Abstract
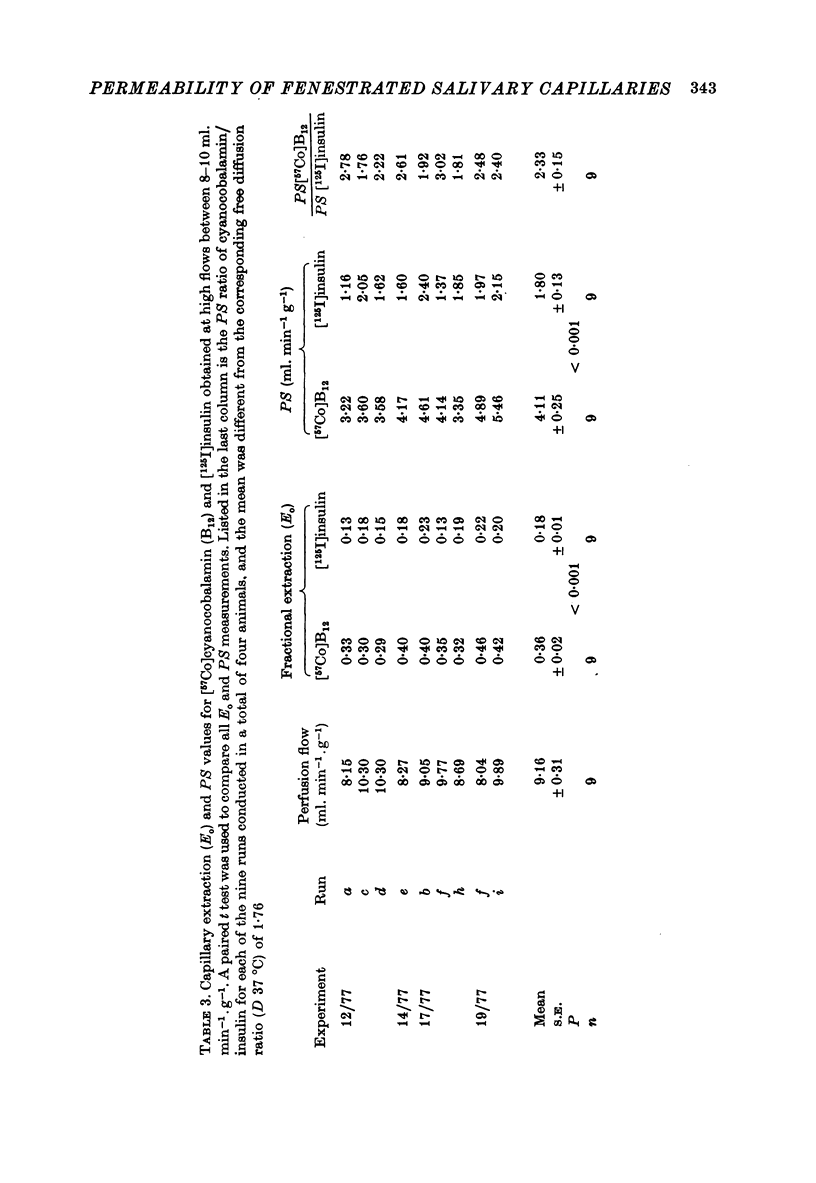
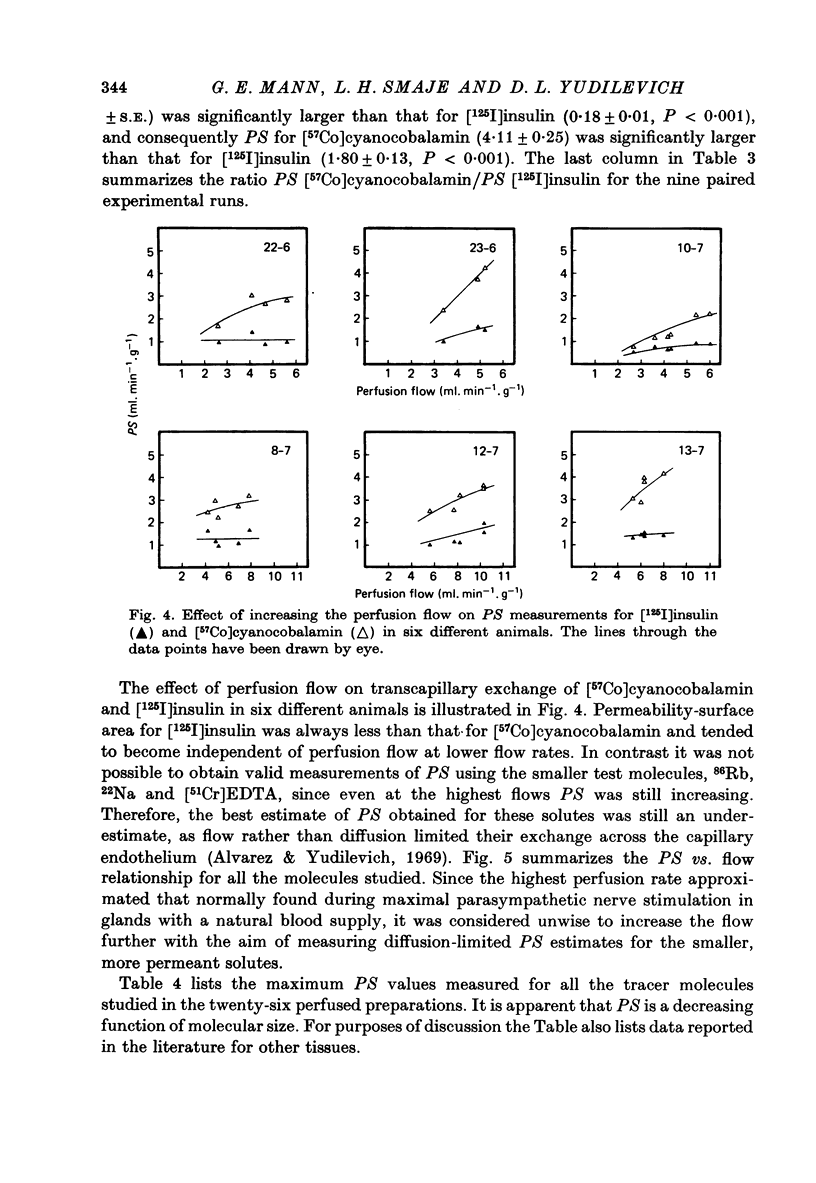
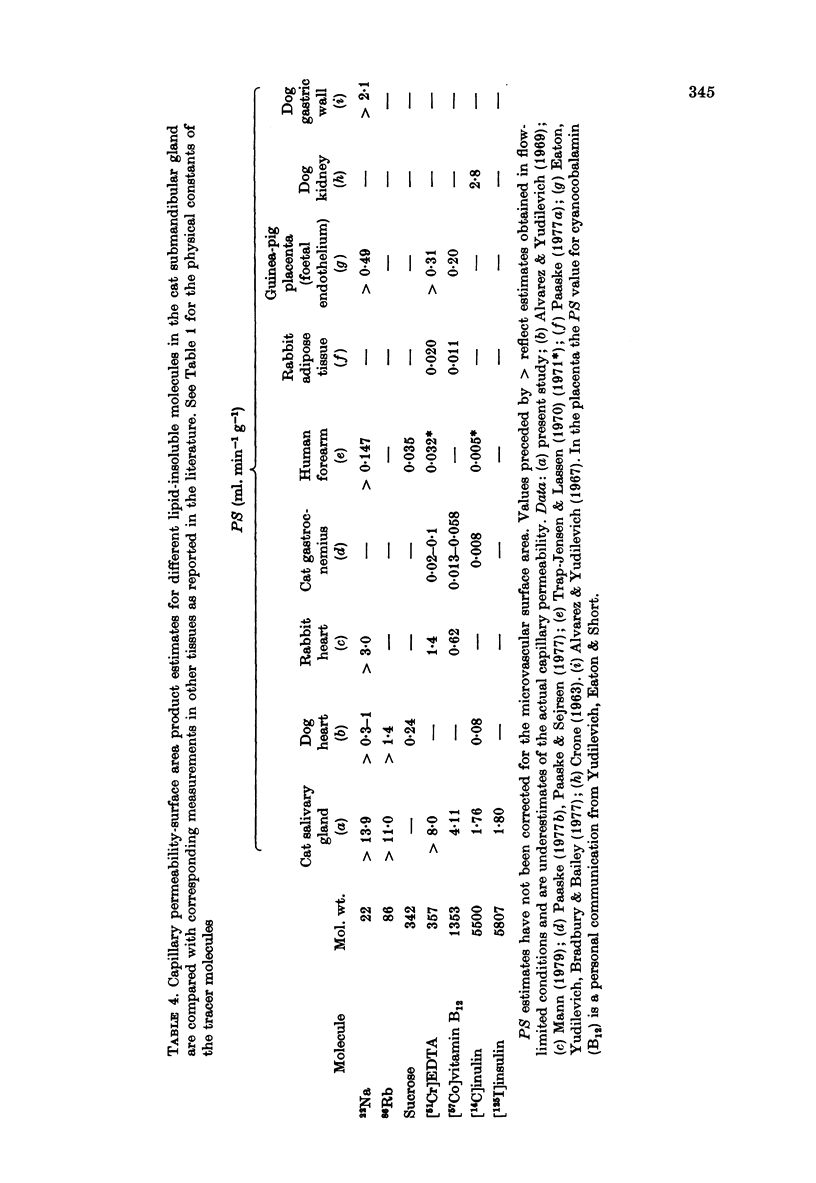
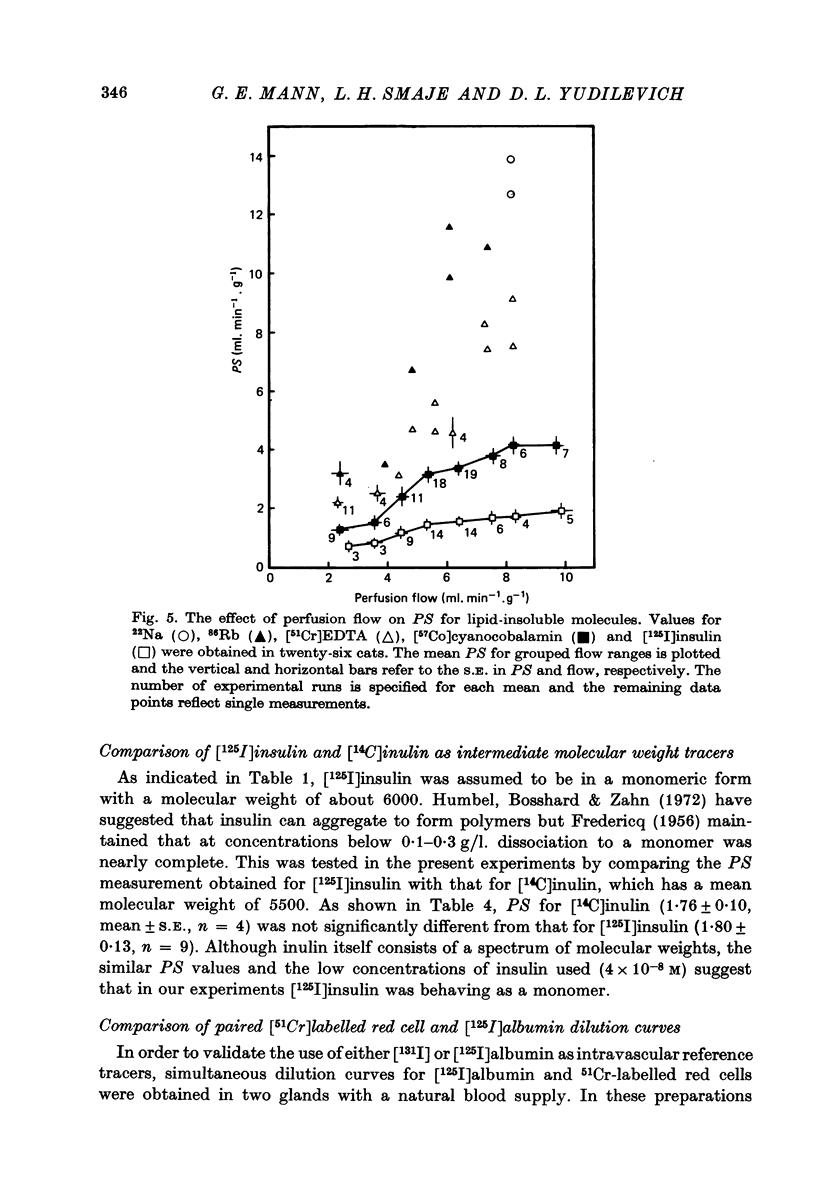
1. Permeability-surface area products for the fenestrated capillaries in the perfused cat submandibular gland have been measured for graded lipid-insoluble molecules using the single-passage, multiple-tracer dilution technique. 2. The permeability-surface area for [57Co]cyanocobalamin (mol. wt. 1353) increased as the perfusion flow was increased, but reached a constant value of 4.11 +/- 0.25 ml.min-1.g-1 (mean +/- S.E., n = 9) at flows above 8 ml. min-1.g-1. For [125I]insulin (approximate mol. wt. 6000) it was 1.80 +/- 0.13 ml.min-1.g-1 (mean +/- S.E., n = 9) and apparently diffusion-limited at all the high flow rates studied. A similar permeability-surface area product was measured for [14C]inulin (mol. wt. 5500): 1.76 +/- 0.10 (mean +/- S.E., n = 4). 3. Permeability-surface area values for cyanocobalamin and insulin in the salivary gland are respectively about 20 and 200 times larger than the estimates reported for the continuous capillaries of cardiac and skeletal muscle. 4. The permeability-surface area (PS) ratio [57Co]cyanocobalamin/[125I]insulin (2.33 +/- 0.15, mean +/- S.E., n = 9) was significantly greater than the apparent ratio of their free diffusion coefficients (1.76), suggesting restricted diffusion of insulin relative to cyanocobalamin across the capillary endothelium. 5. Permeability-surface area products for the smaller molecular weight tracers (22Na, 86Rb and 51Cr-EDTA (mol. wt. 357)) increased continuously with perfusion rate, indicating flow-limited solute exchange. The PS ratio of Rb/EDTA was close to unity whereas the corresponding free diffusion ratio is 3.85. 6. The high permeability-surface area values measured were thought to be associated with the fenestrae which appeared to act as high concentrations of 'small pores' rather than as 'large pores'.
Full text
PDF


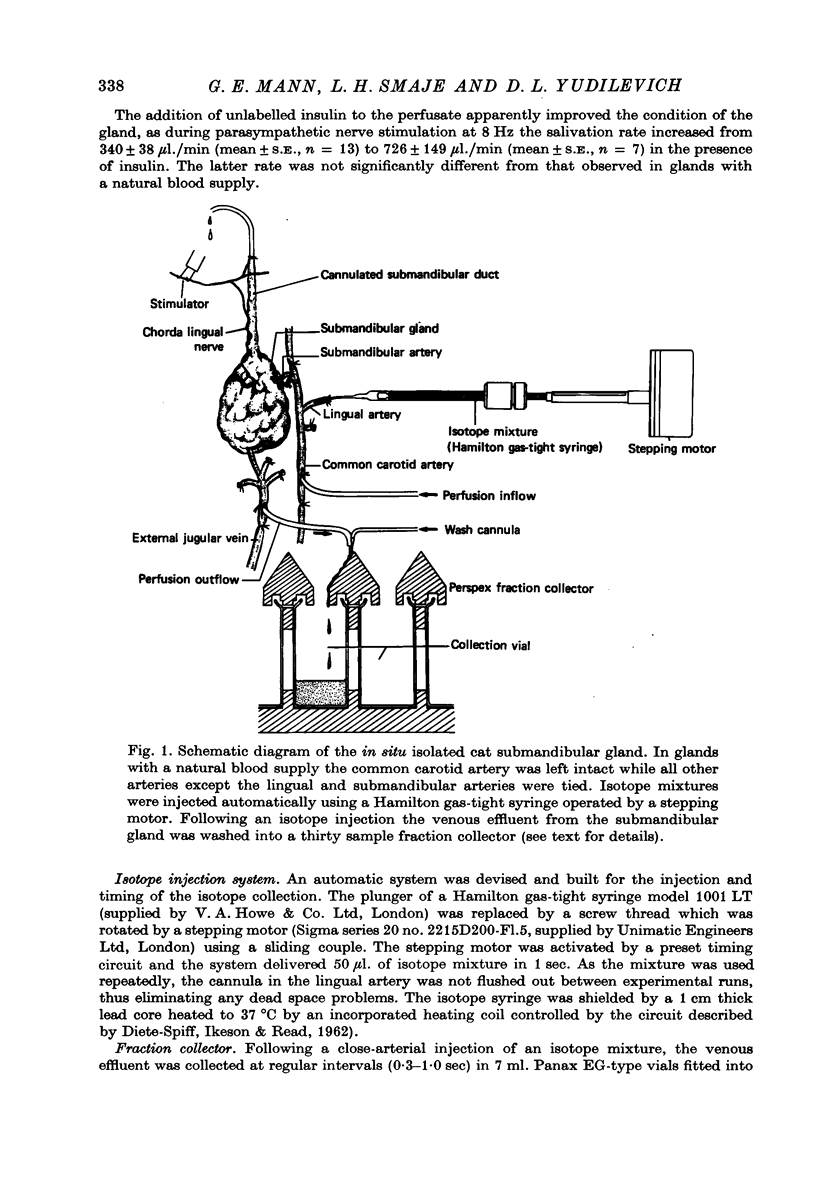
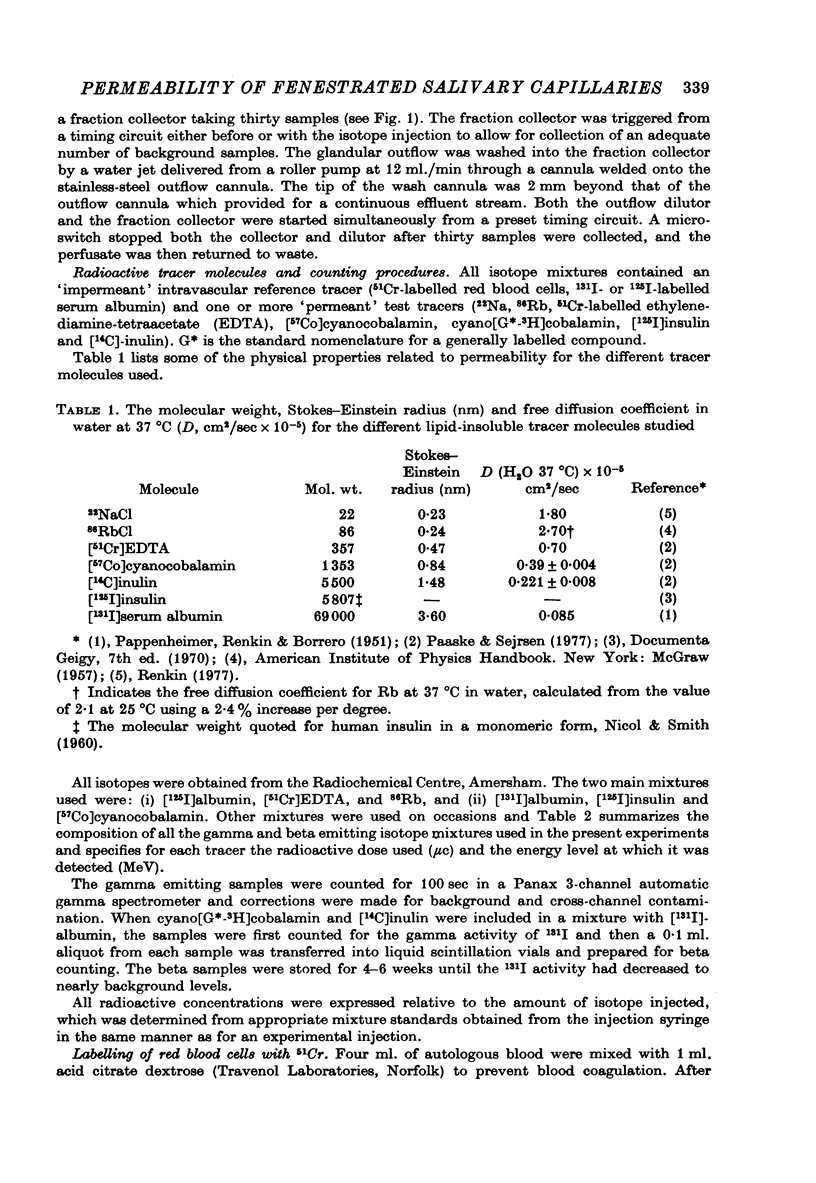

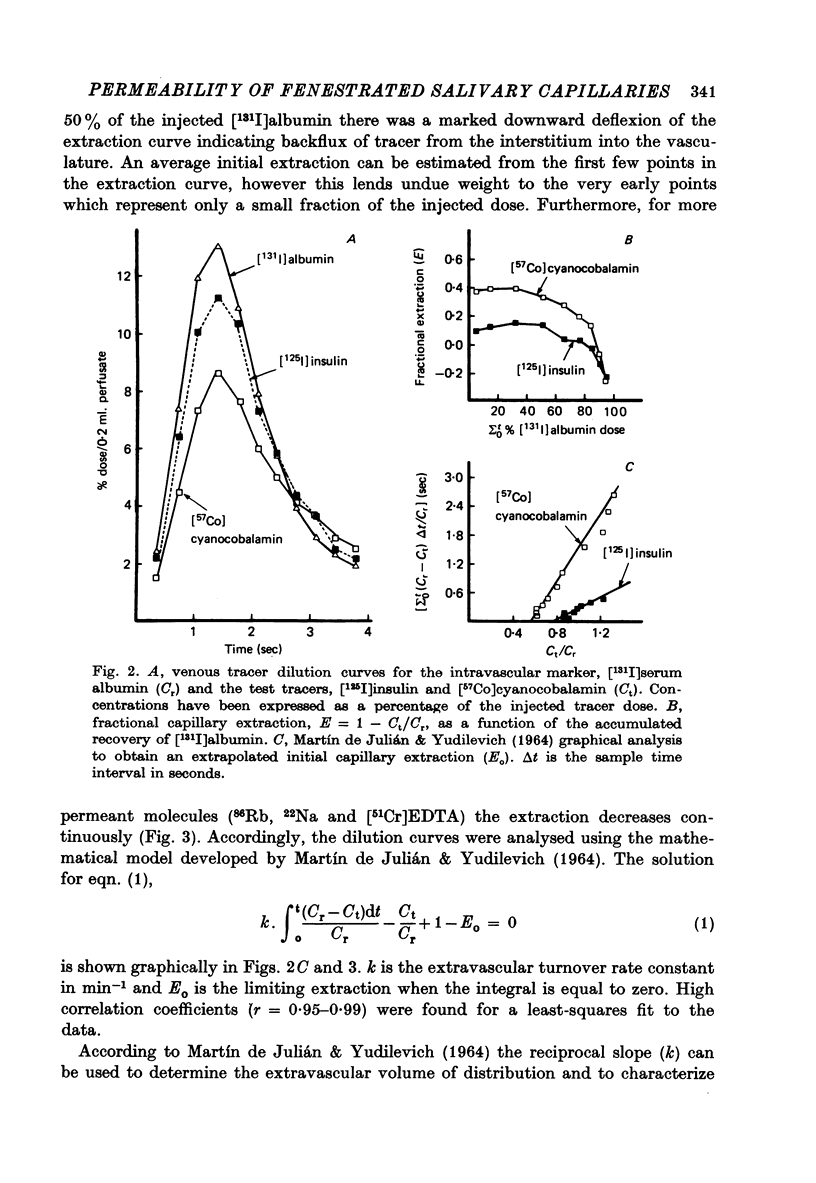
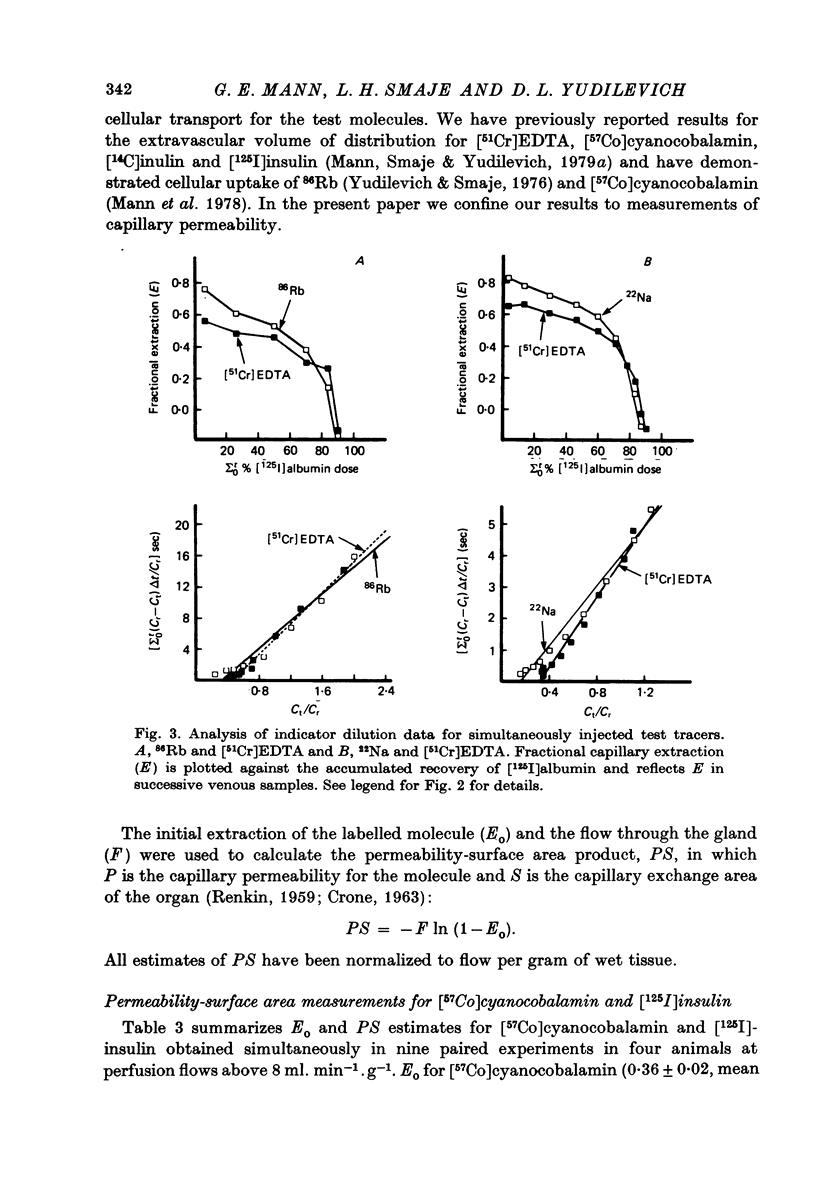
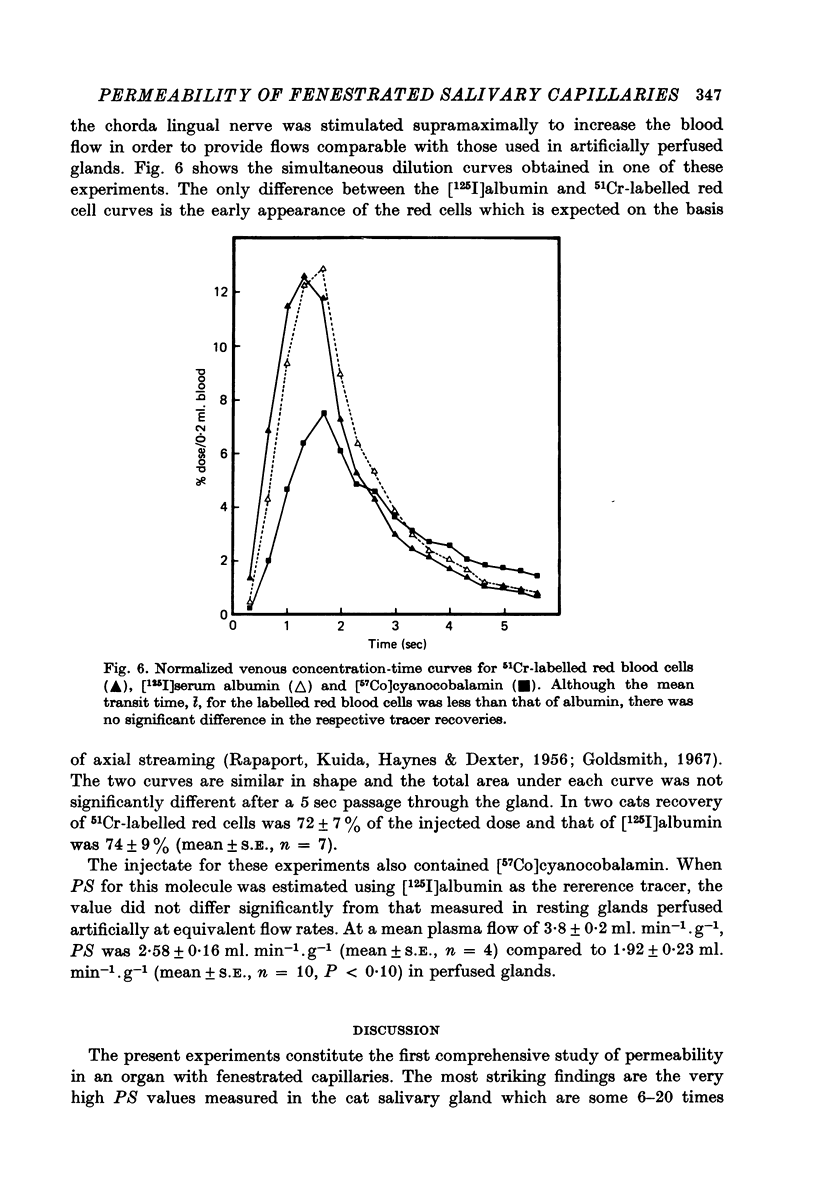

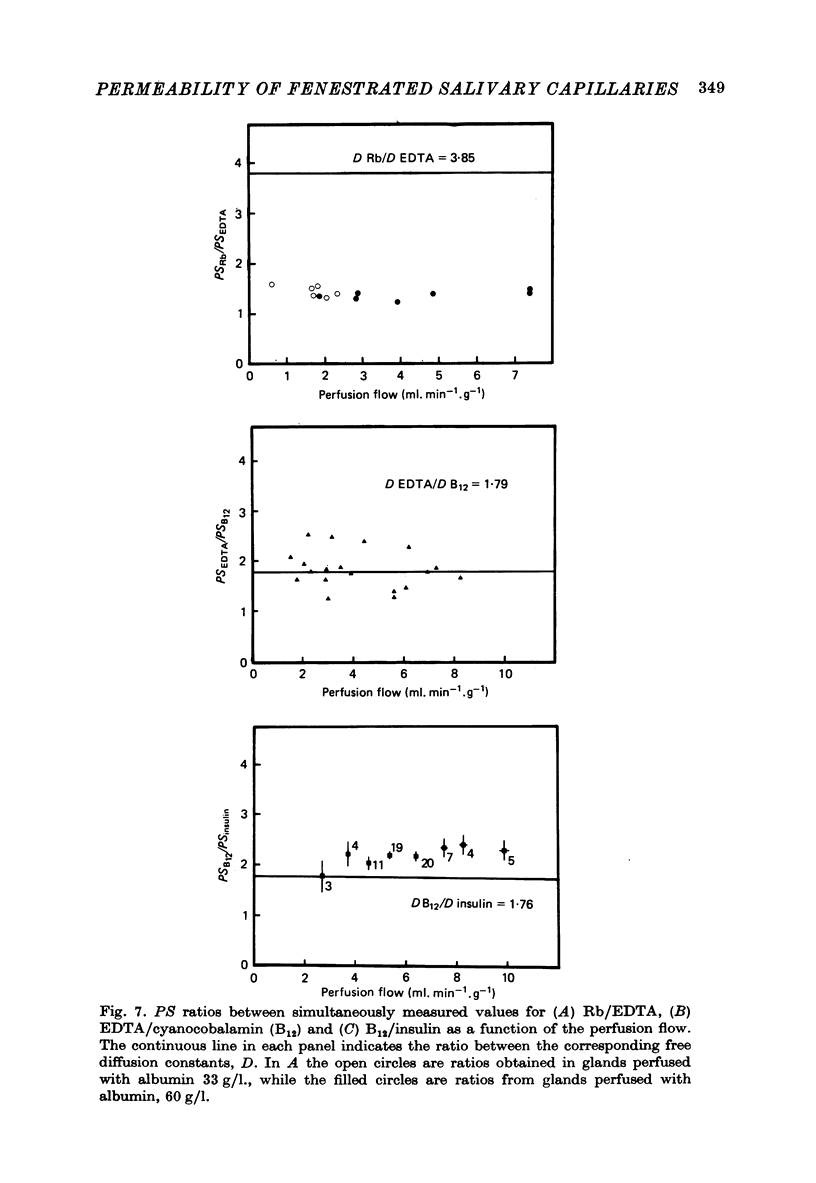





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez O. A., Yudilevich D. L. Heart capillary permeability to lipid-insoluble molecules. J Physiol. 1969 May;202(1):45–58. doi: 10.1113/jphysiol.1969.sp008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEEHEN N. M., FISHER R. B. The action of insulin in the isolated rat heart. J Physiol. 1954 Feb 26;123(2):260–276. doi: 10.1113/jphysiol.1954.sp005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHINARD F. P., ENNS T., GORESKY C. A., NOLAN M. F. RENAL TRANSIT TIMES AND DISTRIBUTION VOLUMES OF T-1824, CREATININE, AND WATER. Am J Physiol. 1965 Aug;209:243–252. doi: 10.1152/ajplegacy.1965.209.2.243. [DOI] [PubMed] [Google Scholar]
- CHINARD F. P., VOSBURGH G. J., ENNS T. Transcapillary exchange of water and of other substances in certain organs of the dog. Am J Physiol. 1955 Nov;183(2):221–234. doi: 10.1152/ajplegacy.1955.183.2.221. [DOI] [PubMed] [Google Scholar]
- CRONE C. THE PERMEABILITY OF CAPILLARIES IN VARIOUS ORGANS AS DETERMINED BY USE OF THE 'INDICATOR DIFFUSION' METHOD. Acta Physiol Scand. 1963 Aug;58:292–305. doi: 10.1111/j.1748-1716.1963.tb02652.x. [DOI] [PubMed] [Google Scholar]
- Coulson R. L., Rusy B. F. A system for assessing mechanical performance, heat production, and oxygen utilization of isolated perfused whole hearts. Cardiovasc Res. 1973 Nov;7(6):859–869. doi: 10.1093/cvr/7.6.859. [DOI] [PubMed] [Google Scholar]
- Curry F. E., Michel C. C., Mason J. C. Osmotic reflextion coefficients of capillary walls to low molecular weight hydrophilic solutes measured in single perfused capillaries of the frog mesentery. J Physiol. 1976 Oct;261(2):319–336. doi: 10.1113/jphysiol.1976.sp011561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke A. C., Smaje L. H. Dependence of functional vasodilatation in the cat submaxillary gland upon stimulation frequency. J Physiol. 1972 Oct;226(1):191–203. doi: 10.1113/jphysiol.1972.sp009980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDERICQ E. The association of insulin molecular units in aqueous solutions. Arch Biochem Biophys. 1956 Nov;65(1):218–228. doi: 10.1016/0003-9861(56)90189-8. [DOI] [PubMed] [Google Scholar]
- FREIS E. D., SCHNAPER H. W., ROSE J. C., LILIENFIELD L. S. Renal, transcapillary, net exchange in the dog. Circ Res. 1958 Jul;6(4):432–437. doi: 10.1161/01.res.6.4.432. [DOI] [PubMed] [Google Scholar]
- Firth J. A., Farr A. Structural features and quantitative age-dependent changes in the intervascular barrier of the guinea-pig haemochorial placenta. Cell Tissue Res. 1977 Nov 23;184(4):507–516. doi: 10.1007/BF00220974. [DOI] [PubMed] [Google Scholar]
- Fleming B. P., Diana J. N. Interaction between fluid movement and solute exchange in the isolated dog hindlimb. Bibl Anat. 1977;(15 Pt 1):483–488. [PubMed] [Google Scholar]
- GROTTE G. Passage of dextran molecules across the blood-lymph barrier. Acta Chir Scand Suppl. 1956;211:1–84. [PubMed] [Google Scholar]
- Ganrot P. O., Laurell C. B., Ohlsson K. Concentration of trypsin inhibitors of different molecular size and of albumin and haptoglobin in blood and in lymph of various organs in the dog. Acta Physiol Scand. 1970 Jun;79(2):280–286. doi: 10.1111/j.1748-1716.1970.tb04727.x. [DOI] [PubMed] [Google Scholar]
- Goldsmith H. L. Microscopic flow properties of red cells. Fed Proc. 1967 Nov-Dec;26(6):1813–1820. [PubMed] [Google Scholar]
- MARTIN P., YUDILEVICH D. A THEORY FOR THE QUANTIFICATION OF TRANSCAPILLARY EXCHANGE BY TRACER-DILUTION CURVES. Am J Physiol. 1964 Jul;207:162–168. doi: 10.1152/ajplegacy.1964.207.1.162. [DOI] [PubMed] [Google Scholar]
- Mann G. E. Capillary permeability-surface area products in the isolated, perfused rabbit heart [proceedings]. J Physiol. 1979 Jun;291:7P–8P. [PubMed] [Google Scholar]
- Mann G. E., Smaje L. H., Yudilevich D. L. Epithelial uptake of vitamin B12 in the cat salivary gland [proceedings]. J Physiol. 1978 Dec;285:32P–32P. [PubMed] [Google Scholar]
- Mann G. E., Smaje L. H., Yudilevich D. L. Microvascular exchange in the cat salivary gland using single passage multiple tracer dilution. Bibl Anat. 1977;(15 Pt 1):469–471. [PubMed] [Google Scholar]
- Mann G. E., Smaje L. H., Yudilevich D. L. Transcapillary exchange in the cat salivary gland during secretion, bradykinin infusion and after chronic duct ligation. J Physiol. 1979 Dec;297(0):355–367. doi: 10.1113/jphysiol.1979.sp013044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A. H. Ultrastructural permeability studies in capillaries of rabbit oral mucosa and salivary glands. Microvasc Res. 1975 May;9(3):287–303. doi: 10.1016/0026-2862(75)90065-5. [DOI] [PubMed] [Google Scholar]
- NICOL D. S., SMITH L. F. Amino-acid sequence of human insulin. Nature. 1960 Aug 6;187:483–485. doi: 10.1038/187483a0. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., RENKIN E. M., BORRERO L. M. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am J Physiol. 1951 Oct;167(1):13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- Paaske W. P. Capillary permeability in skeletal muscle. Acta Physiol Scand. 1977 Sep;101(1):1–14. doi: 10.1111/j.1748-1716.1977.tb05977.x. [DOI] [PubMed] [Google Scholar]
- Paaske W. P., Sejrsen P. Transcapillary exchange of 14C-inulin by free diffusion in channels of fused vesicles. Acta Physiol Scand. 1977 Aug;100(4):437–445. doi: 10.1111/j.1748-1716.1977.tb05968.x. [DOI] [PubMed] [Google Scholar]
- RAPAPORT E., KUIDA H., HAYNES F. W., DEXTER L. Pulmonary red cell and plasma volumes and pulmonary hematocrit in the normal dog. Am J Physiol. 1956 Apr;185(1):127–132. doi: 10.1152/ajplegacy.1956.185.1.127. [DOI] [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- RENKIN E. M. Transport of potassium-42 from blood to tissue in isolated mammalian skeletal muscles. Am J Physiol. 1959 Dec;197:1205–1210. doi: 10.1152/ajplegacy.1959.197.6.1205. [DOI] [PubMed] [Google Scholar]
- Renkin E. M. Multiple pathways of capillary permeability. Circ Res. 1977 Dec;41(6):735–743. doi: 10.1161/01.res.41.6.735. [DOI] [PubMed] [Google Scholar]
- Rippe B., Kamiya A., Folkow B. Simultaneous measurements of capillary diffusion and filtration exchange during shifts in filtration-absorption and at graded alterations in the capillary permeability surface area products (PS). Acta Physiol Scand. 1978 Nov;104(3):318–336. doi: 10.1111/j.1748-1716.1978.tb06284.x. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Siminoescu M., Palade G. E. Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J Cell Biol. 1975 Mar;64(3):586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Permeability of intestinal capillaries. Pathway followed by dextrans and glycogens. J Cell Biol. 1972 May;53(2):365–392. doi: 10.1083/jcb.53.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Permeability of muscle capillaries to exogenous myoglobin. J Cell Biol. 1973 May;57(2):424–452. doi: 10.1083/jcb.57.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M. Fenestrated venules of the large salivary glands. Anat Rec. 1970 Apr;166(4):605–609. doi: 10.1002/ar.1091660406. [DOI] [PubMed] [Google Scholar]
- Trap-Jensen J., Lassen N. A. Restricted diffusion in skeletal muscle capillaries in man. Am J Physiol. 1971 Feb;220(2):371–376. doi: 10.1152/ajplegacy.1971.220.2.371. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Alvarez O. A. Water, sodium, and thiourea transcapillary diffusion in the dog heart. Am J Physiol. 1967 Aug;213(2):308–314. doi: 10.1152/ajplegacy.1967.213.2.308. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Renkin E. M., Alvarez O. A., Bravo I. Fractional extraction and transcapillary exchange during continuous and instantaneous tracer administration. Circ Res. 1968 Aug;23(2):325–336. doi: 10.1161/01.res.23.2.325. [DOI] [PubMed] [Google Scholar]


