Abstract
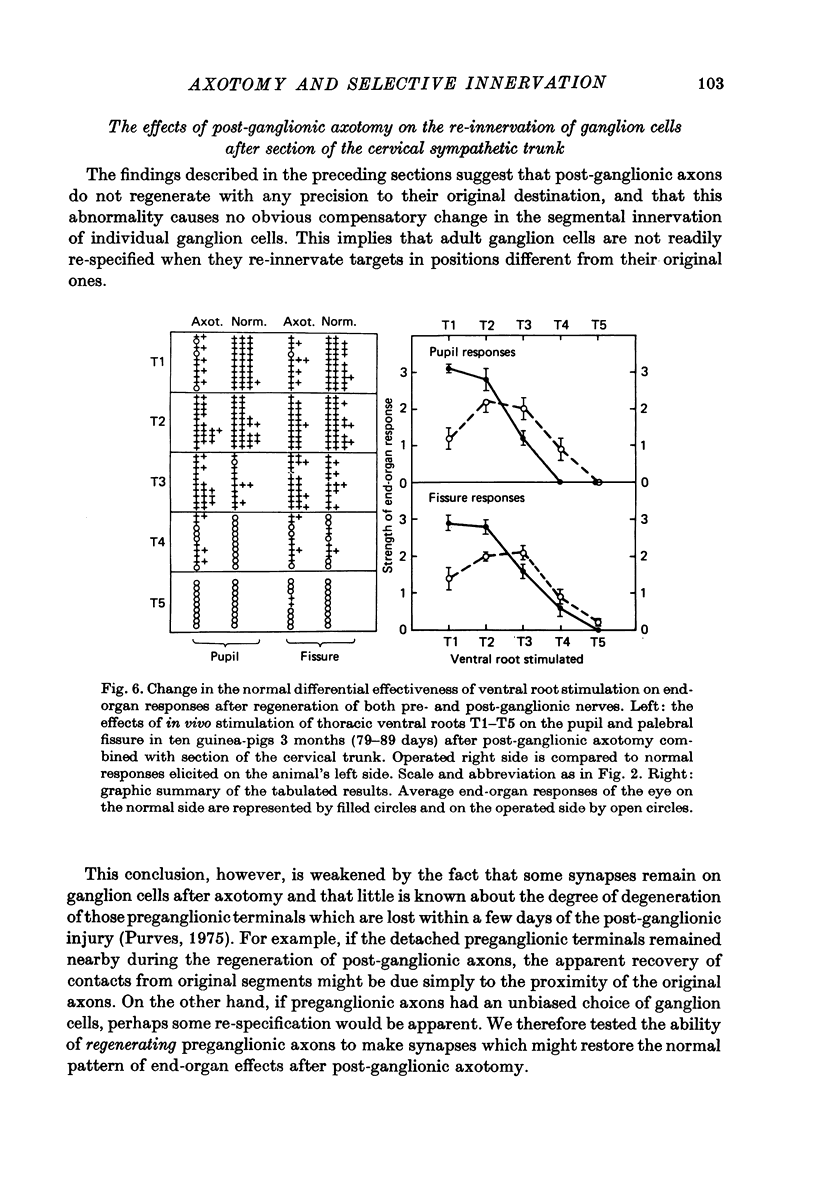
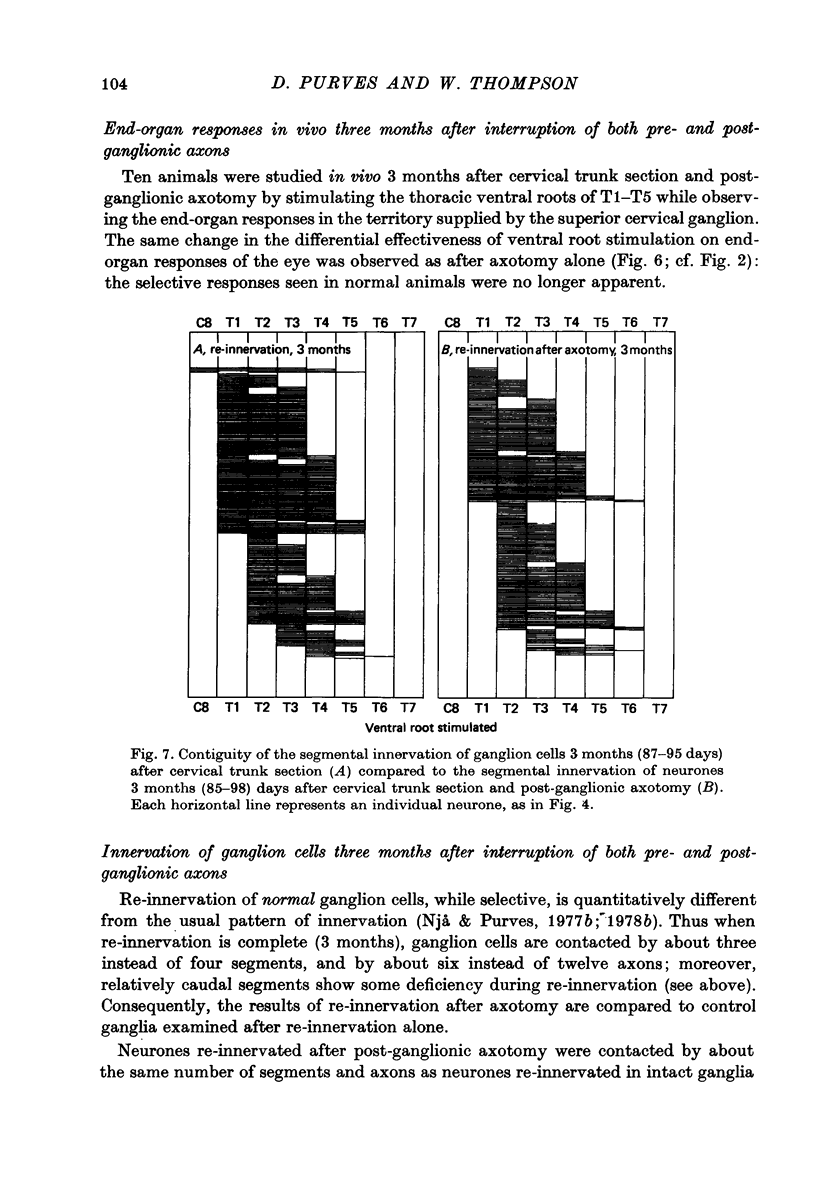
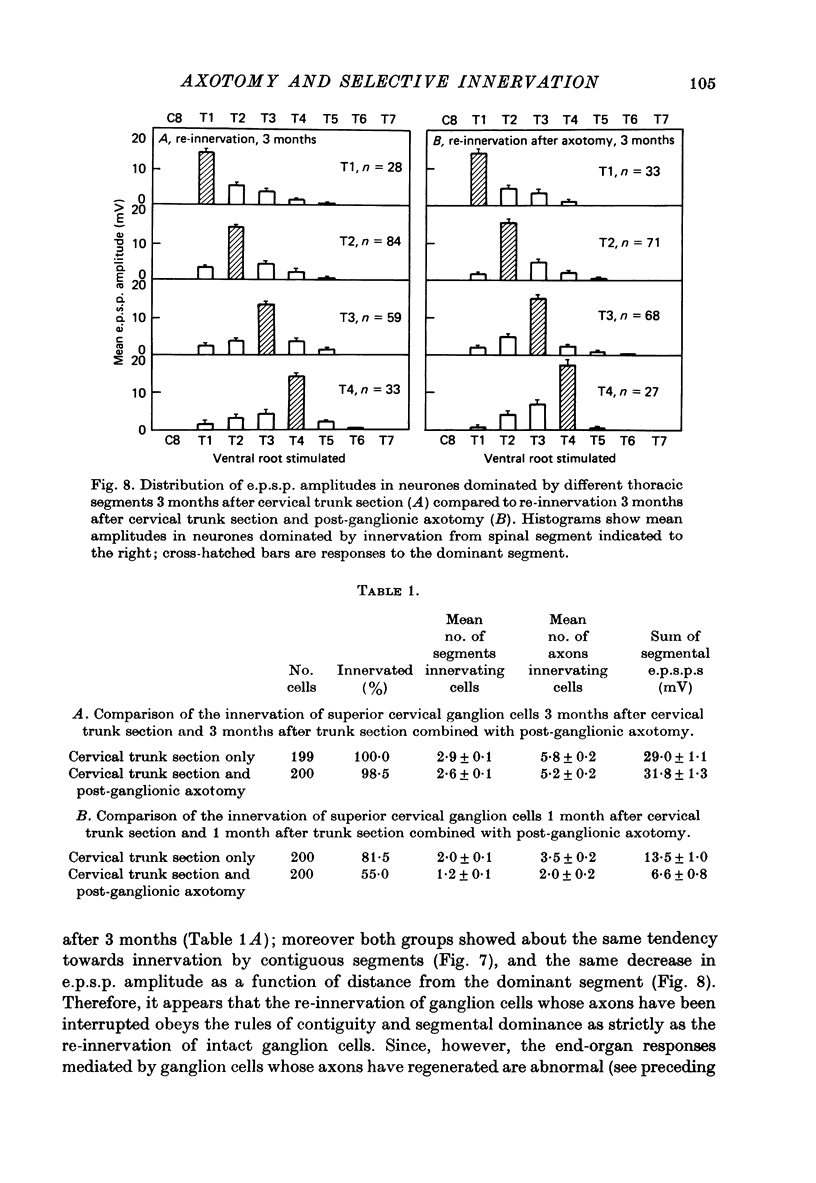
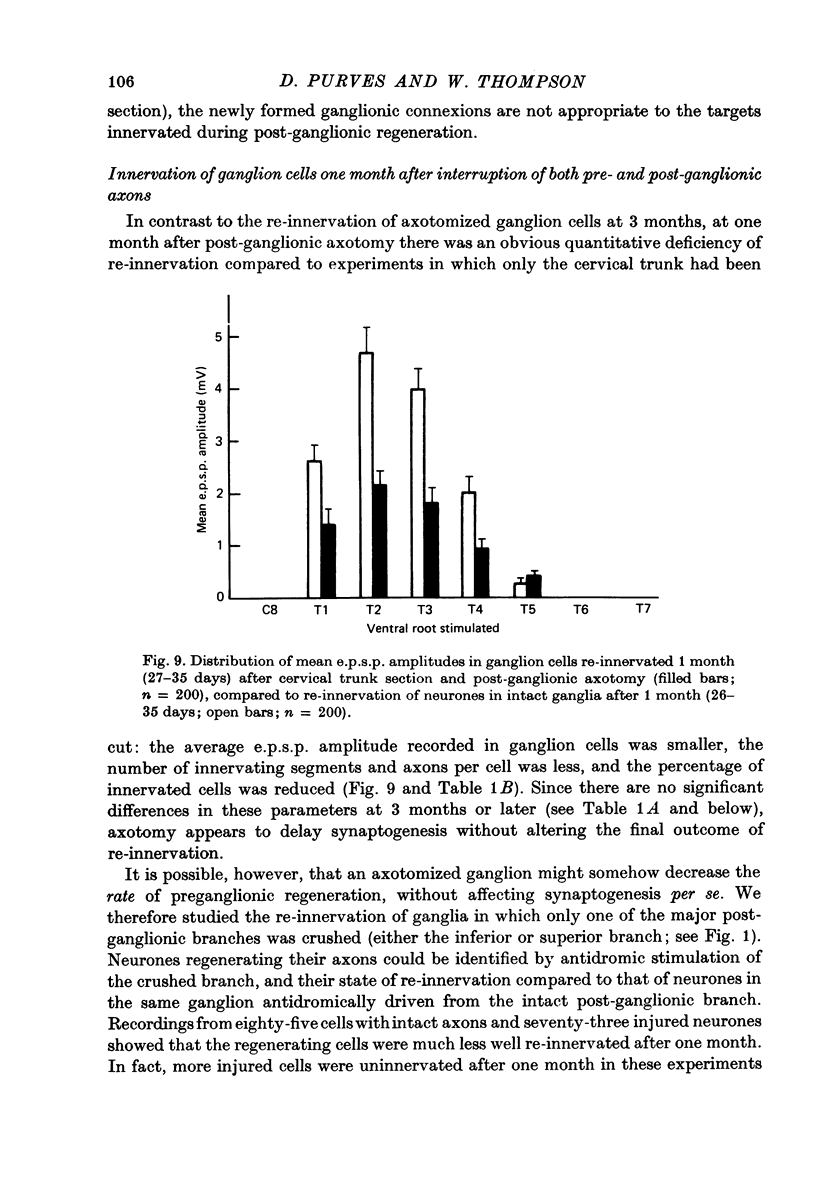
Stimulation of preganglionic axons arising from different levels of the thoracic spinal cord causes different effects on end-organs supplied by the superior cervical ganglion (Langley, 1892; Nja & Purves, 1977a; Lichtman, Purves & Yip, 1979). For example, stimulation of the first thoracic ventral root (T1) causes pupillary dilatation and widening of the palpebral fissure; stimulation of T4, on the other hand, has little effect on the eye, even though axons arising from this level innervate about as many superior cervical ganglion cells as those from T1. Thus ganglion cell innervation is selective. (1) Three months after crushing the major post-ganglionic branches of the superior cervical ganglion this differential effectiveness is lost: T1 and T4 stimulation have approximately equal effects on the end-organs of the eye. (2) In normal animals, the cellular counterpart of selective end-organ effects is the innervation of each ganglion cell by a contiguous subset of the spinal segments that innervate the ganglion as a whole. One of these segments is usually dominant, the strength of innervation from adjacent segments falling off as a function of distance from the dominant one (Nja & Purves, 1977a). Intracellular recordings from ganglion cells 3 months after post-ganglionic axotomy showed that this selective pattern is re-established. (3) Since the innervation of ganglion cells appears normal, the abnormal end-organ responses after post-ganglionic axotomy suggest that ganglion cell axons are not limited to their original targets during peripheral re-innervation. This suggestion is supported by the finding that ganglion cells sending axons to different peripheral destinations via the second and third cervical spinal nerves were no longer distinguishable on the basis of their segmented inputs 3 months after post-ganglionic axotomy. (4) Similar results were obtained when the preganglionic cervical trunk was cut at the same time as the post-ganglionic axons were crushed; the pattern of end-organ responses was abnormal, whereas individual ganglion cells were re-innervated according to the rules of contiguity and segmental dominance. (5) These results indicate that ganglion cells do not undergo a compensatory change in the segmental innervation they receive when their axons regenerate to targets different from, or in addition to those they originally innervated, even when an entirely new set of ganglionic connexions is formed. This suggests that ganglion cells, or some aspect of their immediate environment, possess a permanent label that determines the segmental innervation they receive.
Full text
PDF

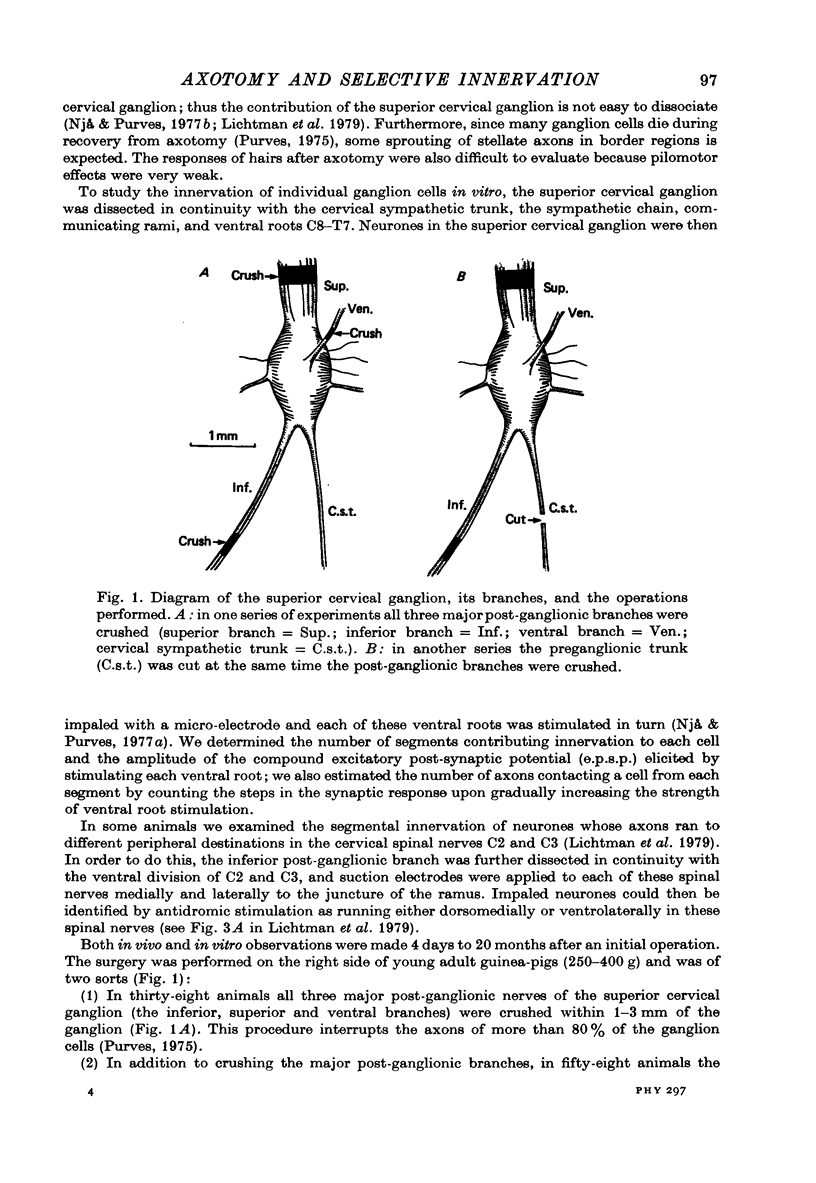

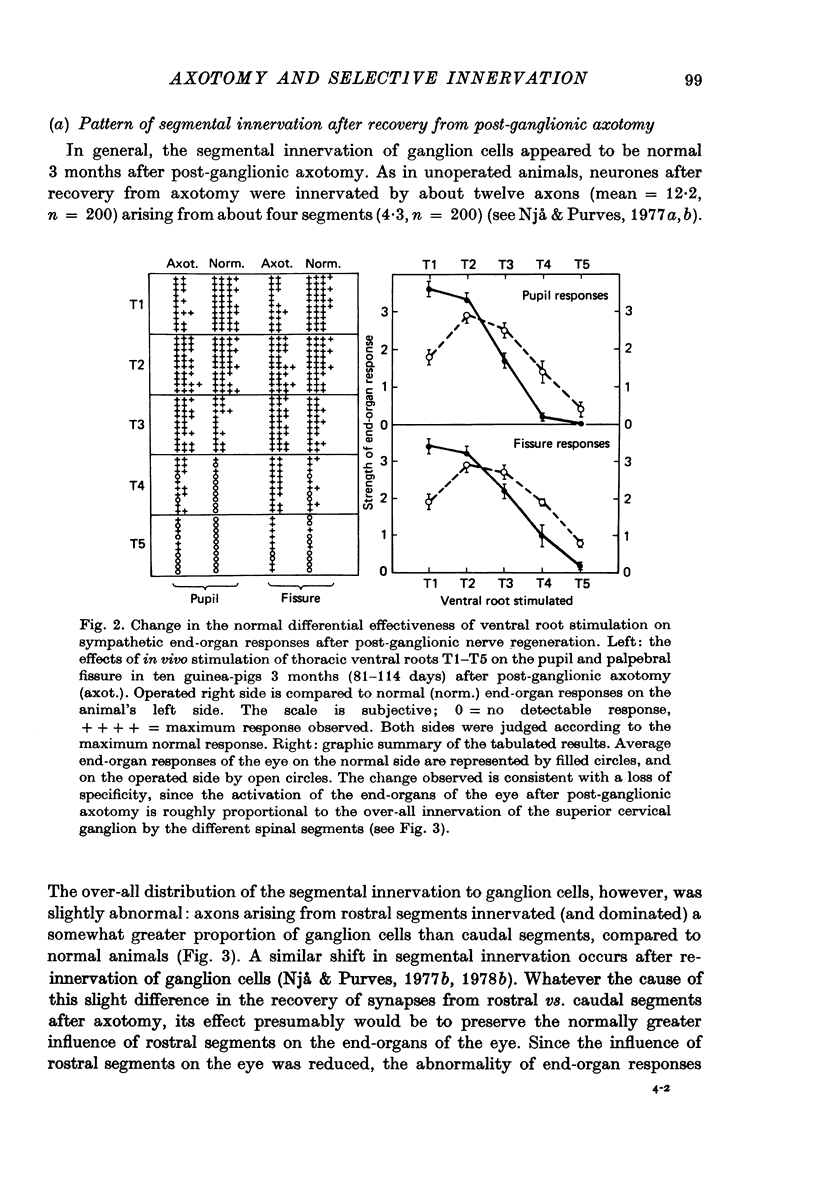
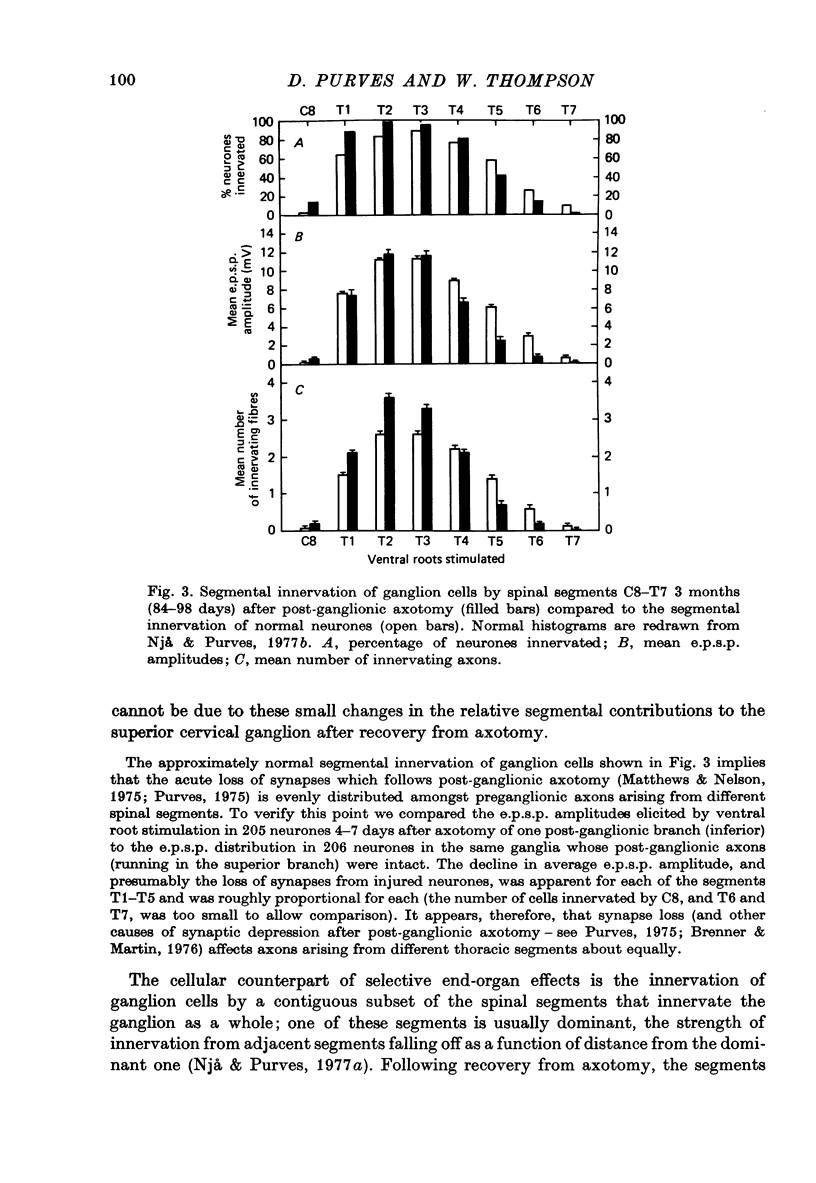

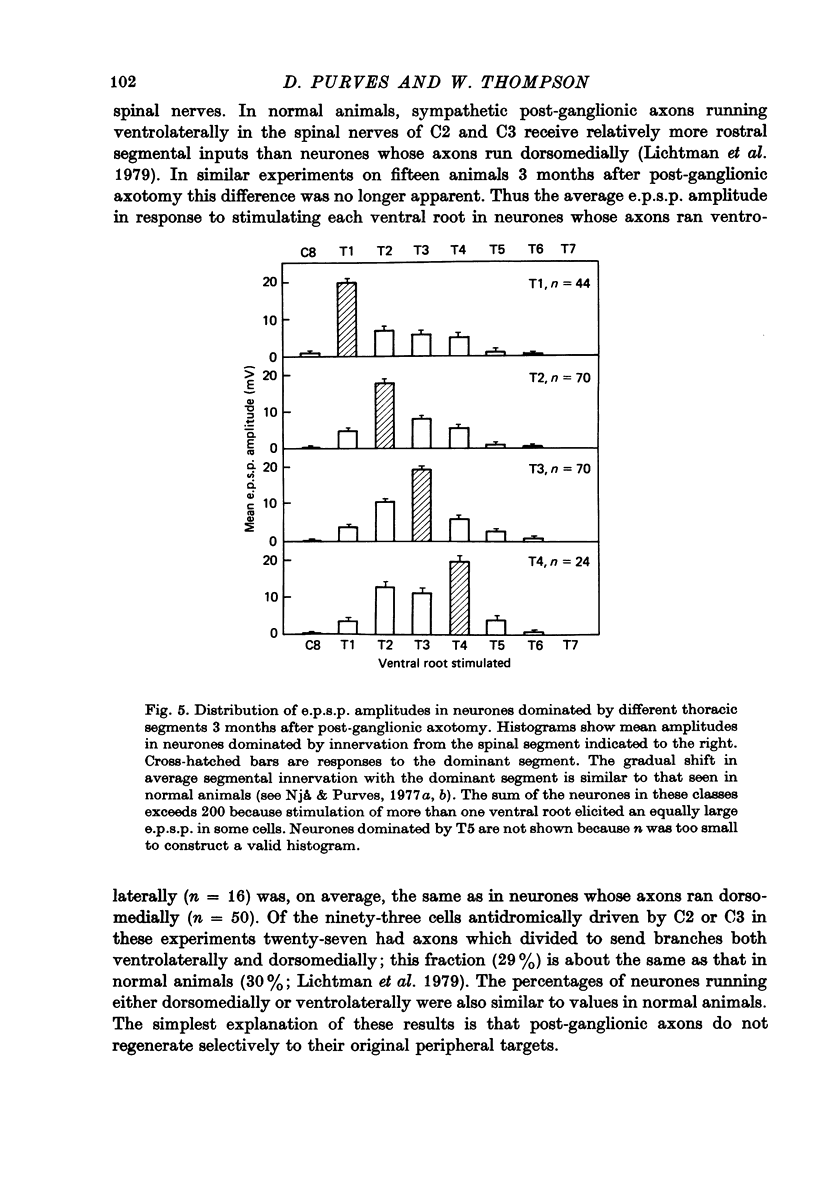




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNSTEIN J. J., GUTH L. Nonselectivity in establishment of neuromuscular connections following nerve regeneration in the rat. Exp Neurol. 1961 Sep;4:262–275. doi: 10.1016/0014-4886(61)90047-4. [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. The role of post-synaptic neurones in the biochemical maturation of presynaptic cholinergic nerve terminals in a mouse sympathetic ganglion. J Physiol. 1972 Feb;221(1):149–159. doi: 10.1113/jphysiol.1972.sp009745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H. R., Martin A. R. Reduction in acetylcholine sensitivity of axotomized ciliary ganglion cells. J Physiol. 1976 Aug;260(1):159–175. doi: 10.1113/jphysiol.1976.sp011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Interactions between neurons and their targets during in vivo synaptogenesis. Fed Proc. 1978 May 15;37(7):2016–2022. [PubMed] [Google Scholar]
- Langley J. N. Note on Regeneration of Prae-Ganglionic Fibres of the Sympathetic. J Physiol. 1895 Jul 18;18(3):280–284. doi: 10.1113/jphysiol.1895.sp000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. On the Regeneration of Pre-Ganglionic and of Post-Ganglionic Visceral Nerve Fibres. J Physiol. 1897 Nov 20;22(3):215–230. doi: 10.1113/jphysiol.1897.sp000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D., Yip J. W. On the purpose of selective innervation of guinea-pig superior cervical ganglion cells. J Physiol. 1979 Jul;292:69–84. doi: 10.1113/jphysiol.1979.sp012839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M. R., Nelson V. H. Detachment of structurally intact nerve endings from chromatolytic neurones of rat superior cervical ganglion during the depression of synaptic transmission induced by post-ganglionic axotomy. J Physiol. 1975 Feb;245(1):91–135. doi: 10.1113/jphysiol.1975.sp010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. Specific innervation of guinea-pig superior cervical ganglion cells by preganglionic fibres arising from different levels of the spinal cord. J Physiol. 1977 Jan;264(2):565–583. doi: 10.1113/jphysiol.1977.sp011683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. Specificity of initial synaptic contacts made on guinea-pig superior cervical ganglion cells during regeneration of the cervical sympathetic trunk. J Physiol. 1978 Aug;281:45–62. doi: 10.1113/jphysiol.1978.sp012408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. The effects of nerve growth factor and its antiserum on synapses in the superior cervical ganglion of the guinea-pig. J Physiol. 1978 Apr;277:53–75. [PMC free article] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following colchicine application to post-ganglionic nerves. J Physiol. 1976 Jul;259(1):159–175. doi: 10.1113/jphysiol.1976.sp011459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Formation and maintenance of synaptic connections in autonomic ganglia. Physiol Rev. 1978 Oct;58(4):821–862. doi: 10.1152/physrev.1978.58.4.821. [DOI] [PubMed] [Google Scholar]


