215193a0. [DOI] [PubMed] [Google Scholar]
- Price G. B., Makinodan T. Immunologic deficiencies in senescence. I. Characterization of intrinsic deficiencies. J Immunol. 1972 Feb;108(2):403–412. [PubMed] [Google Scholar]
- Price G. B., Makinodan T. Immunologic deficiencies in senescence. II. Characterization of extrinsic deficiencies. J Immunol. 1972 Feb;108(2):413–417. [PubMed] [Google Scholar]
- Steel C. M., Evans J., Smith M. A. Letter: Age-related changes in T and B cells. Lancet. 1975 Apr 19;1(7912):914–915. doi: 10.1016/s0140-6736(75)91705-5. [DOI] [PubMed] [Google Scholar]
- Sörén L. Variability of the time at which PHA-stimulated lymphocytes initiate DNA synthesis. Exp Cell Res. 1973 Mar 30;78(1):201–208. doi: 10.1016/0014-4827(73)90055-4. [DOI] [PubMed] [Google Scholar]
- Toyoshima S., Iwata M., Osawa T. Kinetics of lymphocyte stimulation by concanavalin A. Nature. 1976 Dec 2;264(5585):447–449. doi: 10.1038/264447a0. [DOI] [PubMed] [Google Scholar]
- Weksler M. E., Hütteroth T. H. Impaired lymphocyte function in aged humans. J Clin Invest. 1974 Jan;53(1):99–104. doi: 10.1172/JCI107565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney R. B., Sutherland R. M. Characteristics of calcium accumulation by lymphocytes and alterations in the process induced by phytohemagglutinin. J Cell Physiol. 1973 Aug;82(1):9–20. doi: 10.1002/jcp.1040820103. [DOI] [PubMed] [Google Scholar]
- Whitney R. B., Sutherland R. M. Effects of chelating agents on the initial interaction of phytohemagglutinin with lymphocytes and the subsequent stimulation of amino acid uptake. Biochim Biophys Acta. 1973 Apr 16;298(4):790–797. doi: 10.1016/0005-2736(73)90383-0. [DOI] [PubMed] [Google Scholar]
- Whitney R. B., Sutherland R. M. Requirement for calcium ions in lymphocyte transformation stimulated by phytohemagglutinin. J Cell Physiol. 1972 Dec;80(3):329–337. doi: 10.1002/jcp.1040800303. [DOI] [PubMed] [Google Scholar]


