Abstract
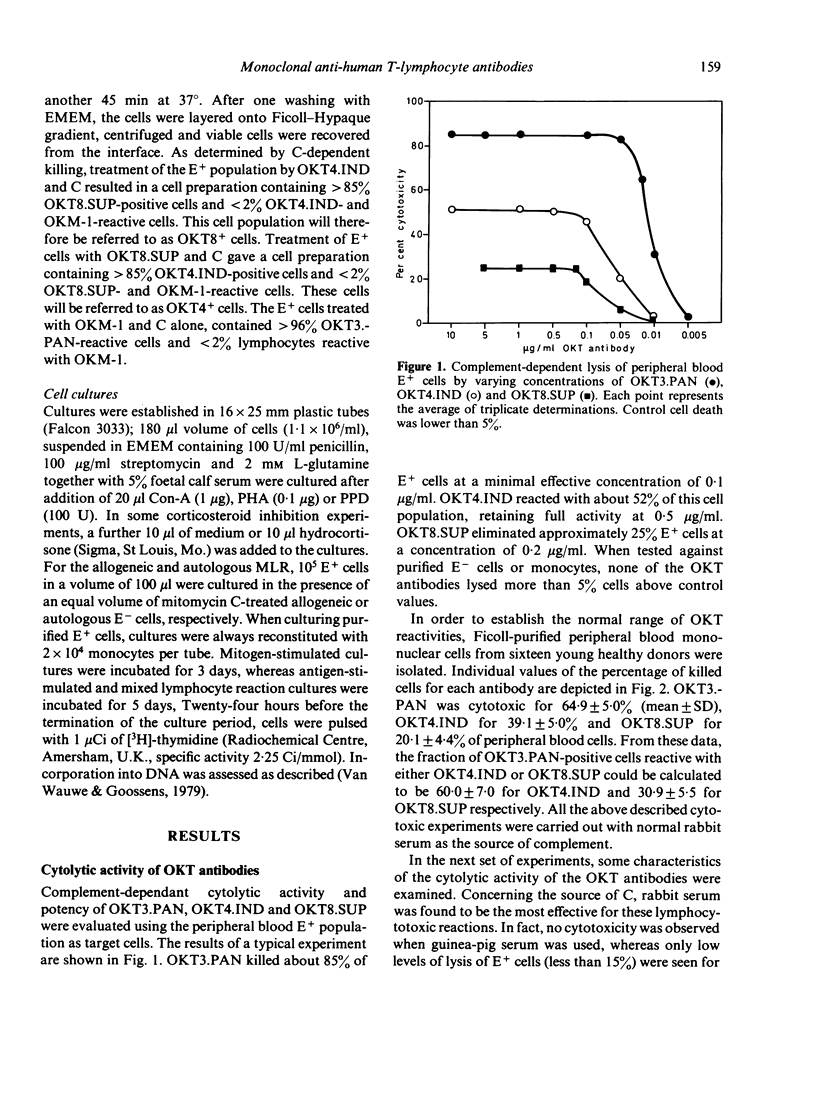
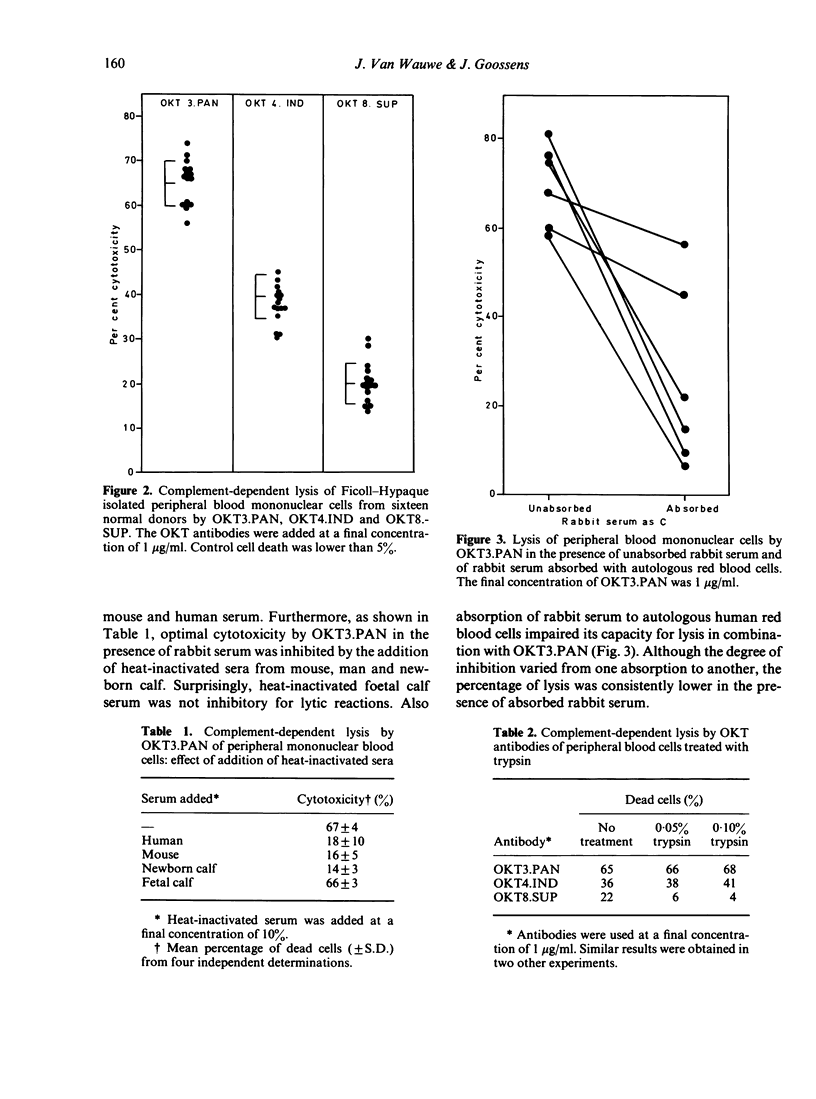
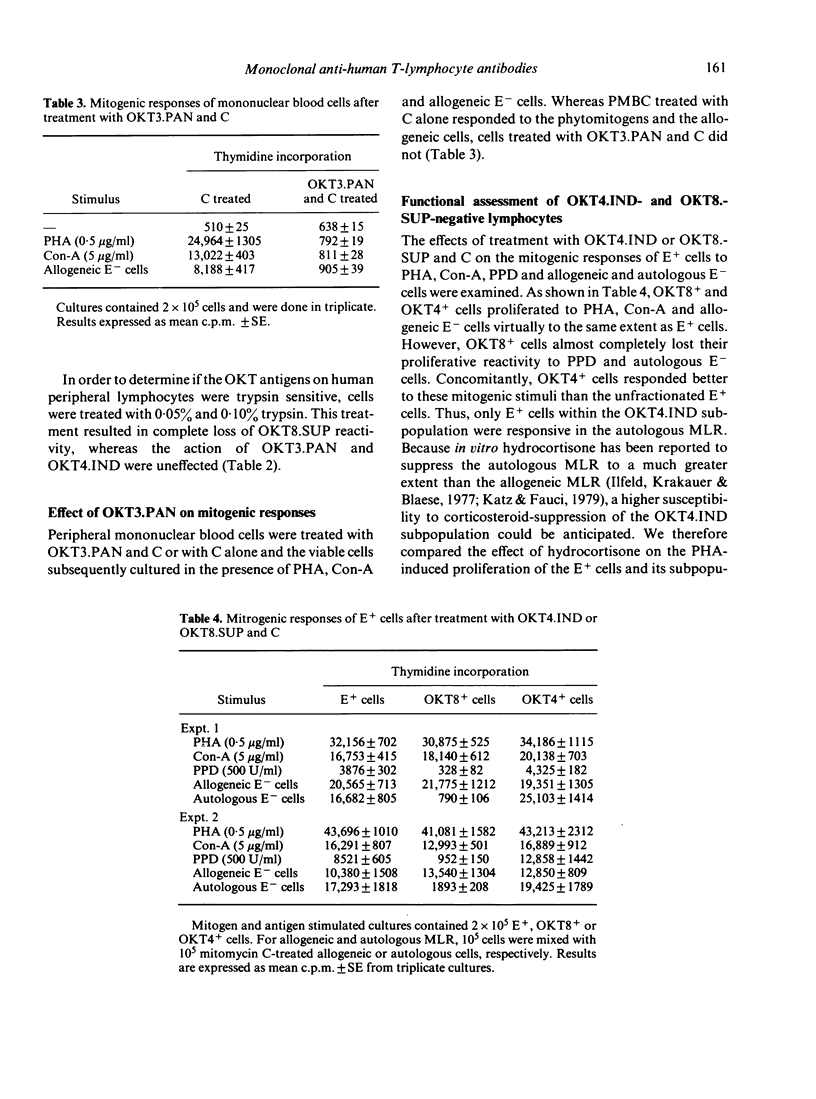
The complement-mediated lysis of human lymphocytes by three monoclonal anti-human T-cell antibodies OKT3.PAN, OKT4.INd and OKT8.SUP was studied. The percentages of Ficoll-Hypaque-isolated mononuclear cells lysed by these antibodies were respectively: 65% for OKT3.PAN, 39% for OKT4.IND and 20% for OKT8.SUP. Optimal lymphocytotoxic reactions were noticed when unabsorbed rabbit serum was used as the source of complement (C). Addition of heat-inactivated human, mouse and newborn calf sera but not of foetal calf serum inhibited the lytic activity of the antibodies. Treatment of peripheral mononuclear blood cells with OKT3.PAN and C abrogated their mitotic response to PHA and Con-A. Sheep erythrocyte rosetting lymphocytes (E+ cells) treated with OKT4.IND or OKT8.SUP and C exhibited no marked changes in responsiveness to PHA, Con-A or allogeneic non-T cells. However, only E+ cells enriched with OKT4.IND-reactive cells responded to purified protein derivative, proliferated in the autologous mixed lymphocyte reaction and were highly sensitive to hydrocortisone suppression when stimulated by PHA. Our data indicate that these monoclonal antibodies can be regarded as invaluable tools for enumeration, characterization and functional assessment of human T cells and their subclasses.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiuti F., Wigzell H. Function and distribution pattern of human T lymphocytes. II. Presence of T lymphocytes in normal humans and in humans with various immunodeficiency disorders. Clin Exp Immunol. 1973 Feb;13(2):183–189. [PMC free article] [PubMed] [Google Scholar]
- Bobrove A. M., Strober S., Herzenberg L. A., DePamphilis J. D. Identification and quantitation of thymus-derived lymphocytes in human peripheral blood. J Immunol. 1974 Feb;112(2):520–527. [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Brochier J., Abou-Hamed Y. A., Gueho J. P., Revillard J. P. Study of human T and B lymphocytes with heterologous antisera. I Preparation, specificity and properties of antisera. Immunology. 1976 Nov;31(5):749–758. [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Shen F. W., Boyse E. A. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Breard J. M., Lazarus H., Schlossman S. F., Chess L. Detection, isolation, and functional characterization of two human T-cell subclasses bearing unique differentiation antigens. J Exp Med. 1977 Jan 1;145(1):221–233. doi: 10.1084/jem.145.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrone S., Cooper N. R., Pellegrino M. A., Reisfeld R. A. The lymphocytotoxic reaction: the mechanism of rabbit complement action. J Immunol. 1971 Oct;107(4):939–947. [PubMed] [Google Scholar]
- Hausman P. B., Stobo J. D. Specificity and function of a human autologous reactive T cell. J Exp Med. 1979 Jun 1;149(6):1537–1542. doi: 10.1084/jem.149.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B. Inhibition of natural cytotoxic rabbit antibody by human IgM: production of nontoxic rabbit serum for use as complement source. J Immunol. 1970 Apr;104(4):805–809. [PubMed] [Google Scholar]
- Hirano T., Kishimoto T., Kuritani T., Muraguchi A., Yamamura Y., Ralph P., Good R. A. In vitro immune response of human peripheral lymphocytes. IV. Specific induction of human suppressor T cells by an antiserum to the T leukemia cell line HSB. J Immunol. 1979 Sep;123(3):1133–1140. [PubMed] [Google Scholar]
- Howard J. C., Butcher G. W., Galfre G., Milstein C. Monoclonal anti-rat MHC (H-1) alloantibodies. Curr Top Microbiol Immunol. 1978;81:54–60. doi: 10.1007/978-3-642-67448-8_9. [DOI] [PubMed] [Google Scholar]
- Ilfeld D. N., Krakauer R. S., Blaese R. M. Suppression of the human autologous mixed lymphocyte reaction by physiologic concentrations of hydrocortisone. J Immunol. 1977 Aug;119(2):428–434. [PubMed] [Google Scholar]
- Innes J. B., Kuntz M. M., Kim Y. T., Weksler M. E. Induction of suppressor activity in the autologous mixed lymphocyte reaction and in cultures with concanavalin A. J Clin Invest. 1979 Dec;64(6):1608–1613. doi: 10.1172/JCI109622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P., Fauci A. S. Autologous and allogeneic intercellular interactions: modulation by adherent cells, irradiation, and in vitro and in vivo corticosteroids. J Immunol. 1979 Nov;123(5):2270–2277. [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Linscott W. D. An antigen density effect on the hemolytic efficiency of complement. J Immunol. 1970 May;104(5):1307–1309. [PubMed] [Google Scholar]
- Moody C. E., Casazza B. A., Christenson W. N., Weksler M. E. Lymphocyte transformation induced by autologous cells. VIII. Impaired autologous mixed lymphocyte reactivity in patients with acute infectious mononucleosis. J Exp Med. 1979 Dec 1;150(6):1448–1455. doi: 10.1084/jem.150.6.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen F. L., Fanger M. W. Studies on the human T-lymphocyte population. I. The development and characterization of a specific anti-human T-cell antibody. J Immunol. 1974 Oct;113(4):1128–1137. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Green I. Specificity and suppressor function of human T cells responsive to autologous non-T cells. J Immunol. 1979 Aug;123(2):584–589. [PubMed] [Google Scholar]
- Strelkauskas A. J., Callery R. T., Borel Y., Schlossman S. F. Functional characteristics of human T-lymphocyte subsets identified by sera from patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1979 Sep;14(1):47–55. doi: 10.1016/0090-1229(79)90124-7. [DOI] [PubMed] [Google Scholar]
- Van Wauwe J. P., De Mey J. R., Goossens J. G. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980 Jun;124(6):2708–2713. [PubMed] [Google Scholar]
- Van Wauwe J., Goossens J. The effects of antioxidants on the stimulation of mouse thymocytes by concanavalin A. Int J Immunopharmacol. 1979;1(3):233–237. doi: 10.1016/0192-0561(79)90047-x. [DOI] [PubMed] [Google Scholar]
- Vande Stouwe R. A., Kunkel H. G., Halper J. P., Weksler M. E. Autologous mixed lymphocyte culture reactions and generation of cytotoxic T cells. J Exp Med. 1977 Dec 1;146(6):1809–1814. doi: 10.1084/jem.146.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]


