Abstract
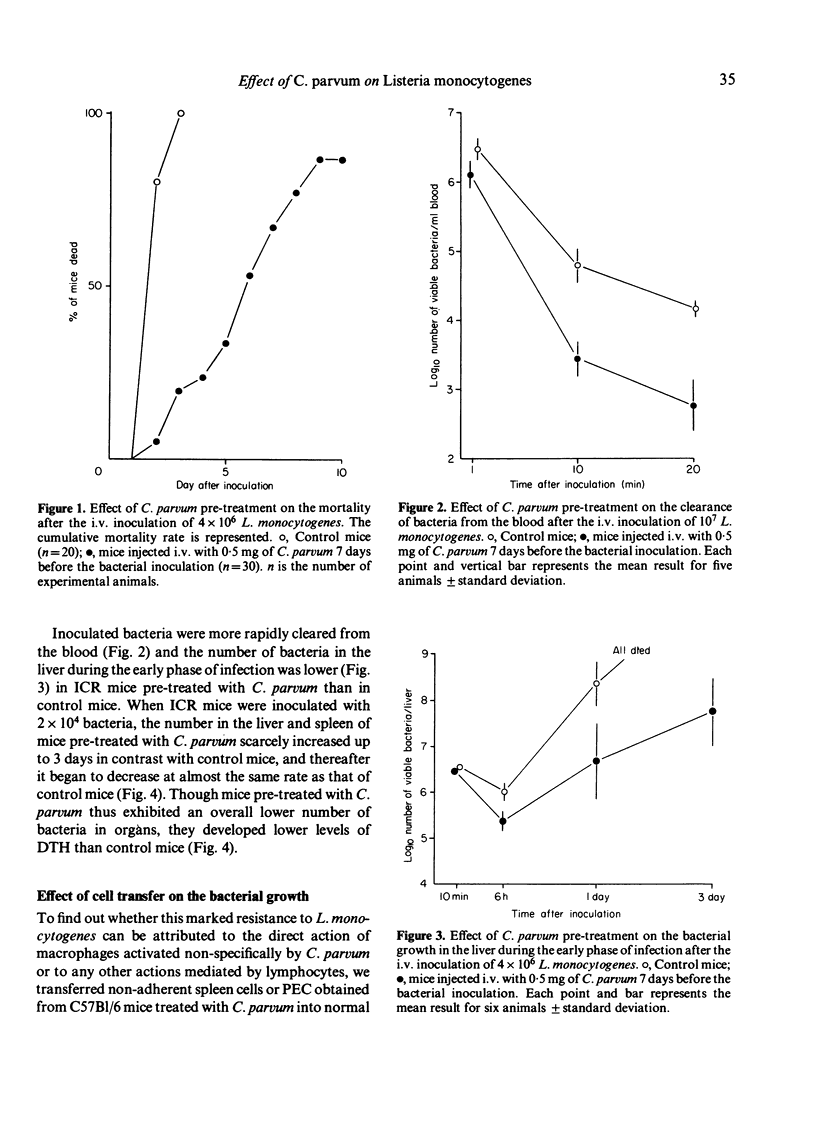
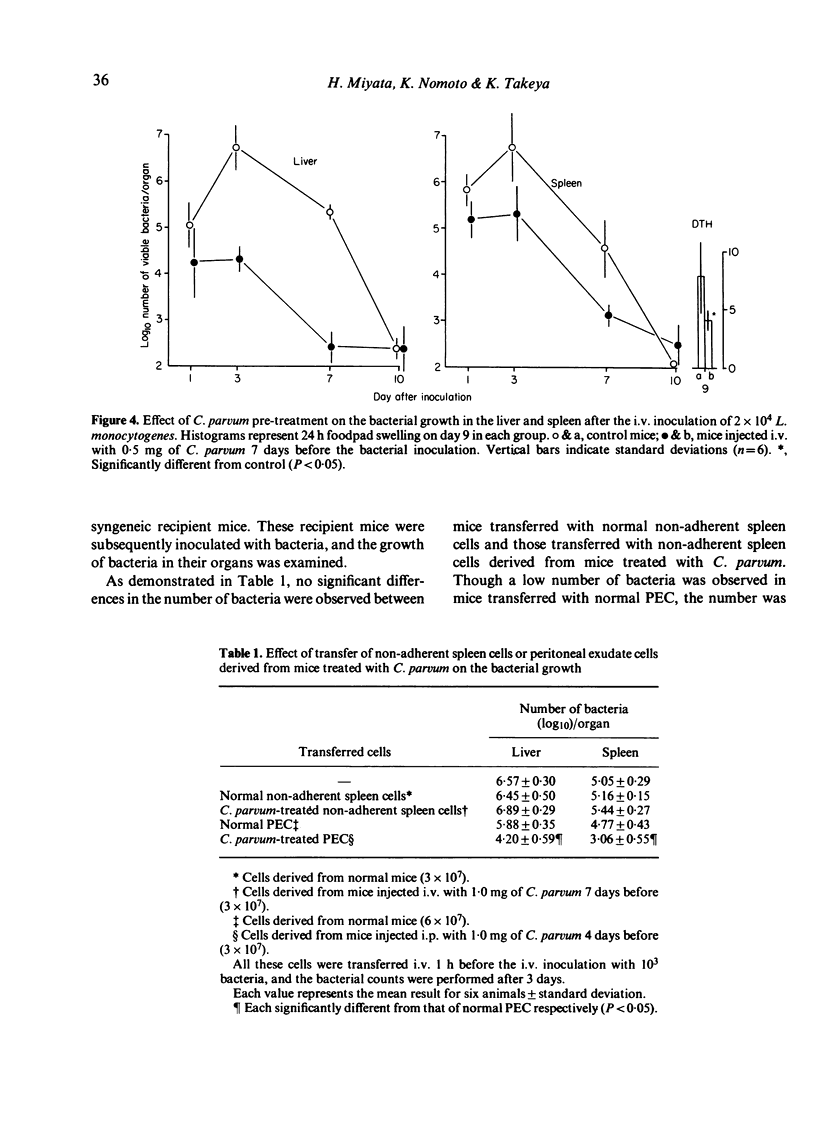
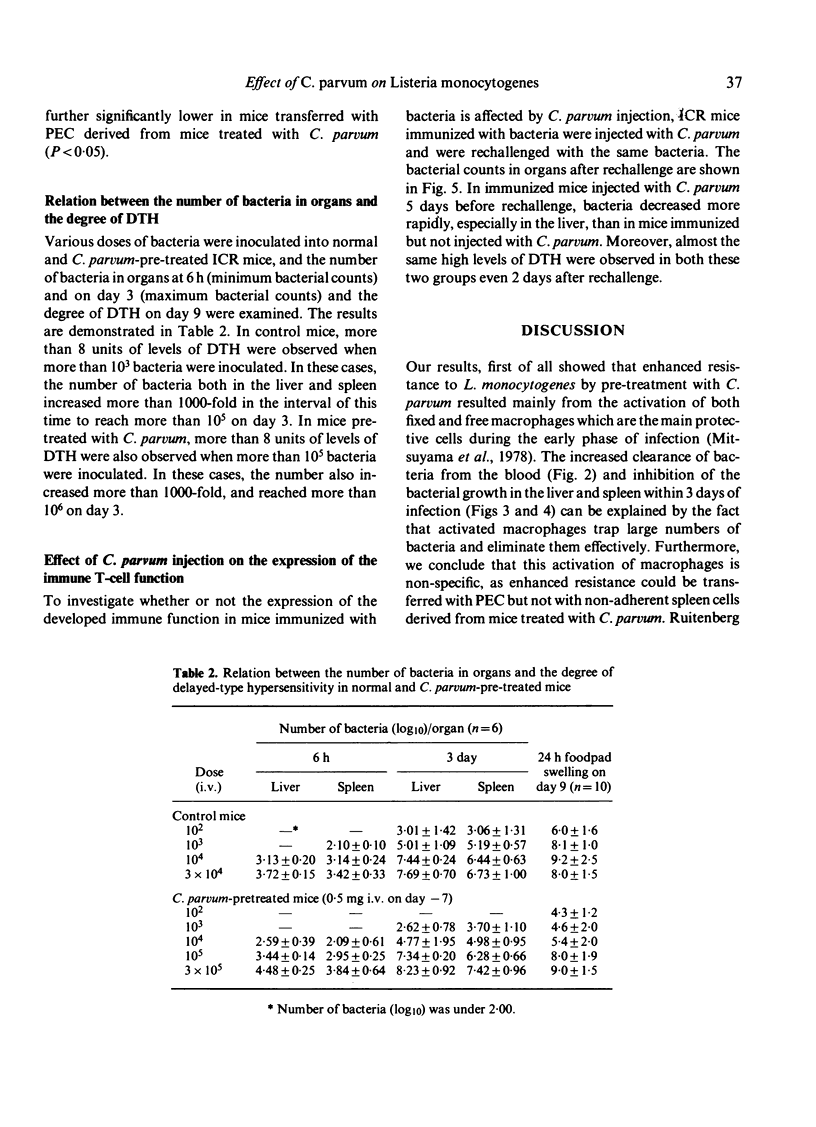
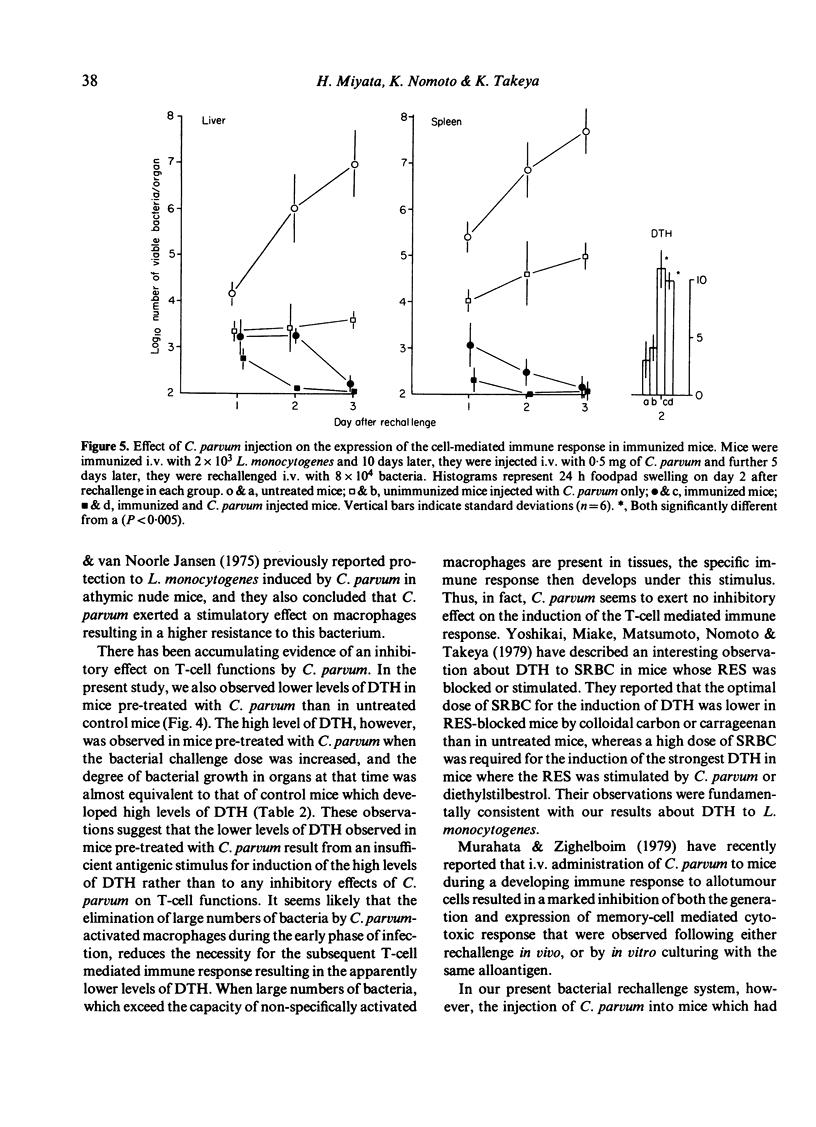
In mice pre-treated with Corynebacterium parvum, Listeria monocytogenes was cleared rapidly from the blood and bacterial growth in the liver and spleen was inhibited effectively during the early phase of infection. This enhanced resistance could be transferred with peritoneal exudate cells (PEC) but not with non-adherent spleen cells. In spite of earlier elimination of bacteria, pre-treated mice developed lower levels of delayed-type hypersensitivity (DTH) to bacteria than untreated immune control mice, but the control levels of DTH could be reached by increasing the challenge dose of bacteria in C. parvum-pre-treated mice. Additionally, C. parvum did not inhibit the expression of antibacterial immunity when immune mice were rechallenged. It appeared that the active suppression of the T-cell mediated immune response by C. parvum-activated macrophages was not seen during the course of L. monocytogenes infection, and that the lower levels of DTH seen in mice pre-treated with C. parvum were attributable to an insufficient antigenic stimulus following the accelerated elimination of bacteria by non-specifically activated macrophages during the early phase of infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlam C., Broughton E. S., Scott M. T. Enhanced resistance of mice to infection with bacteria following pre-treatment with Corynebacterium parvum. Nat New Biol. 1972 Feb 16;235(59):219–220. doi: 10.1038/newbio235219a0. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Allwood G. G. Depression of delayed hypersensitivity by pretreatment with Freund-type adjuvants. I. Description of the phenomenon. Clin Exp Immunol. 1971 Aug;9(2):249–258. [PMC free article] [PubMed] [Google Scholar]
- Berd D. A., Mitchell M. S. Immunological enhancement of leukemia L1210 by Corynebacterium parvum in allogeneic mice. Cancer Res. 1976 Nov;36(11 Pt 1):4119–4124. [PubMed] [Google Scholar]
- Collins F. M., Scott M. T. Effect of Corynebacterium parvum treatment on the growth of Salmonella enteritidis in mice. Infect Immun. 1974 May;9(5):863–869. doi: 10.1128/iai.9.5.863-869.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow L. A., Fischbach J., Bryant S. M., Kern E. R. Immunomodulation of host resistance to experimental viral infections in mice: effects of Corynebacterium acnes, Corynebacterium parvum, and Bacille calmette-guérin. J Infect Dis. 1977 May;135(5):763–770. doi: 10.1093/infdis/135.5.763. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F. Enhancement of resistance and suppression of immunization against experimental Trypanosoma cruzi infection by Corynebacterium parvum. Infect Immun. 1975 Nov;12(5):1227–1229. doi: 10.1128/iai.12.5.1227-1229.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Holden H. T., Herberman Splenic suppressor macrophages induced in mice by injection of Corynebacterium parvum. J Immunol. 1975 Nov;115(5):1212–1216. [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Miake S., Mitsuyama M., Takeya K., Nomoto K. Augmented resistance to Listeria monocytogenes in mice at an early stage of aging. J Clin Lab Immunol. 1979 Apr;2(1):55–58. [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Murahata R. I., Zighelboim J. Inhibition of memory cell-mediated cytotoxic response by systemic administration of Corynebacterium parvum. Cell Immunol. 1979 Feb;42(2):289–297. doi: 10.1016/0008-8749(79)90194-1. [DOI] [PubMed] [Google Scholar]
- Nussenzweig R. S. Increased nonspecific resstance to malaria produced by administration of killed Corynebacterium parvum. Exp Parasitol. 1967 Oct;21(2):224–231. doi: 10.1016/0014-4894(67)90084-7. [DOI] [PubMed] [Google Scholar]
- Ruittenberg E. J., van Noorle Jansen L. M. Effect of Corynebacterium parvum on the course of a Listeria monocytogenes infection in normal and congenitally athymic (nude) mice. Zentralbl Bakteriol Orig A. 1975;231(1-3):197–205. [PubMed] [Google Scholar]
- Scott M. T. Biological effects of the adjuvant Corynebacterium parvum. I. Inhibition of PHA, mixed lymphocyte and GVH reactivity. Cell Immunol. 1972 Nov;5(3):459–468. doi: 10.1016/0008-8749(72)90072-x. [DOI] [PubMed] [Google Scholar]
- Scott M. T. Biological effects of the adjuvant Corynebacterium parvum. II. Evidence for macrophage-T-cell interaction. Cell Immunol. 1972 Nov;5(3):469–479. doi: 10.1016/0008-8749(72)90073-1. [DOI] [PubMed] [Google Scholar]
- Scott M. T. Depression of delayed-type hypersensitivity by Corynebacterium parvum: mandatory role of the spleen. Cell Immunol. 1974 Aug;13(2):251–263. doi: 10.1016/0008-8749(74)90243-3. [DOI] [PubMed] [Google Scholar]
- Sher N. A., Chaparas S. D., Greenberg L. E., Bernard S. Effects of BCG, Corynebacterium parvum, and methanol-extration residue in the reduction of mortality from Staphylococcus aureus and Candida albicans infections in immunosuppressed mice. Infect Immun. 1975 Dec;12(6):1325–1330. doi: 10.1128/iai.12.6.1325-1330.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzberg J. E., Krahenbuhl J. L., Remington J. S. Dichotomy between macrophage activation and degree of protection against Listeria monocytogenes and Toxoplasma gondii in mice stimulated with Corynebacterium parvum. Infect Immun. 1975 Nov;12(5):1037–1043. doi: 10.1128/iai.12.5.1037-1043.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikai Y., Miake S., Matsumoto T., Nomoto K., Takeya K. Effect of stimulation and blockade of mononuclear phagocyte system on the delayed footpad reaction to SRBC in mice. Immunology. 1979 Nov;38(3):577–583. [PMC free article] [PubMed] [Google Scholar]


