Abstract
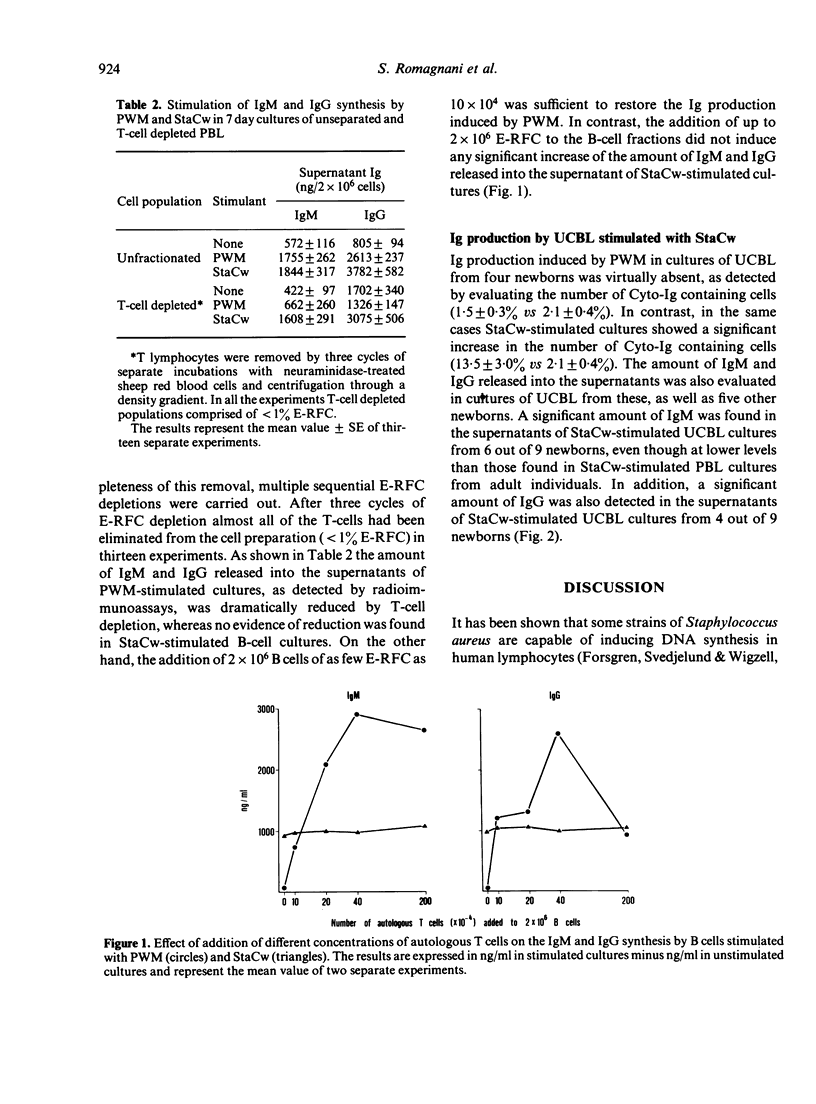
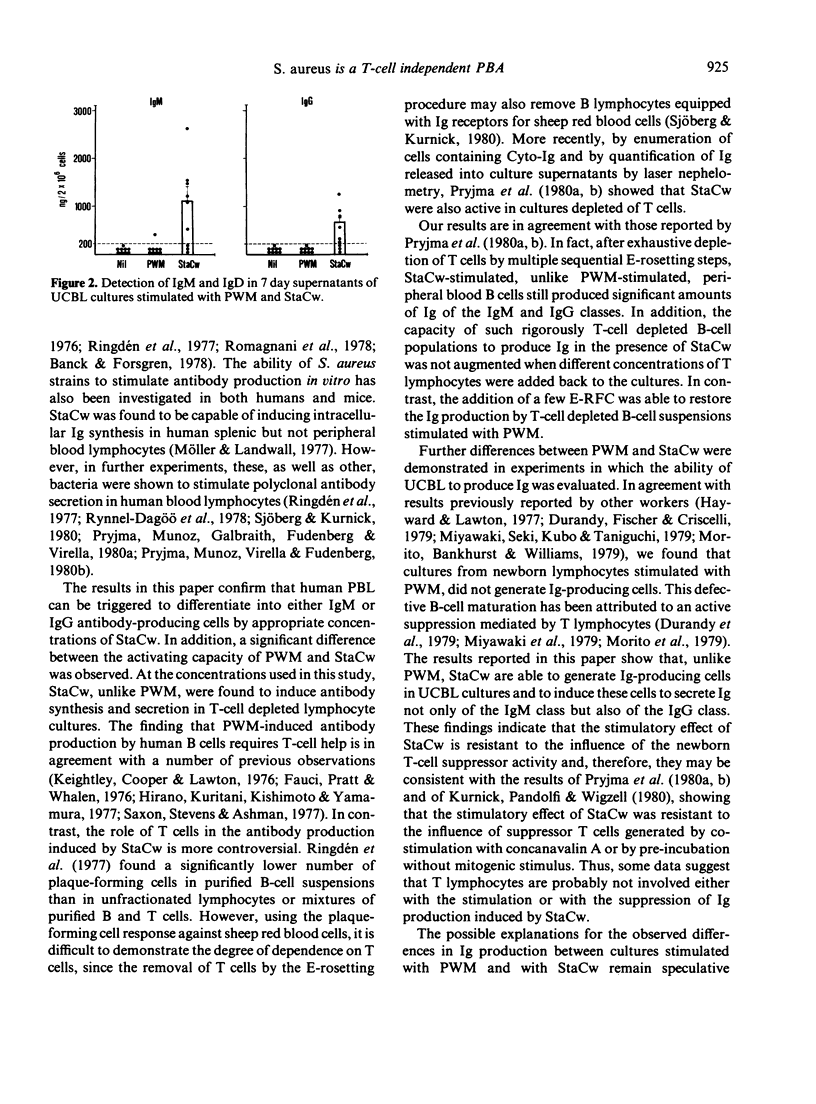
Unfractionated and T-cell depleted human peripheral blood lymphocytes (PBL) were cultured in vitro in the presence of pokeweed mitogen (PWM) and Staphylococcus aureus strain Cowan I (StaCw). After 7 days of culture, the cells were assayed for cytoplasmic immunoglobulins (Cyto-Ig) by direct staining using fluorescein-labelled F(ab')2 fragments prepared from specific antisera against human IgG F(ab')2. The amount of immunoglobulin of the IgM and IgG class released into the cell-free supernatants was also measured by radioimmunoassay. In unfractionated PBL StaCw, like PWM, was able to induce a significant increase of either the number of Cyto-Ig containing cells for the amount of IgM and IgG secreted into the supernatant. In contrast, the amount of IgM and IgG immunoglobulin released into the supernatant of T-cell depleted suspensions stimulated with PWM was significantly reduced in comparison with that of unfractionated populations, whereas it was unchanged in T-cell depleted vs unfractionated suspensions stimulated with StaCw. The addition of a few T lymphocytes restored the ability of T-cell depleted suspensions to produce Ig in the presence of PWM, whereas despite addition of high numbers of T cells no further augmentation of the Ig production induced by StaCw on T-cell depleted suspensions was observed. Cultures of umbilical cord blood lymphocytes (UCBL) stimulated with PWM did not generate Ig-producing cells, whereas UCBL stimulated with StaCw showed significant production of Ig of both IgM and IgG classes. The results indicate that T lymphocytes are probably not involved either with stimulation or with the suppression of Ig production induced by StaCw.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banck G., Forsgren A. Many bacterial species are mitogenic for human blood B lymphocytes. Scand J Immunol. 1978;8(4):347–354. doi: 10.1111/j.1365-3083.1978.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Ceska M., Lundkvist U. A new and simple radioimmunoassay method for the determination of IgE. Immunochemistry. 1972 Oct;9(10):1021–1030. doi: 10.1016/0019-2791(72)90112-7. [DOI] [PubMed] [Google Scholar]
- Chiorazzi N., Fu S. M., Kunkel H. G. Stimulation of human B lymphocytes by antibodies to IgM and IgG: functional evidence for the expression of IgG on B-lymphocyte surface membranes. Clin Immunol Immunopathol. 1980 Mar;15(3):301–313. doi: 10.1016/0090-1229(80)90042-2. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Möller G. Thymus-independent B-cell induction and paralysis. Adv Immunol. 1975;21:113–236. doi: 10.1016/s0065-2776(08)60220-5. [DOI] [PubMed] [Google Scholar]
- Durandy A., Fischer A., Griscelli C. Active suppression of B lymphocyte maturation by two different newborn T lymphocyte subsets. J Immunol. 1979 Dec;123(6):2644–2650. [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R., Whalen G. Activation of human B lymphocytes. II. Cellular interactions in the PFC response of human tonsillar and peripheral blood B lymphocytes to polyclonal activation by pokeweed mitogen. J Immunol. 1976 Dec;117(6):2100–2104. [PubMed] [Google Scholar]
- Forsgren A., Svedjelund A., Wigzell H. Lymphocyte stimulation by protein A of Staphylococcus aureus. Eur J Immunol. 1976 Mar;6(3):207–213. doi: 10.1002/eji.1830060312. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Lawton A. R. Induction of plasma cell differentiation of human fetal lymphocytes: evidence for functional immaturity of T and B cells. J Immunol. 1977 Oct;119(4):1213–1217. [PubMed] [Google Scholar]
- Hirano T., Kuritani T., Kishimoto T., Yamamura Y. In vitro immune response of human peripheral lymphocytes. I. The mechanism(s) involved in T cell helper functions in the pokeweed mitogen-induced differentiation and proliferation of B cells. J Immunol. 1977 Oct;119(4):1235–1241. [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- Luzzati A. L., Hengartner H., Schreier M. H. Induction of plaque-forming cells in cultured human lymphocytes by combined action of antigen and EB virus. Nature. 1977 Sep 29;269(5627):419–420. doi: 10.1038/269419a0. [DOI] [PubMed] [Google Scholar]
- Lêthibichthuy, Ciorbaru R., Brochier J. Human B cell differentiation. I. Immunoglobulin synthesis induced by Nocardia mitogen. Eur J Immunol. 1978 Feb;8(2):119–123. doi: 10.1002/eji.1830080209. [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Seki H., Kubo M., Taniguchi N. Suppressor activity of T lymphocytes from infants assessed by co-culture with unfractionated adult lymphocytes in the pokeweed mitogen system. J Immunol. 1979 Sep;123(3):1092–1096. [PubMed] [Google Scholar]
- Morito T., Bankhurst A. D., Williams R. C., Jr Studies of human cord blood and adult lymphocyte interactions with in vitro immunoglobulin production. J Clin Invest. 1979 Oct;64(4):990–995. doi: 10.1172/JCI109565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G., Landwall P. The polyclonal B-cell-activating property of protein A is not due to its interaction with the FC part of immunoglobulin receptors. Scand J Immunol. 1977;6(4):357–366. doi: 10.1111/j.1365-3083.1977.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Pryjma J., Muñoz J., Galbraith R. M., Fudenberg H. H., Virella G. Induction and suppression of immunoglobulin synthesis in cultures of human lymphocytes: effects of pokeweed mitogen and Staphylococcus aureus Cowan I. J Immunol. 1980 Feb;124(2):656–661. [PubMed] [Google Scholar]
- Pryjma J., Muñoz J., Virella G., Fudenberg H. H. Evaluation of IgM, IgG, IgA, IgD, and IgE secretion by human peripheral blood lymphocytes in cultures stimulated with pokeweed mitogen and Staphylococcus aureus Cowan I. Cell Immunol. 1980 Mar 1;50(1):115–124. doi: 10.1016/0008-8749(80)90011-8. [DOI] [PubMed] [Google Scholar]
- Ringdén O., Rynnel-Dagö Activation of human B and T lymphocytes by protein A of Staphylococcus aureus. Eur J Immunol. 1978 Jan;8(1):47–52. doi: 10.1002/eji.1830080110. [DOI] [PubMed] [Google Scholar]
- Ringdén O., Rynnel-Dagö B., Waterfield E. M., Möller E., Möller G. Polyclonal antibody secretion in human lymphocytes induced by killed staphylococcal bacteria and by lipopolysaccharide. Scand J Immunol. 1977;6(11):1159–1169. doi: 10.1111/j.1365-3083.1977.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Ringeén O., Möller E. B-cell mitogenic effects on human lymphocytes of rabbit anti-human beta 2-microglobulin. Scand J Immunol. 1975;4(2):171–179. doi: 10.1111/j.1365-3083.1975.tb02614.x. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Almerigogna F., Giudizi G. M., Ricci M. Rosette formation with protein A-coated erythrocytes: a method for detecting both IgG-bearing cells and another subset of human peripheral blood B lymphocytes. J Immunol Methods. 1980;33(1):11–21. doi: 10.1016/0022-1759(80)90078-2. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Amadori A., Giudizi M. G., Biagiotti R., Maggi E., Ricci M. Different mitogenic activity of soluble and insoluble staphylococcal protein A (SPA). Immunology. 1978 Sep;35(3):471–478. [PMC free article] [PubMed] [Google Scholar]
- Romagnani S., Del Prete G. F., Giudizi G. M., Almerigogna F., Ricci M. A simple solid-phase radioimmunoassay for the measurement of IgG secreted in vitro by human lymphocytes. J Immunol Methods. 1979;29(3):263–270. doi: 10.1016/0022-1759(79)90314-4. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Giudizi G. M., Almerigogna F., Nicoletti P. L., Ricci M. Protein A of Staphylococcus aureus is mitogenic for IgG-bearing, but also for a subpopulation of IgM- and/or IgD-bearing human lymphocytes. Immunology. 1980 Mar;39(3):417–425. [PMC free article] [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Almerigogna F., Ricci M. Interaction of staphylococcal protein A with membrane components of IgM- and/or IgD-bearing lymphocytes from human tonsil. J Immunol. 1980 Apr;124(4):1620–1626. [PubMed] [Google Scholar]
- Rynnel-Dagö B., Ringdén O., Alfredsson H., Möller E. The use of bacteria for the functional characterization of human lymphocyte subpopulations in various lymphoid organs. Scand J Immunol. 1978;8(5):369–375. doi: 10.1111/j.1365-3083.1978.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Sjöberg O., Kurnick J. Conditions for induction of specific and polyclonal antibody production by Cowan 1 bacteria and by pokeweed mitogen. Scand J Immunol. 1980;11(1):47–51. doi: 10.1111/j.1365-3083.1980.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Sjödahl J., Møller G. The Fc binding regions in protein A are not responsible for the polyclonal B cell activating property of protein A. Scand J Immunol. 1979;10(6):593–596. doi: 10.1111/j.1365-3083.1979.tb01394.x. [DOI] [PubMed] [Google Scholar]


