Abstract
The chemical composition of the sexual communication signals of female moths is thought to be under strong stabilizing selection, because females that produce atypical pheromone blends suffer lower success in finding mates. This intraspecific selection pressure cannot explain the high diversity of moth pheromone blends found in nature. We conducted experiments to determine whether communication interference from males of closely related species could exert strong enough directional selection to cause evolution of these signals. Attraction and mating success of Heliothis subflexa (Hs) females with a normal quantitative trait locus for production of acetate pheromone components (norm-OAc) were compared with Hs females with an introgressed quantitative trait locus from Heliothis virescens (Hv) that dramatically decreased the amount of acetate esters in their pheromone glands (low-OAc). In field experiments with natural Hv and Hs populations, 10 times more Hv males were captured in traps baited with live low-OAc Hs females than in traps with norm-OAc Hs females. This pattern was confirmed in mate-choice assays in cages. Hybrids resulting from Hv–Hs matings have effectively zero fitness in the field. Combining our results with the extensive data set gathered in the past 40 years on the reproductive biology of Hv, we can quantitatively estimate that the directional selection exerted by Hv males on Hs females to produce relatively high amounts (>5%) of acetates can range from 0.135 to 0.231. Such intense interspecific selection may counteract intraspecific stabilizing selection that impedes evolutionary changes in pheromone blends and could lead to diversification of sexual signals.
Keywords: communication interference, Heliothis subflexa, Heliothis virescens, sexual communication, speciation
Sexual communication signals that vary little within species have been found to be under strong stabilizing selection in diverse animal taxa, including frogs (1–4), crickets and grasshoppers (5–9), and moths (10–14). Because the signaler and responder need to be finely tuned to each other for optimal mutual recognition (6, 14, 15), a population converges to the most attractive signal–response combination (16). In most nocturnal moths, females are the pheromone signalers and males are the responders. Because males are behaviorally tuned to their species-specific pheromone blend (10, 11), a mutation that alters the female’s pheromone blend is likely to lower her reproductive fitness (12, 13). Such stabilizing selection forces make it difficult to understand how one sexual communication system evolves to another (13, 14, 17, 18) and what led to the high diversity of moth pheromone blends found in nature.
Directional selection through communication interference between sympatric species that use similar premating signals has been proposed to counteract stabilizing selection (14, 19–25) and cause reproductive character displacement. In the past decade, researchers have described patterns in reproductive traits that are in accordance with reproductive character displacement, i.e., greater divergence has been found in mate recognition signals of closely related species in areas of sympatry than in areas of allopatry: in the songs of tree frogs (21, 26, 27), in the mate recognition cues in Drosophila (28–31) and in pheromone communication in moths (22–24). However, these studies fall short of addressing whether selection due to interspecific interference could have been strong enough to overcome stabilizing selection by conspecifics.
We developed a new approach to (i) test whether interspecific communication interference can be a directional selection force and (ii) estimate the intensity of such a selection force. Instead of starting from existing patterns in nature, we constructed one specific change in the mate-recognition signal of a moth species and tested its impact in the field. Through hybridizing and backcrossing two closely related species, Heliothis virescens (Fabricius 1777) (Hv) and Heliothis subflexa (Guenée 1852) (Hs) (Lepidoptera: Noctuidae), we genetically altered one specific part of the Hs pheromone, after which we experimentally assessed whether, and to what extent, this specific change elicited attraction of and mating with sympatric Hv males. Hv and Hs co-occur throughout the Americas, but hybridization is not known to occur in nature, and cross-attraction has not been found in traps baited with synthetic pheromone lures (e.g., refs. 32–37). The fitness cost of cross-fertilization would be high, because laboratory matings between Hs females and Hv males result in sterile F1 males (38). In addition, only 30% of the F1 progeny are females, and a large percentage of these females have been found to enter a protracted diapause lasting up to 2 years (38, 39). The F1 females that manage to reproduce have very low fecundity, and they oviposit many of their eggs on unsuitable host plants (40). It is reasonable to surmise that Hs eggs fertilized by Hv males have close to zero fitness.
The pheromone blends of both species contain (Z)-11-hexadecenal (Z11–16:Ald) as the major component and different relative amounts of tetradecanal (14:Ald), (Z)-9-tetradecenal (Z9–14:Ald), (Z)-7-hexadecenal (Z7–16:Ald), (Z)-9-hexadecenal (Z9–16:Ald), and (Z)-11-hexadecenol (Z11–16:OH) (34, 41 –50). In addition to the major component, Z9–16:Ald and Z11–16:OH are essential for the attraction of Hs males (34, 49, 51), whereas Hv males are attracted to a minimal blend consisting of Z11–16:Ald and Z9–14:Ald (41–43, 48). Hs females also produce three acetate esters, (Z)-7-hexadecenyl acetate (Z7–16:OAc), (Z)-9-hexadecenyl acetate (Z9–16:OAc), and (Z)-11-hexadecenyl acetate (Z11–16:OAc) (collectively denoted “acetates”), which are all absent in Hv (43, 45, 48–50, 52). The addition of Z11–16:OAc to the minimal Hs pheromone blend (three components) results in an insignificant increase in attraction of Hs males (51, 53), so the intraspecific role of this compound has been unclear. Addition of Z11–16:OAc to an Hv pheromone blend strongly antagonizes attraction of Hv males (54). However, no studies have examined whether the acetates are essential compounds to antagonize attraction of Hv males to the Hs blend, which is qualitatively and quantitatively different from the Hv blend. If so, Hs’s acetates could play an important role in decreasing communication interference from sympatric Hv males.
We introgressed one quantitative trait locus (QTL) for low production of the acetate compounds (which we denote low-OAc QTL) into an Hs genomic background (55, 56). We then conducted field and cage experiments with the backcross female offspring [with the introgressed low-OAc QTL and without (norm-OAc QTL)] to measure their relative capacity to attract and mate with Hs and Hv males. We found that Hv males were much more attracted to Hs females with the low-OAc QTL, even though the other five pheromone gland components were produced in typical Hs-like ratios, which differ substantially from Hv. This experimentally demonstrates that Hv males can exert directional selection on Hs females to produce a relatively high amount of acetates as part of their pheromone blend. We use the field results to quantitatively estimate the potential intensity of the directional selection force that Hv males exert on Hs females for the production of acetate pheromone components.
Results
Genotype and Pheromone Phenotype of Experimental Females.
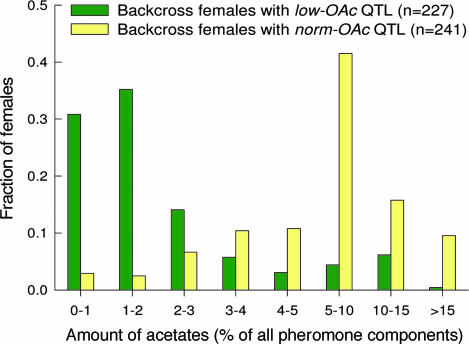
Fig. 1 shows the distribution of acetate levels (combined Z7–16:OAc, Z9–16:OAc and Z11–16:OAc) in the pheromone glands of all backcross females that were used in both the cage and field experiments and for which the genotype (presence/absence of the low-OAc QTL) was determined. In the field experiments, all 237 females were phenotyped and 212 females were genotyped, whereas in the cage experiments all 256 females were genotyped and phenotyped. Overall, backcross females with the low-OAc QTL contained extremely low amounts of acetates, with most (202 of 227 females) containing acetate levels of <5% of the total pheromone components. However, production of the three acetate esters was not completely eliminated in backcross females in which the low-OAc QTL was isolated in an Hs genomic background. This is probably because an additional QTL is involved in lower production of these acetates (56), and the low-OAc QTL may not be completely dominant. The relative amounts of the other five pheromone components that differ between the two species showed the Hs-like pattern and were not significantly different between the backcross females with the norm-OAc QTL and those with the low-OAc QTL (see also ref. 55).
Fig. 1.
Frequency distributions of acetate esters in backcross females with and without the low-OAc QTL. All females genotyped in the field experiments (n = 212) and cage experiments (n = 256) were combined in this analysis.
Field Experiments: Attraction of Males.
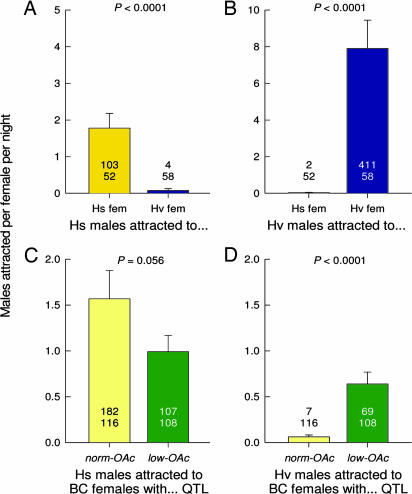
103 Hs males were caught in traps baited with Hs females, and only four Hs males were caught in traps baited with Hv females (Fig. 2A). Hv males were likewise attracted to conspecific females: 411 Hv males were captured in traps baited with Hv females, and only two Hv males were caught in traps baited with Hs females (Fig. 2B). Unbaited control traps (n = 19) caught a total of one Hv male and one Hs male. Hs males were marginally more attracted (P = 0.056) to the backcross females; with the norm-OAc QTL than to females with the low-OAc QTL (Fig. 2C). Dramatically fewer Hv males were attracted to both sets of backcross females, but ≈10 times more Hv males were caught in traps baited with backcross females containing the low-OAc QTL than in traps baited with females with the norm-OAc QTL (Fig. 2D).
Fig. 2.
Mean number of Hs and Hv males attracted in the field experiments to the parental species females (fem) (A and B) and to the backcross females (C and D). Hs males were captured more in traps baited with Hs females (A) or with backcross females with the norm-OAc QTL (C), whereas Hv males were attracted more to traps baited with Hv females (B) or with backcross females with the low-OAc QTL (D). The upper numbers in the bar graphs represent the number of males attracted. The lower numbers represent the number of females tested. Error bars represent SEM.
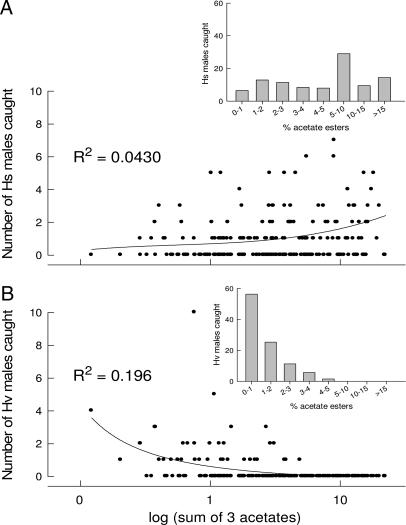
A small but highly significant positive relationship (R2 = 0.043, P = 0.0026) was found between the number of Hs males trapped and the relative amount of acetates in the pheromone gland of females to which they were attracted (Fig. 3A). In contrast, a highly significant negative relationship (R2 = 0.196, P < 0.0001) was found between the number of Hv males caught and the relative amount of the three acetates in the pheromone gland (Fig. 3B); no Hv males were caught when females produced >5% acetates.
Fig. 3.
Regression and frequencies (insets) of the number of males caught in traps baited with Hs-like females with the norm-OAc QTL or the low-OAc QTL against the acetate content in the pheromone glands of these females. (A) For Hs males, a significant positive relationship is seen between the relative amount of the three acetates and the number of males lured to live females. (B) A significant negative relationship is evident between the acetate content of glands and the number of Hv males caught per female per night. These regressions show that backcross females that produced intermediate amounts of the acetate esters attracted Hs and Hv males approximately equally [females with 1% acetate esters caught 0.7 ± 0.02 (SEM) Hs males and 0.4 ± 0.01 Hv males per female per night], whereas females producing more acetate esters selectively attracted Hs males (females with 10% acetates caught 1.8 ± 0.1 Hs males per female per night and no Hv males).
Cage Experiments: Female Mating.
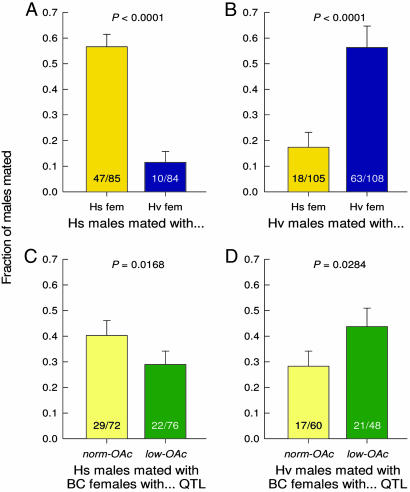
In all dissected females, either no spermatophore or one red spermatophore was found, indicating that all matings were with virgin males, and that females did not mate more than once in the 24-h experiments. When Hs males were confined with Hs and Hv females, only the variable “mating status” (mated/unmated females) showed a significant effect; significantly more Hs females were mated (55.3%, 47 of 85), whereas only 11.9% (10 of 84) Hv females were mated (Fig. 4A). When Hv males were confined with Hs and Hv females, both the mating status and experiment (each experiment being one cage) variables were significant, whereas their interaction effect was not. Overall, 58.3% of the Hv males mated with conspecific females (63 of 108 available Hv females), and 17.1% mated with heterospecific females (18 of 105 available Hs females) (Fig. 4B). Thus, even within the confines of a small cage, and with laboratory-reared moths, conspecific matings occurred significantly more often than heterospecific matings (P < 0.0001 in both cases) (Fig. 4).
Fig. 4.
Fraction of matings in the cage experiments with the parental species females (A and B) and the backcross females without and with the low-OAc QTL (C and D). In separate cages, Hs males (A and C) or Hv males (B and D) were offered a concurrent choice of Hs and Hv females (A and B) or backcross females without and with the low-OAc QTL (C and D). Mating was confirmed by presence of a spermatophore in the female’s bursa. Error bars represent SEM.
A lower fraction of backcross than parental females were mated in 24 h when confined with Hs or Hv males. When Hs males were confined with the backcross females, again only the mating status (mated/unmated) variable showed a significant effect. Hs males mated significantly more (P = 0.0168) with backcross females containing the norm-OAc QTL than with females with the low-OAc QTL (Fig. 4C), as expected, because the low-OAc QTL alters the pheromone blend away from the species normal blend. When Hv males were confined with the backcross females, both the mating status and experiment variables were significant, whereas their interaction effect was not, similar to the experiments with the parental species. Hv males mated significantly more (P = 0.0284) with females that had the low-OAc QTL (21 of 48 females) than with females with the norm-OAc QTL (17 of 60 females) (Fig. 4D). These results demonstrate that a single genetic change that reduces one set of related pheromone components can increase heterospecific attraction and matings.
Discussion
The construction of an introgression line has commonly been used in Drosophila to determine the genetic architecture of mating signals (57–64). Fitness consequences of differences between allopatric and sympatric phenotypes have been measured in birds (65) and butterflies (66). However, to our knowledge, the fitness consequences of a single introgressed QTL have never been tested in the field. Because mate-finding and reproductive isolation of moth species is typically based solely on pheromonal communication (67, 68) and can be measured in the field, moths offer an ideal system for examining selection pressures on single components of sexual communication signals.
Our field experiments demonstrate that a relative gland content of 5% acetates in backcross females is sufficient to completely prevent cross-attraction of Hv males (Fig. 3B). As the relative amount of acetates declined in backcross Hs females with the low-OAc QTL, they surprisingly attracted more Hv males. Although the addition of Z11–16:OAc, the major acetate component in Hs females, to a synthetic Hv pheromone blend has been shown to suppress upwind flight of Hv males (54), we had expected species-specificity to remain encoded in the other five Hs pheromone gland components that were equally represented in norm- and low-OAc Hs females, because the relative amounts of these components differ substantially from the Hv pheromone blend (55, 56). (Indeed, Hv females that do not produce any component that is behaviorally antagonistic to Hs males attracted 7.9 Hv males per female per night and only 0.08 Hs males per female per night in the field.) By producing >5% acetates, Hs females prevent the attraction of heterospecific Hv males, which in general can decrease the female’s fitness through harassment, interference with conspecific males, and nonfertile matings. Hence, Hv males exert directional selection on Hs females for the production of >5% acetate esters in their pheromone blend.
Our data on responses of conspecific and heterospecific males to Hs females with known genotypes can be coupled with previous data on Hv and Hs sexual reproduction traits to provide the unique opportunity to quantitatively estimate the fitness consequences of producing <5% acetate esters in Hs females. Specifically, the fitness consequences of attracting heterospecific males on the female’s lifetime reproductive success depend on (i) the chance that attraction is followed by mating, (ii) her ability to resume calling behavior (pheromone emission) after mating, (iii) her age, (iv) whether she mates multiple times, (v) how many eggs are oviposited after each mating, (vi) the presence and form of sperm competition and whether sperm precedence occurs, and (vii) the reproductive success of her offspring. Such data are sparse for Hs but abundant for Hv because it is an important agricultural pest. Research in the past 40 years, which aimed to assess the feasibility of suppressing Hv populations by releasing hybrid sterile males (38), has generated an extensive data set on all of the above traits in Hv. When we guardedly extend the information on life history and behavioral traits of Hv to the very closely related Hs females, we can calculate the selection intensity of our measured communication interference (see also the supporting information, which is published on the PNAS web site).
First, we need to calculate the probability that Hs females with a low amount of acetates attract Hv males. In the field, live Hs and Hv females effectively attracted almost exclusively conspecific males: Hs females attracted 103 Hs males and only two Hv males, and Hv females attracted 411 Hv males and only four males (Fig. 2A). Likewise, Hs-like backcross females with the norm-OAc QTL attracted 182 Hs males and only seven Hv males during the same period (Fig. 2B). In contrast, backcross females with the low-OAc QTL in an Hs genomic background attracted 107 Hs males and 69 Hv males (Fig. 2B), indicating that the introgressed low-OAc QTL was specifically responsible for the observed diminished discrimination and communication interference by Hv males. Even when we take into account that females with the low-OAc QTL were less attractive to Hs males (1.6 times more Hs males were attracted to females with the norm-OAc QTL than to females with the low-OAc QTL), the probability that Hs females with the low-OAc QTL would attract Hs males versus Hv males declined from 96.3% [182/(182 + 7)] to 71.2% {(107·1.6)/[(107·1.6)+ 69]}. The relative trap catches of Hv and Hs males also depend on their respective population densities, which can be estimated from the number of Hv and Hs males caught in traps baited with the parental species females throughout the season. During the same trapping period, 1.23 times more Hv than Hs males were captured by Hv and Hs females, respectively (2.52 and 2.05 males per female). If we consider equal population densities of Hv and Hs and conservatively estimate the possible communication interference of Hv males on the Hs communication channel, the probability that Hs females with the low-OAc QTL would attract Hv males is 23.4% [(100–71.2%)/1.23].
The chance that a heterospecific attraction is followed by mating can be deduced from our cage experiments: 58.3% (63 of 108) of Hv females and 43.8% (21 of 48) of Hs-like females with the low-OAc QTL mated when confined with Hv males (Fig. 4), indicating that Hv males were likely to mate with low-OAc females once they were encountered. What happens after attraction and the first mating can be estimated from previously published research. Most Hv females mate on the first or second night after eclosion; they oviposit eggs throughout the next night and resume calling the second night after mating (69). Both Hv and Hs females mate repeatedly (69–71). Based on the number of spermatophores found in field-collected females, Hv females mate on average 2.6 times, and up to 7 times (70). They exhibit a curvilinear asymptotic relationship of cumulative oviposition as a function of age, ovipositing more as young females than as older females (39). Cumulatively, an Hv female is likely to oviposit ≈50% of her eggs before her second mating and 83% of her eggs before her third mating.
Sperm competition and sperm precedence have been measured in Hv as well (72–76). Sperm precedence typically occurs, although the eggs are not always fertilized by sperm from the last male to mate but by sperm from the oldest male (74). We estimate fitness reduction for the three most extreme scenarios: (i) there is always conspecific sperm precedence, (ii) the first male always has sperm precedence, and (iii) the last male always has sperm precedence. The mean fitness reduction for each scenario can be calculated based on the 23% probability that an acetate-deficient Hs female will attract an Hv male when population densities of Hv and Hs are equal. Given that an Hs female mates on average three times during her life and Hs eggs fertilized by Hv males have close to zero fitness, her average fitness loss will be 0.135 in the first scenario and 0.23 in the other two scenarios (see the supporting information). Hv males can thus exert potent directional selection on Hs females to elevate acetate production to >5% of the pheromone blend, even if we assume equal population densities of Hv and Hs.
We acknowledge the limitations of our extrapolations from Hv to Hs. We also recognize that our results do not enable us to determine whether acetate production in Hs females initially evolved in response to selection for more efficient attraction of Hs males or selection to repel heterospecific males. Despite these caveats our results are important because they represent a quantitative estimate of the directional selection force exerted by heterospecific males on one single introgressed QTL controlling one subset of biochemically related pheromone components of a pheromone blend. The interspecific directional selection pressure on this single QTL is substantial. Our approach could be used with other moth species to determine the generality of this evolutionary pathway. If directional selection forces from heterospecific males are frequently found to be strong, they may explain how moth sex pheromone communication systems diversified even in the face of stabilizing selection from conspecific males.
Materials and Methods
Genotype and Pheromone Phenotype of Experimental Females.
Colonies of Hv (strain YDK) and Hs have been reared in the laboratory since 1988 and 1997, respectively (40). In 2001, single-pair matings were set up between Hv females and Hs males. Females of the hybrid cross that produced the most offspring (family DD23) were backcrossed to Hs males in single-pair matings. The backcross-1 females were phenotyped by extracting their pheromone glands and analyzing their content on an HP6890 gas chromatograph equipped with a splitless inlet, capillary column, and a flame ionization detector, and genetically mapped using amplified fragment length polymorphism markers (see the supporting information for further details). Because there is no recombination in female Lepidoptera (77) and Heliothis spp. contain 31 chromosomes (78), a linkage group can be considered a chromosome, corresponding to ≈3% of the genome, which is equivalent to, or more fine-scaled than, that found in most other QTL analyses (e.g., refs. 59, 62, 63, and 79).
After identifying Hv chromosome 22 as a major QTL (explaining 23% of the phenotypic variance) and Hv chromosome 4 as a minor QTL (explaining 10% of the variance) for decreased production of acetates, backcross females were selected based on the presence of the major QTL and a corresponding pheromone blend (55). The selected females were further backcrossed to Hs males (see the supporting information). The female offspring of backcross generations 4 (in 2002, n = 60), 10, and 11 (in 2003, n = 196) were used in the cage experiments, and the female offspring of backcrosses 17 and 18 (in 2004, n = 237) were used in the field experiments. Mating backcross females with the low-OAc QTL to Hs males was predicted to generate female offspring with and without the low-OAc QTL in a 1:1 ratio through Mendelian segregation.
Field Experiments.
Attraction assays were conducted at the North Carolina State University field station in Clayton, NC, from July 20 to September 26, 2004. The field site (60 × 80 m) alternated rows of cotton with rows of Physalis, including P. angulata, P. pubescens, P. cordata, and P. heterophylla. Attraction of naturally occurring Hv and Hs males to backcross females with and without the low-OAc QTL was measured by using Hartstack wire-mesh cone traps (32). Thirteen traps were distributed at least 15 m apart throughout the field. Virgin females were used as lures: one live 1- to 2-d-old backcross female was placed in a small open cylinder sealed with gauze on both sides. Controls for attractiveness of females of the parental species, Hs and Hv, to conspecific and heterospecific males were tested by deploying traps baited with one live virgin 1- to 2-d-old Hv or Hs female. Empty containers served as negative controls. One container was deployed at the opening of each trap. Containers with backcross and parental species females as lures and empty containers were distributed randomly over the 13 traps. Males caught in the traps during 1–2 nights were sorted by species under a microscope. A total of 141 backcross females were deployed in the field for 1 night each, and 96 females were left in the field for 2 nights each. When containers were left in the field for 2 nights, all containers were rotated among all trap locations after 1 night to minimize possible position effects and odorant contamination. The pheromone glands of all backcross females that were used as lures were extracted and analyzed by gas chromatography, and the presence/absence of the low-OAc QTL was determined by using a codominant marker developed from the sequence of one of the four amplified fragment length polymorphism markers that identified this QTL (see the supporting information).
The numbers of Hv or Hs males that were attracted by the different females were compared by using ANOVA (proc glm in sas 9.1). Because backcross females with the norm-OAc QTL were genetically and phenotypically identical to Hs females, our alternative hypothesis was that Hs males would be attracted significantly more, and Hv males would be attracted significantly less, to these females. Our a priori hypothesis structure allowed us to use one-tailed statistical tests. To determine the relationship between the amount of acetates in the backcross females and the number of males caught, we conducted a regression analysis, using proc glm in sas 9.2, after log-transforming the percentages of acetates present in the female glands.
Cage Experiments.
Cages (118 × 118 × 225 cm, constructed of window screening) were placed on greenhouse tables near an exhaust fan. Another fan was positioned in front of each cage to facilitate air flow. Five to 10 Hs or Hv virgin males and twice as many virgin backcross females were released into each cage in the afternoon. Hs males were used in 13 replicate assays and Hv males were used in 12 replicate assays. All insects were recaptured 24 h later. Both males and females were dissected to determine whether they had mated. Virgin males contain red seminal fluid, which is transferred to the female only during the first mating (80). Therefore, presence of a red spermatophore in the female’s bursa indicates mating with a virgin male, whereas a white spermatophore indicates mating with a previously mated male (80–82). The pheromone glands of the females were extracted and chemically analyzed (50), and presence/absence of the low-OAc QTL was ascertained based on the four amplified fragment length polymorphism markers. The cage bioassay was validated with similar experiments using the parental species: equal numbers of Hs and Hv females were released in each cage for 24 h with either Hs males (12 tests) or Hv males (11 tests).
For statistical analysis, we first determined whether the female offspring of backcrosses 4, 10, and 11 with and without the low-OAc QTL produced similar amounts and relative ratios of pheromone gland components by using ANOVA (proc glm in sas 9.1). Because no significant differences were found among the different backcrosses in any of the compounds, including the acetates, all backcross generations were pooled in subsequent analyses. Differences between the number of Hs and Hv females that mated in trials with Hs and Hv males and between the number of mated backcross females with and without the low-OAc QTL were statistically tested by using a generalized linear model (proc genmod in sas 9.1) that assumed a binomial distribution with a logit link, where mating status (mated/unmated) and experiment (one cage) were considered the explanatory variables. As in the field experiments, our hypothesis was that Hs males would mate significantly more and that Hv males would mate significantly less with females with the norm-OAc QTL. Our a priori hypothesis structure allowed us to use one-tailed statistical tests.
Supplementary Material
Acknowledgments
We thank Reid Evans and Cathy Herring from Central Crops Research Station (Clayton, NC) and Nicole Benda and Melanie Bateman for assisting with maintenance of the field plot; Maude Rowland, Kate Dulaney, and Natalie Taylor for help with the cage experiments; Cavell Brownie for guidance with the statistical analyses; and Mohammed Noor, Maria Servedio, Marcel Dicke, Neil Vickers, and two anonymous reviewers for valuable comments on the manuscript. This research was supported by National Science Foundation Population Biology Grant 0235400; the National Research Initiative through U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service Grant 2005-00896; the North Carolina State University W. M. Keck Center for Behavioral Biology; and the Blanton J. Whitmire Endowment at North Carolina State University.
Abbreviations
- Hs
Heliothis subflexa
- Hv
Heliothis virescens
- QTL
quantitative trait locus
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ryan M. J., Wilczynski W. Science. 1988;240:1786–1788. [Google Scholar]
- 2.Gerhardt H. C. Anim. Behav. 1991;42:615–635. [Google Scholar]
- 3.Wollerman L. Anim. Behav. 1998;55:1619–1630. doi: 10.1006/anbe.1997.0697. [DOI] [PubMed] [Google Scholar]
- 4.Ryan M. J., Rand A. S. Evolution. 2003;57:2608–2618. doi: 10.1111/j.0014-3820.2003.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 5.von Helverson O., von Helverson D. In: Neural Basis of Behavioural Adaptation. Schildberger K., Elsner N., editors. Stuttgart, Germany: Fisher; 1994. pp. 253–284. [Google Scholar]
- 6.Ritchie M. G. Proc. Natl. Acad. Sci. USA. 1996;93:14628–14631. doi: 10.1073/pnas.93.25.14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw K. L., Herlihy D. P. Proc. R. Soc. London Ser. B; 2000. pp. 577–584. [Google Scholar]
- 8.Fereira M., Ferguson J. W. H. J. Zool. (London) 2002;257:163–170. [Google Scholar]
- 9.Klappert K., Reinhold K. Anim. Behav. 2003;65:225–233. [Google Scholar]
- 10.Cossé A. A., Campbell M., Glover T. J., Linn C. E., Jr, Todd J. L., Baker T. C., Roelofs W. L. Experientia. 1995;51:809–816. [Google Scholar]
- 11.Linn C. E., Jr, Young M. S., Gendle M., Glover T. J., Roelofs W. L. Physiol. Entomol. 1997;22:212–223. [Google Scholar]
- 12.Zhu J., Chastain B. B., Spohn B. G., Haynes K. F. J. Insect Behav. 1997;10:805–817. [Google Scholar]
- 13.Butlin R., Trickett A. J. In: Insect Pheromone Research: New Directions. Cardé R. T., Minks A. K., editors. New York: Chapman & Hall; 1997. pp. 548–562. [Google Scholar]
- 14.Butlin R. In: Speciation and the Recognition Concept: Theory and Application. Lambert D. M., Spencer H. G., editors. Baltimore: Johns Hopkins Univ. Press; 1995. pp. 327–366. [Google Scholar]
- 15.Butlin R. K., Hewitt G. M., Webb S. F. Anim. Behav. 1985;33:1281–1292. [Google Scholar]
- 16.Brooks R., Hunt J., Blows M. W., Smith M. J., Bussière L. F., Jennions M. D. Evolution. 2005;59:871–880. [PubMed] [Google Scholar]
- 17.Löfstedt C. Proc. R. Soc. London Ser. B; 1993. pp. 167–177. [Google Scholar]
- 18.Phelan P. L. In: Evolution of Mating Systems in Insects and Arachnids. Choe J., Crespi B., editors. Cambridge: Cambridge Univ. Press; 1997. pp. 240–256. [Google Scholar]
- 19.Cardé R. T., Cardé A. M., Hill A. S., Roelofs W. L. J. Chem. Ecol. 1977;3:71–84. [Google Scholar]
- 20.Löfstedt C., Herrebout W. M., Menken S. B. J. Chemoecology. 1991;2:20–28. [Google Scholar]
- 21.Howard D. J. In: Hybrid Zones and the Evolutionary Process. Harrison R. G., editor. New York: Oxford Univ. Press; 1993. pp. 46–69. [Google Scholar]
- 22.McElfresh J. S., Millar J. C. J. Chem. Ecol. 1999;25:2505–2525. [Google Scholar]
- 23.McElfresh J. S., Millar J. C. Ecology. 2001;82:3505–3518. [Google Scholar]
- 24.Gries G., Schaefer P. W., Gries R., Liska J., Gotoh T. J. Chem. Ecol. 2001;27:1163–1176. doi: 10.1023/a:1010316029165. [DOI] [PubMed] [Google Scholar]
- 25.Shaw K. L., Parson Y. M. Am. Nat. 2002;159:S61–S75. doi: 10.1086/338373. [DOI] [PubMed] [Google Scholar]
- 26.Gerhardt H. C. Anim. Behav. 1994;47:959–969. [Google Scholar]
- 27.Höbel G., Gerhardt H. C. Evolution. 2003;57:894–904. doi: 10.1111/j.0014-3820.2003.tb00300.x. [DOI] [PubMed] [Google Scholar]
- 28.Coyne J. A., Orr H. A. Evolution. 1989;43:362–381. doi: 10.1111/j.1558-5646.1989.tb04233.x. [DOI] [PubMed] [Google Scholar]
- 29.Coyne J. A., Orr H. A. Evolution. 1997;51:295–303. doi: 10.1111/j.1558-5646.1997.tb02412.x. [DOI] [PubMed] [Google Scholar]
- 30.Noor M. A. Nature. 1995;375:674–675. doi: 10.1038/375674a0. [DOI] [PubMed] [Google Scholar]
- 31.Higgie M., Chenoweth S., Blows M. W. Science. 2000;290:519–521. doi: 10.1126/science.290.5491.519. [DOI] [PubMed] [Google Scholar]
- 32.Hartstack A. W., Witz J. A., Buck D. R. J. Econ. Entomol. 1979;72:519–522. [Google Scholar]
- 33.Heath R. R., Mitchell E. R., Tovar J. C. J. Chem. Ecol. 1990;16:1259–1268. doi: 10.1007/BF01021024. [DOI] [PubMed] [Google Scholar]
- 34.Klun J. A., Leonardt B. A., Lopez J. D., LaChance L. E. Environ. Entomol. 1982;11:1084–1090. [Google Scholar]
- 35.Lopez J. D., Goodenough J. L., Beerwinkle K. R. J. Econ. Entomol. 1994;87:793–801. [Google Scholar]
- 36.Chapin J. B., Ganaway D. R., Leonard B. R., Micinsky S., Burrise E., Graves J. B. Southwest Entomol. 1997;22:223–231. [Google Scholar]
- 37.Parajulee M. N., Rummel D. R., Arnold M. D., Carrol S. C. J. Econ. Entomol. 2004;97:668–677. doi: 10.1093/jee/97.2.668. [DOI] [PubMed] [Google Scholar]
- 38.Laster M. Environ. Entomol. 1972;1:682–687. [Google Scholar]
- 39.Proshold F. I., Karpenko C. P., Graham C. K. Ann. Entomol. Soc. Am. 1982;75:51–55. [Google Scholar]
- 40.Sheck A. L., Gould F. Environ. Entomol. 1995;24:341–347. [Google Scholar]
- 41.Roelofs W. L., Hill A. S., Cardé R. T., Baker T. C. Life Sci. 1974;14:1555–1562. doi: 10.1016/0024-3205(74)90166-0. [DOI] [PubMed] [Google Scholar]
- 42.Tumlinson J. H., Hendricks P. E., Mitchell E. R., Doolittle R. E., Brennan M. M. J. Chem. Ecol. 1975;1:203–214. [Google Scholar]
- 43.Tumlinson J. H., Heath R. R., Teal P. E. A. In: Insect Pheromone Technology: Chemistry and Applications. Leonhardt B. A., Beroza M., editors. Washington D.C.: Am. Chem. Soc.; 1982. pp. 1–25. [Google Scholar]
- 44.Vetter R. S., Baker T. C. J. Chem. Ecol. 1983;9:747–759. doi: 10.1007/BF00988780. [DOI] [PubMed] [Google Scholar]
- 45.Klun J. A., Plimmer J. R., Bierl-Leonhardt B. A. Science. 1979;204:1328–1330. doi: 10.1126/science.204.4399.1328. [DOI] [PubMed] [Google Scholar]
- 46.Klun J. A., Bierl-Leonhardt B. A., Plimmer J. R., Sparks A. N., Primiani M., Chapman O. L., Lepone G., Lee G. H. J. Chem. Ecol. 1980;6:177–183. [Google Scholar]
- 47.Teal P. E. A., Tumlinson J. H. Arch. Insect Biochem. Physiol. 1987;4:261–269. [Google Scholar]
- 48.Teal P. E. A., Tumlinson J. H., Heath R. R. J. Chem. Ecol. 1986;12:107–125. doi: 10.1007/BF01045595. [DOI] [PubMed] [Google Scholar]
- 49.Heath R. R., McLaughlin J. R., Proshold F., Teal P. E. A. Ann. Entomol. Soc. Am. 1991;84:182–189. [Google Scholar]
- 50.Groot A. T., Fan Y., Brownie C., Jurenka R. A., Gould F., Schal C. J. Chem. Ecol. 2005;31:15–28. doi: 10.1007/s10886-005-0970-8. [DOI] [PubMed] [Google Scholar]
- 51.Vickers N. J. J. Chem. Ecol. 2002;28:1255–1267. doi: 10.1023/a:1016242019571. [DOI] [PubMed] [Google Scholar]
- 52.Pope M. M., Gaston L. K., Baker T. C. J. Chem. Ecol. 1982;8:1043–1055. doi: 10.1007/BF00987885. [DOI] [PubMed] [Google Scholar]
- 53.Teal P. E. A., Heath R. R., Tumlinson J. H., McLaughlin J. R. J. Chem. Ecol. 1981;7:1011–1022. doi: 10.1007/BF00987623. [DOI] [PubMed] [Google Scholar]
- 54.Vickers N. J., Baker T. C. J. Comp. Physiol. A. 1997;180:523–536. [Google Scholar]
- 55.Groot A. T., Ward C., Wang J., Pokrzywa A., O’Brien J., Bennett J., Kelly J., Santangelo R. G., Schal C., Gould F. J. Chem. Ecol. 2004;30:2495–2514. doi: 10.1007/s10886-004-7946-y. [DOI] [PubMed] [Google Scholar]
- 56.Sheck A. L., Groot A. T., Ward C. M., Gemeno C., Wang J., Schal C., Gould F. J. Evol. Biol. 2006;19:600–617. doi: 10.1111/j.1420-9101.2005.00999.x. [DOI] [PubMed] [Google Scholar]
- 57.Coyne J. A., Mah K., Crittenden A. Genetics. 1994;134:487–496. [Google Scholar]
- 58.Noor M. A. F. Evolution. 1997;51:809–815. doi: 10.1111/j.1558-5646.1997.tb03663.x. [DOI] [PubMed] [Google Scholar]
- 59.Ting C.-T., Takahashi A., Wu C.-I. Proc. Natl. Acad. Sci. USA. 2001;98:6709–6713. doi: 10.1073/pnas.121418898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doi M., Matsuda M., Tomaru M., Matsubayashi H., Oguma Y. Proc. Natl. Acad. Sci. USA. 2001;98:6714–6719. doi: 10.1073/pnas.091421598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kopp A., Graze R. M., Xu S., Carroll S. B., Nuzhdin S. V. Genetics. 2003;163:771–787. doi: 10.1093/genetics/163.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gleason J. M., Ritchie M. G. Genetics. 2004;166:1303–1311. doi: 10.1534/genetics.166.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moehring A. J., Li J., Schug M. D., Smith S. G., deAngelis M., Mackay T. F. C., Coyne J. A. Genetics. 2004;167:1265–1274. doi: 10.1534/genetics.103.024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ortíz-Barrientos D., Noor M. A. F. Science. 2005;310:1467. doi: 10.1126/science.1121260. [DOI] [PubMed] [Google Scholar]
- 65.Saetre G.-P., Moum T., Bureš S., Král M., Adamjan M., Moreno J. Nature. 1997;387:589–592. [Google Scholar]
- 66.Jiggins C. D., Naisbit R. E., Coe R. L., Mallet J. Nature. 2001;411:302–305. doi: 10.1038/35077075. [DOI] [PubMed] [Google Scholar]
- 67.Roelofs W. L., Cardé R. T. In: Pheromones. Birch M., editor. Amsterdam: North–Holland; 1974. pp. 96–114. [Google Scholar]
- 68.Cardé R. T., Minks A. K. Insect Pheromone Research: New Directions. New York: Chapman & Hall; 1997. [Google Scholar]
- 69.Raina A. K., Stadelbacher E. A. Ann. Entomol. Soc. Am. 1991;83:987–990. [Google Scholar]
- 70.Raulston J. R., Snow W., Graham H. M., Lingren P. D. Ann. Entomol. Soc. Am. 1975;68:701–704. [Google Scholar]
- 71.Klepetka B., Gould F. Environ. Entomol. 1996;25:993–1001. [Google Scholar]
- 72.Flint H. M., Kressin E. L. J. Econ. Entomol. 1968;61:477–483. [Google Scholar]
- 73.Pair S. D., Laster M. L., Martin D. F. Ann. Entomol. Soc. Am. 1977;70:952–954. [Google Scholar]
- 74.LaMunyon C. W. Anim. Behav. 2000;59:395–402. doi: 10.1006/anbe.1999.1294. [DOI] [PubMed] [Google Scholar]
- 75.LaMunyon C. W., Huffman T. S. J. Insect Behav. 2001;14:187–199. [Google Scholar]
- 76.LaMunyon C.W. Ecol. Entomol. 2001;26:388–394. [Google Scholar]
- 77.Heckel D. G. Annu. Rev. Entomol. 1993;38:381–408. [Google Scholar]
- 78.Robinson R. Lepidoptera Genetics. Oxford: Pergamon; 1971. [Google Scholar]
- 79.Hawthorne D. J., Via S. Nature. 2001;412:904–907. doi: 10.1038/35091062. [DOI] [PubMed] [Google Scholar]
- 80.Henneberry T. J., Clayton T. E. Ann. Entomol. Soc. Am. 1984;77:301–305. [Google Scholar]
- 81.Callahan P. S. Ann. Entomol. Soc. Am. 1958;51:413–428. [Google Scholar]
- 82.Callahan P. S., Cascio T. Ann. Entomol. Soc. Am. 1963;56:535–556. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.