Abstract
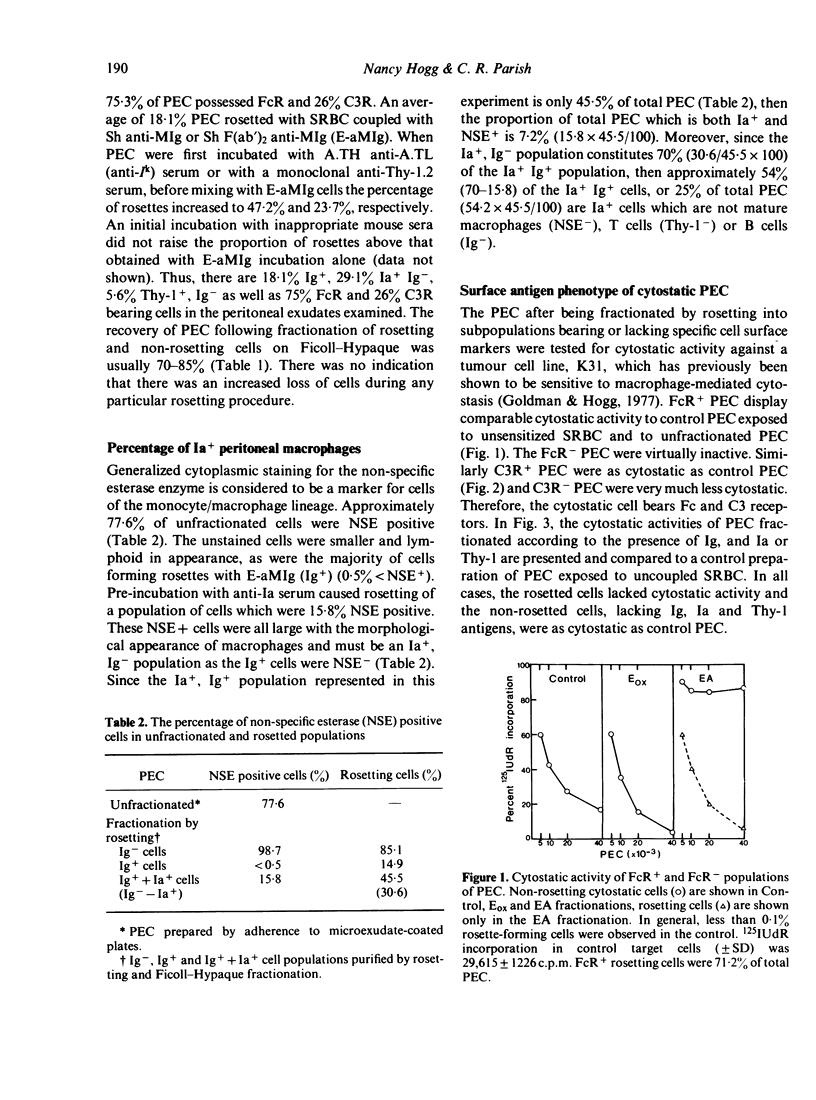
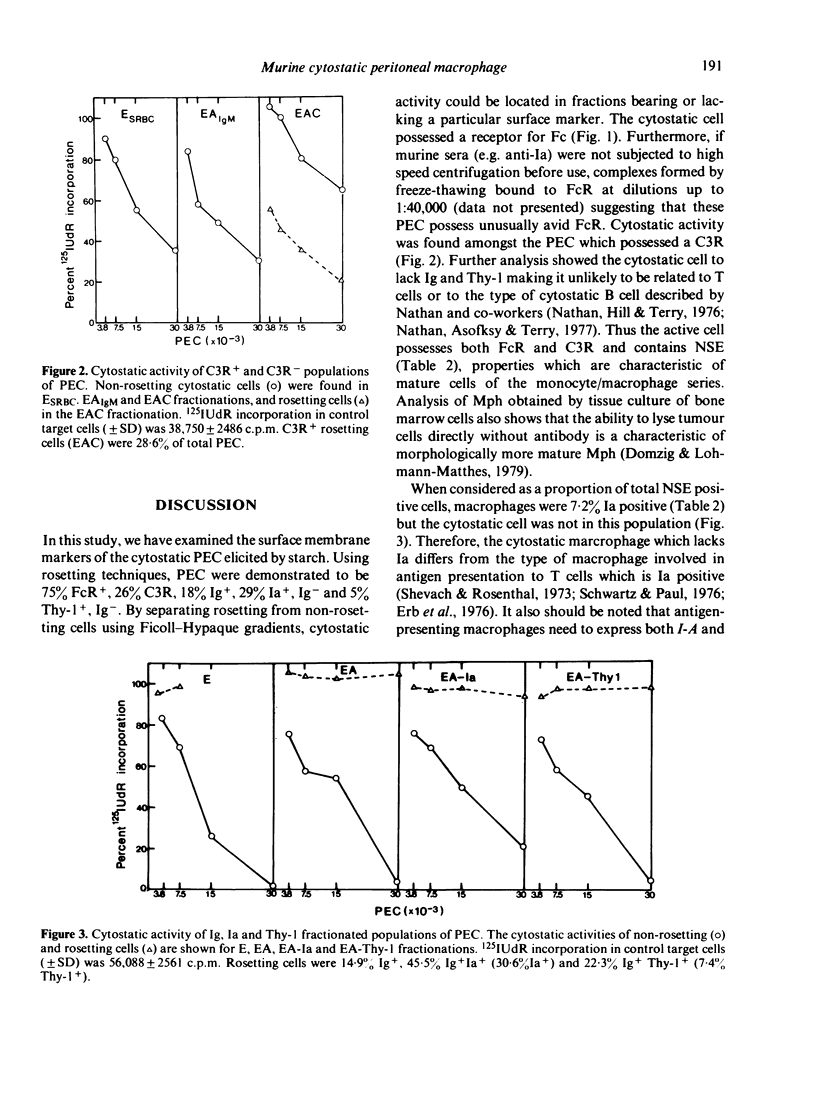
By rosetting techniques, peritoneal exudate cells (PEC) from mice stimulated intraperitoneally with starch have been shown to be a mixed population of cells consisting of the following subpopulations of cells: 75% FcR, 26% C3R, 18% Ig+, 29% Ia+, Ig- and 5% Thy-1+, Ig-. By separating rosetting from non-rosetting cells, it was possible to establish the phenotype of the PEC which was cytostatic for tumour cells. This cell possessed receptors for Fc and C3 but lacked surface Ig, the Thy-1 antigen and I-region controlled antigens and was NSE positive. Thus by presently available criteria, the cytostatic PEC can be identified as a macrophage. The lack of Ia distinguishes this type of macrophage from the antigen-presenting macrophage which bears Ia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Weaver C. A. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971 Nov;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- Ackerman S. K., Douglas S. D. Purification of human monocytes on microexudate-coated surfaces. J Immunol. 1978 Apr;120(4):1372–1374. [PubMed] [Google Scholar]
- Cowing C., Pincus S. H., Sachs D. H., Dickler H. B. A subpopulation of adherent accessory cells bearing both I-A and I-E or C subregion antigens is required for antigen-specific murine T lymphocyte proliferation. J Immunol. 1978 Nov;121(5):1680–1686. [PubMed] [Google Scholar]
- Cowing C., Schwartz B. D., Dickler H. B. Macrophage Ia antigens. I. macrophage populations differ in their expression of Ia antigens. J Immunol. 1978 Feb;120(2):378–384. [PubMed] [Google Scholar]
- Diamond B., Bloom B. R., Scharff M. D. The Fc receptors of primary and cultured phagocytic cells studied with homogeneous antibodies. J Immunol. 1978 Oct;121(4):1329–1333. [PubMed] [Google Scholar]
- Domzig W., Lohmann-Matthes M. L. Antibody-dependent cellular cytotoxicity against tumor cells. II. The promonocyte identified as effector cell. Eur J Immunol. 1979 Apr;9(4):267–272. doi: 10.1002/eji.1830090404. [DOI] [PubMed] [Google Scholar]
- Erb P., Feldmann M., Hogg N. Role of macrophages in the generation of T helper cells. IV. Nature of genetically related factor derived from macrophages incubated with soluble antigens. Eur J Immunol. 1976 May;6(5):365–372. doi: 10.1002/eji.1830060512. [DOI] [PubMed] [Google Scholar]
- Goldman R., Hogg N. Enhanced susceptibility of virus-infected fibroblasts to cytostasis mediated by peritoneal exudate cells. J Immunol. 1978 Nov;121(5):1657–1663. [PubMed] [Google Scholar]
- Goud T. J., Schotte C., van Furth R. Identification and characterization of the monoblast in mononuclear phagocyte colonies grown in vitro. J Exp Med. 1975 Nov 1;142(5):1180–1199. doi: 10.1084/jem.142.5.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes R. J., Ahmann G. B., Hathcock K. S., Dickler H. B., Singer A. Cellular and genetic control of antibody responses in vitro. IV. Expression of Ia antigens on accessory cells required for responses to soluble antigens including a response under Ir gene control. J Immunol. 1978 Oct;121(4):1501–1509. [PubMed] [Google Scholar]
- Horwitz D. A., Niaudet P., Greaves M. F., Dorling J., Deteix P. Surface markers and electron microscopy of human blood L cells: a comparison with T and B lymphocytes. J Immunol. 1978 Aug;121(2):678–684. [PubMed] [Google Scholar]
- Lee K. C., Berry D. Functional heterogeneity in macrophages activated by Corynebacterium parvum: characterization of subpopulations with different activities in promoting immune responses and suppressing tumor cell growth. J Immunol. 1977 May;118(5):1530–1540. [PubMed] [Google Scholar]
- Nathan C. F., Asofsky R., Terry W. D. Characterization of the nonphagocytic adherent cell from the peritoneal cavity of normal and BCG-treated mice. J Immunol. 1977 May;118(5):1612–1621. [PubMed] [Google Scholar]
- Nathan C. F., Hill V. M., Terry W. D. Isolation of a subpopulation of adherent peritoneal cells with anti-tumour activity. Nature. 1976 Mar 11;260(5547):146–148. doi: 10.1038/260146a0. [DOI] [PubMed] [Google Scholar]
- Parish C. R., Chilcott A. B. Functional significance of the complement receptors on B lymphocytes. Cell Immunol. 1975 Dec;20(2):290–303. doi: 10.1016/0008-8749(75)90106-9. [DOI] [PubMed] [Google Scholar]
- Parish C. R., Hayward J. A. The lymphocyte surface. I. Relation between Fc receptors, C'3 receptors and surface immunoglobulin. Proc R Soc Lond B Biol Sci. 1974 Aug 27;187(1086):47–63. doi: 10.1098/rspb.1974.0060. [DOI] [PubMed] [Google Scholar]
- Parish C. R., McKenzie I. F. A sensitive rosetting method for detecting subpopulations of lymphocytes which react with alloantisera. J Immunol Methods. 1978;20:173–183. doi: 10.1016/0022-1759(78)90254-5. [DOI] [PubMed] [Google Scholar]
- Parish C. R. Separation and functional analysis of subpopulations of lymphocytes bearing complement and Fc receptors. Transplant Rev. 1975;25:98–120. doi: 10.1111/j.1600-065x.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Rosenthal A. S. Function of macrophages in antigen recognition by guinea pig T lymphocytes. II. Role of the macrophage in the regulation of genetic control of the immune response. J Exp Med. 1973 Nov 1;138(5):1213–1229. doi: 10.1084/jem.138.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey D. E., Wolfe S. A., Durdik J. M., Henney C. S. BCG-induced murine effector cells. I. Cytolytic activity in peritoneal exudates: an early response to BCG. J Immunol. 1977 Sep;119(3):1145–1151. [PubMed] [Google Scholar]
- Winchester R. J., Meyers P. A., Broxmeyer H. E., Wang C. Y., Moore M. A., Kunkel H. G. Inhibition of human erythropoietic colony formation in culture by treatment with Ia antisera. J Exp Med. 1978 Aug 1;148(2):613–618. doi: 10.1084/jem.148.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Ross G. D., Jarowski C. I., Wang C. Y., Halper J., Broxmeyer H. E. Expression of Ia-like antigen molecules on human granulocytes during early phases of differentiation. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4012–4016. doi: 10.1073/pnas.74.9.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe S. A., Tracey D. E., Henney C. S. BCG-induced murine effector cells. II. Characterization of natural killer cells in peritoneal exudates. J Immunol. 1977 Sep;119(3):1152–1158. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


