Abstract
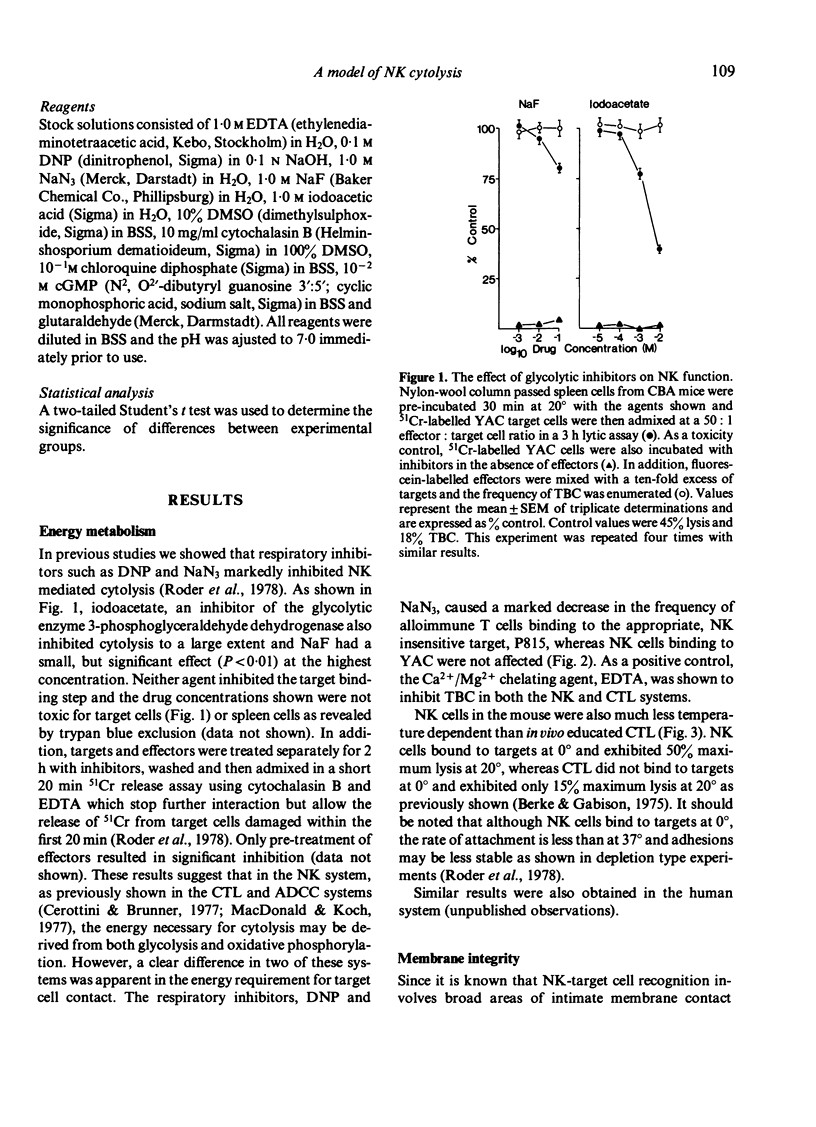
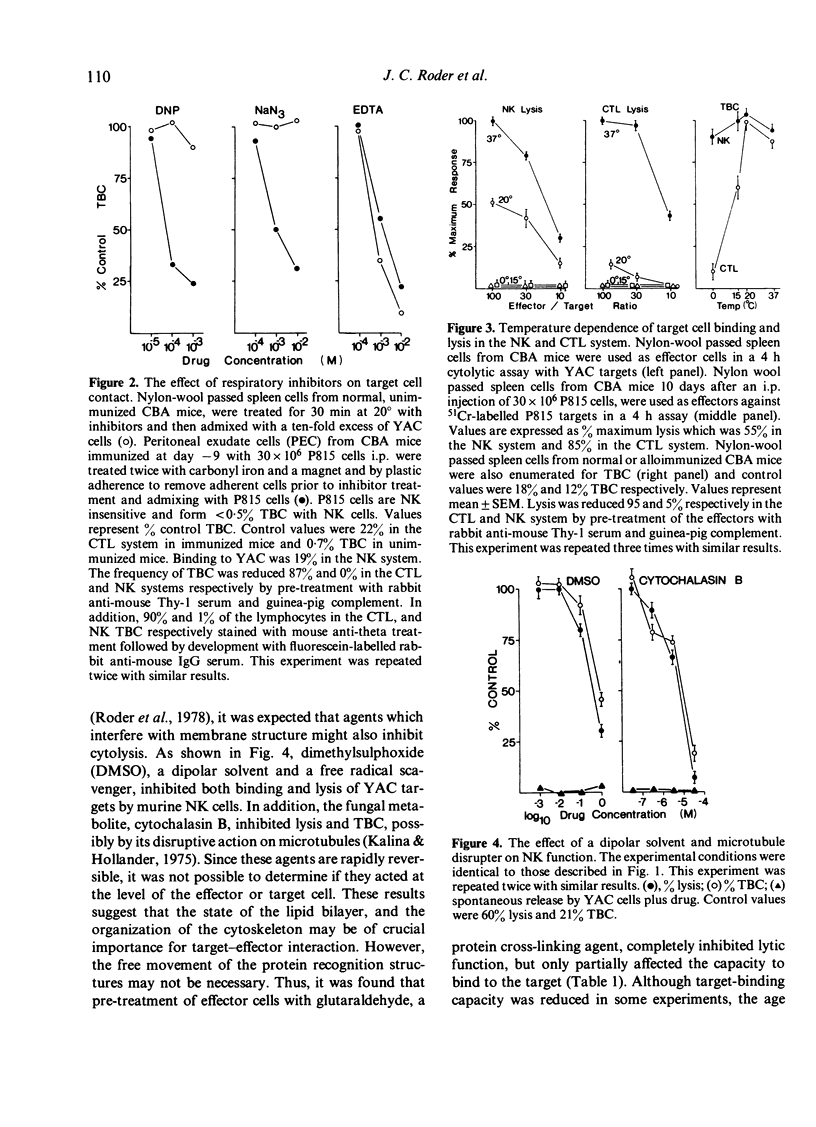
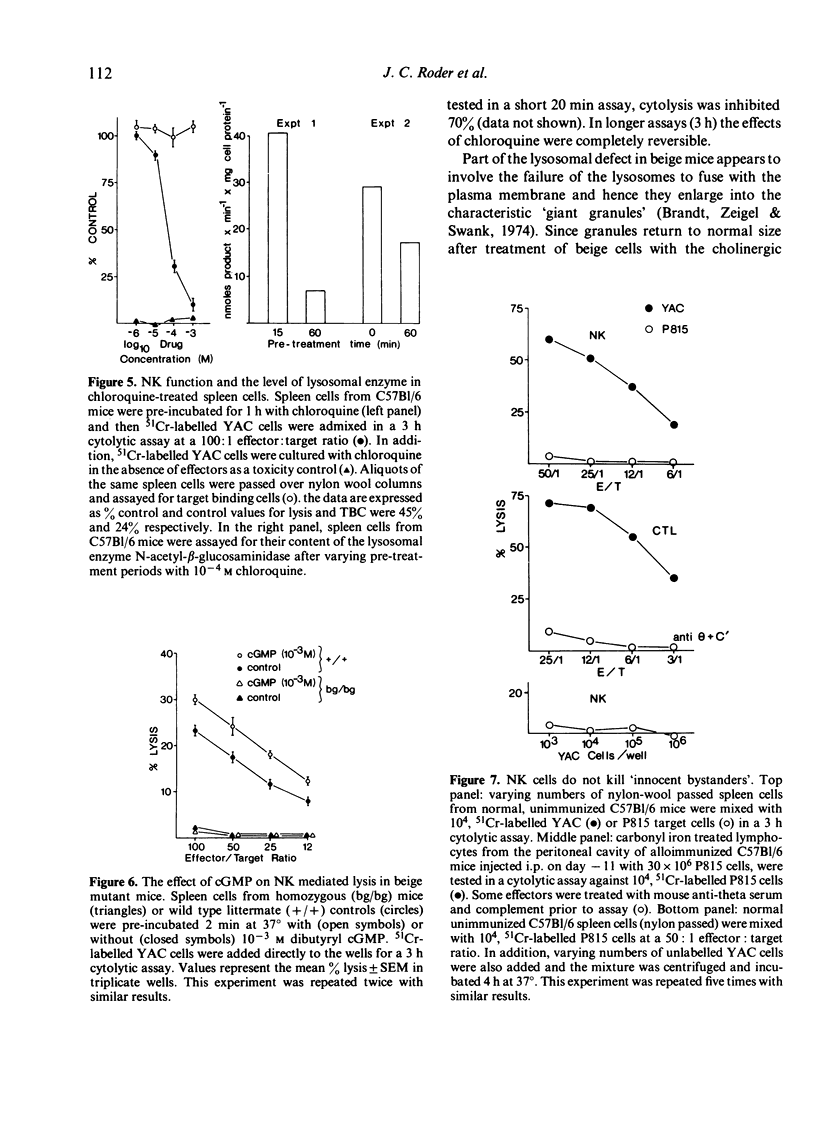
Various inhibitors were used to study further the mechanism of natural killing and to compare it to lympholysis by cytotoxic T lymphocytes (CTL). The respiratory inhibitors DNP and NaN3 or low temperatures (0 degrees) blocked the cell contact phase of target-effector interaction in the CTL system but not the NK system. The lytic stage was also inhibited by the glycolytic inhibitors, iodoacetate and NaF, in the NK system as previously shown in the CTL system. Dimethylsulphoxide, a dipolar solvent, and cytochalasin B, a microtubule disruptor, inhibited NK target binding. Pre-treatment of Nk cells with glutaraldehyde, a protein cross-linking agent, completely prevented lysis, but not the formation of target-effector conjugates. The lytic phase of NK lysis was inhibited by chloroquine which also inhibited lysosomal enzyme function. Lysosome defective, beige mutant mice were also totally deficient in NK lytic function and this defect could not be restored with cGMP. T-cell and macrophage mediated cytolysis was previously shown to be relatively normal in beige mice. These results suggest that (i) the mechanism of NK cytolysis is a complex, multistep process, and (ii) this process is fundamentally different from that occurring in CTL. A 'stimulus-secretion' model of NK cytolysis is presented in which it is postulated that lysosomal enzymes may be the lytic molecules.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker S., Stendahl O., Magnusson K. E. Physico-chemical characteristics of tumour cells susceptible to lysis by natural killer (NK) cells. Immunol Commun. 1979;8(1):73–83. doi: 10.3109/08820137909044708. [DOI] [PubMed] [Google Scholar]
- Berke G., Gabison D. Energy requirements of the binding and lytic steps of T lymphocyte-mediated cytolysis of leukemic cells in vitro. Eur J Immunol. 1975 Oct;5(10):671–675. doi: 10.1002/eji.1830051004. [DOI] [PubMed] [Google Scholar]
- Binz H., Wigzell H. Antigen-binding, idiotypic T-lymphocyte receptors. Contemp Top Immunobiol. 1977;7:113–177. doi: 10.1007/978-1-4684-3054-7_4. [DOI] [PubMed] [Google Scholar]
- Bubbers J. E., Henney C. S. Studies on the mechanism of lymphocyte-mediated cytolysis. V. The use of cytochalasins A and B to dissociate glucose transport from the lytic event. J Immunol. 1975 Jul;115(1):145–149. [PubMed] [Google Scholar]
- Golstein P., Foa C., MacLennan I. C. Mechanism of T cell-mediated cytolysis: the differential impact of cytochalasins at the recognition and lethal hit stages. Eur J Immunol. 1978 May;8(5):302–309. doi: 10.1002/eji.1830080504. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Bartram S., Haskill J. S., Nunn M., Holden H. T., West W. H. Fc receptors on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1977 Jul;119(1):322–326. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kalina M., Hollander N. The effect of cytochalasin B on effector--target cell interaction. Quantitative and ultrastructural study. Immunology. 1975 Oct;29(4):709–717. [PMC free article] [PubMed] [Google Scholar]
- Kiessling R., Petranyi G., Kärre K., Jondal M., Tracey D., Wigzell H. Killer cells: a functional comparison between natural, immune T-cell and antibody-dependent in vitro systems. J Exp Med. 1976 Apr 1;143(4):772–780. doi: 10.1084/jem.143.4.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Koch C. J. Energy metabolism and T-cell-mediated cytolysis. I. Synergism between inhibitors of respiration and glycolysis. J Exp Med. 1977 Sep 1;146(3):698–709. doi: 10.1084/jem.146.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo E., Wigzell H. Natural killer cells may be the only cells in normal mouse lymphoid cell populations endowed with cytolytic ability for antibody-coated tumour target cells. Scand J Immunol. 1978 Apr;7(4):297–306. doi: 10.1111/j.1365-3083.1978.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Krawiec J. A., Berlin R. D. Carbamycholine prevents giant granule-formation in cultured fibroblasts from beige (Chediak-Higashi) mice. J Cell Biol. 1976 Apr;69(1):205–210. doi: 10.1083/jcb.69.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieur D. J., Davis W. C., Padgett G. A. Defective function of renal lysosomes in mice with the Chediak-Higashi syndrome. Am J Pathol. 1972 May;67(2):227–236. [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Kiessling R., Biberfeld P., Andersson B. Target-effector interaction in the natural killer (NK) cell system. II. The isolation of NK cells and studies on the mechanism of killing. J Immunol. 1978 Dec;121(6):2509–2517. [PubMed] [Google Scholar]
- Roder J. C., Klein M. Target-effector interaction in the natural killer cell system. IV. Modulation by cyclic nucleotides. J Immunol. 1979 Dec;123(6):2785–2790. [PubMed] [Google Scholar]
- Roder J. C., Lohmann-Matthes M. L., Domzig W., Wigzell H. The beige mutation in the mouse. II. Selectivity of the natural killer (NK) cell defect. J Immunol. 1979 Nov;123(5):2174–2181. [PubMed] [Google Scholar]
- Roder J. C., Rosén A., Fenyö E. M., Troy F. A. Target-effector interaction in the natural killer cell system: isolation of target structures. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1405–1409. doi: 10.1073/pnas.76.3.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J., Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979 Mar 29;278(5703):451–453. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- SELLINGER O. Z., BEAUFAY H., JACQUES P., DOYEN A., DE DUVE C. Tissue fractionation studies. 15. Intracellular distribution and properties of beta-N-acetylglucosaminidase and beta-galactosidase in rat liver. Biochem J. 1960 Mar;74:450–456. doi: 10.1042/bj0740450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano R. F., Henney C. S. Studies on the mechanism of lymphocyte-mediated cytolysis. X. Enucleated cells as targets for cytotoxic attack. J Immunol. 1978 Jul;121(1):186–191. [PubMed] [Google Scholar]
- Thorn R. M., Henney C. S. Studies on the mechanism of lymphocyte-mediated cytolysis. VI. A reappraisal of the requirement for protein synthesis during T cell-mediated lysis. J Immunol. 1976 Jan;116(1):146–149. [PubMed] [Google Scholar]
- Vassalli J. D., Granelli-Piperno A., Griscelli C., Reich E. Specific protease deficiency in polymorphonuclear leukocytes of Chédiak-Higashi syndrome and beige mice. J Exp Med. 1978 Apr 1;147(4):1285–1290. doi: 10.1084/jem.147.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner F. D., Satir P. The structural basis of ciliary bend formation. Radial spoke positional changes accompanying microtubule sliding. J Cell Biol. 1974 Oct;63(1):35–63. doi: 10.1083/jcb.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Goldstein I., Hoffstein S., Tsung P. K. Reciprocal effects of cAMP and cGMP on microtubule-dependent release of lysosomal enzymes. Ann N Y Acad Sci. 1975 Jun 30;253:750–762. doi: 10.1111/j.1749-6632.1975.tb19243.x. [DOI] [PubMed] [Google Scholar]
- Windhorst D. B., Padgett G. The Chediak-Higashi syndrome and the homologous trait in animals. J Invest Dermatol. 1973 Jun;60(6):529–537. doi: 10.1111/1523-1747.ep12703609. [DOI] [PubMed] [Google Scholar]
- Wolberg G., Hümstra K., Burge J. J., Singler R. C. Reversible inhibition of lymphocyte mediated cytolysis by dimethyl sulfoxide (DMSO). J Immunol. 1973 Nov;111(5):1435–1443. [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]


