Abstract
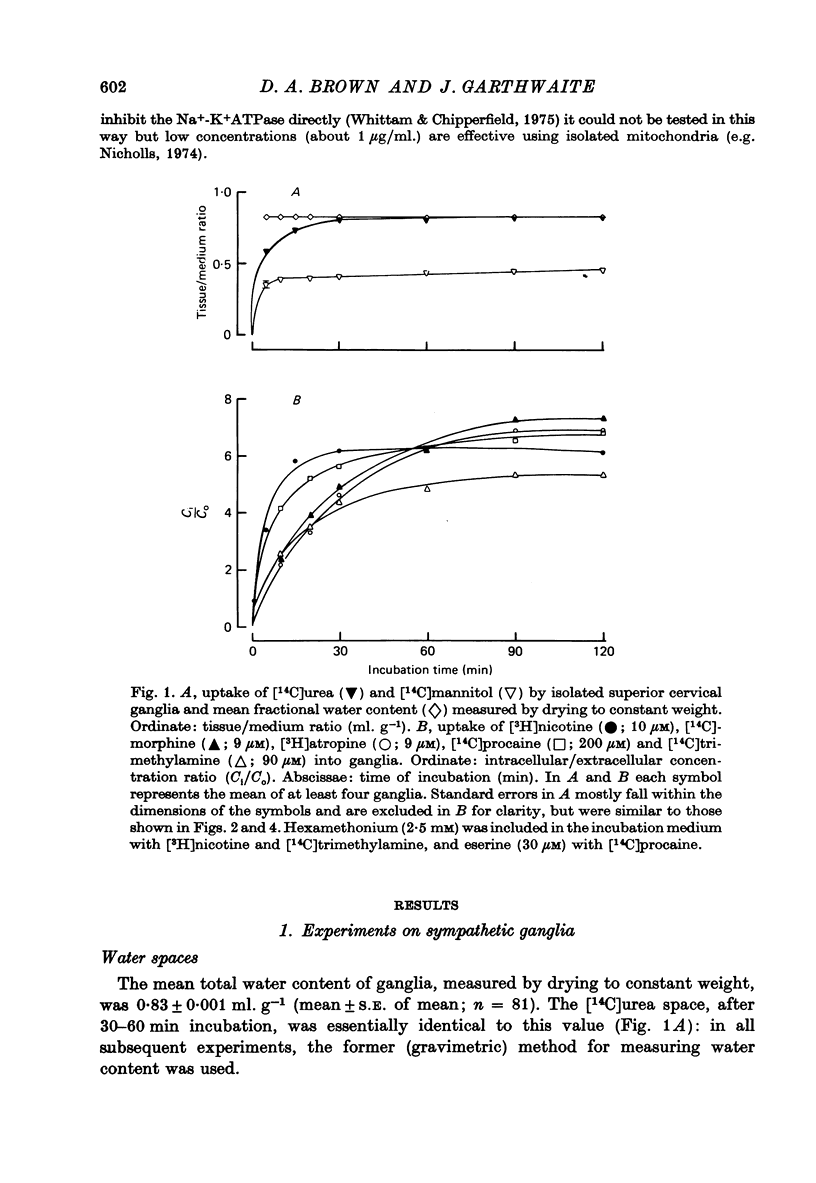
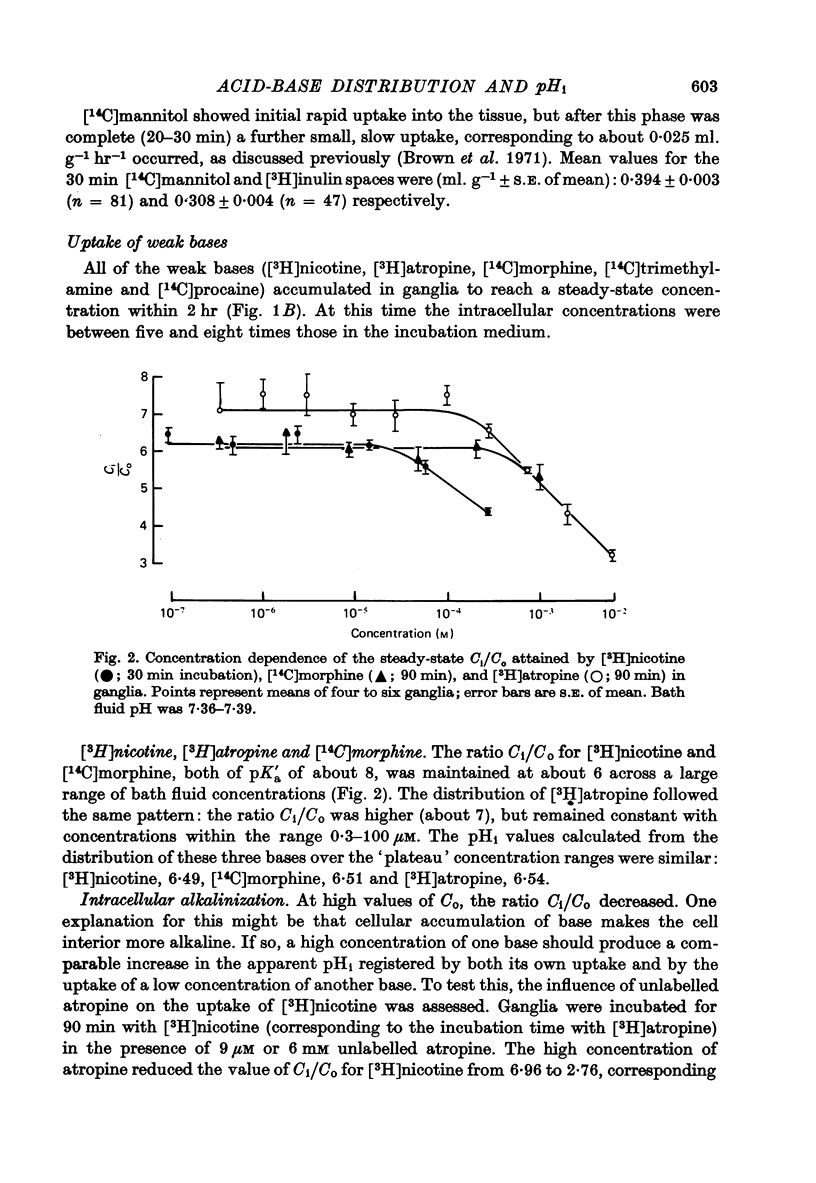
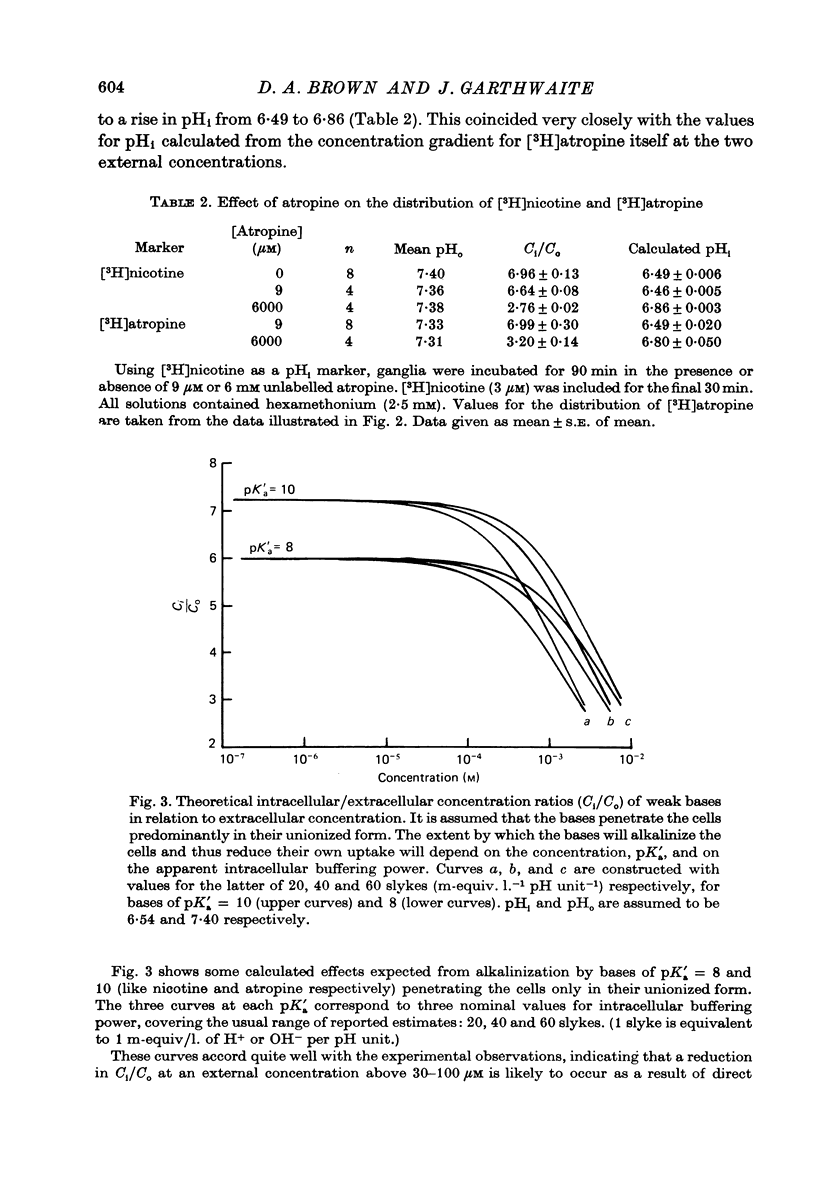
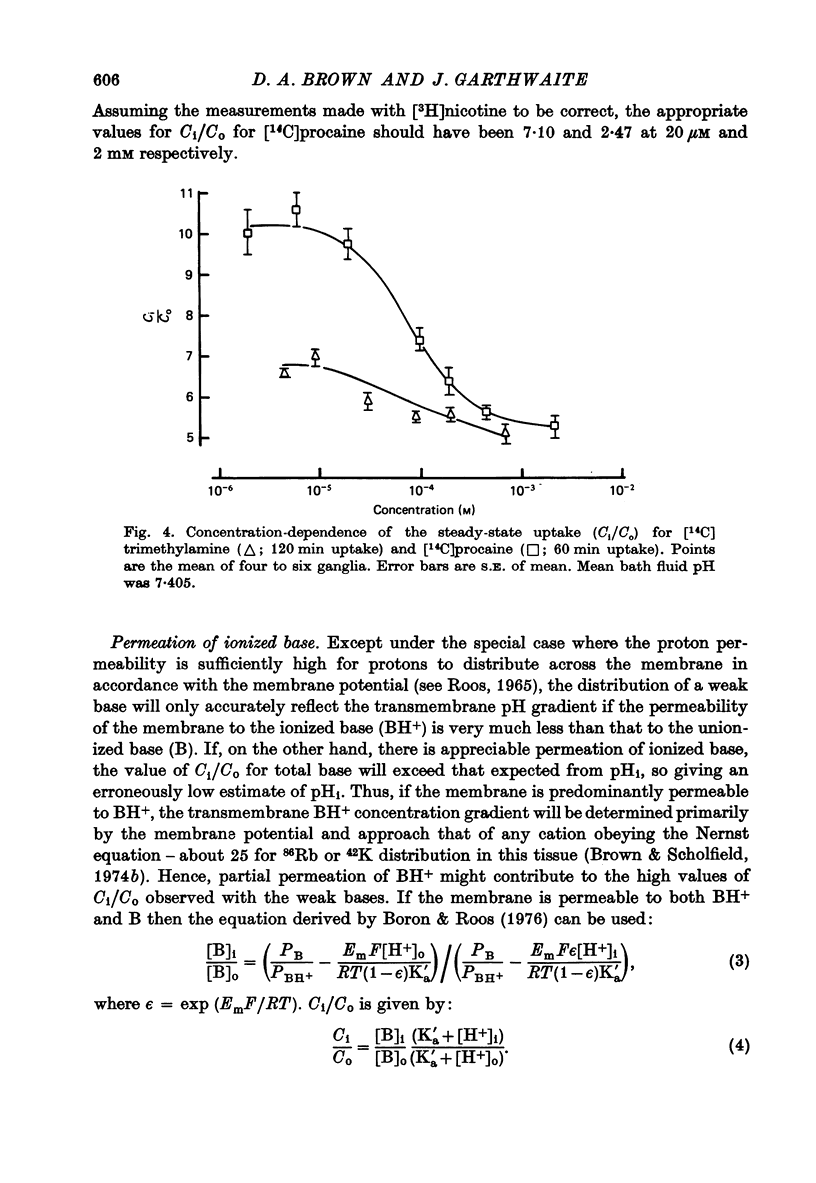
1. The steady-state intracellular/extracellular concentration ratios (Ci/Co) of a number of radiolabelled weak bases in isolated rat superior cervical ganglia were measured. 2. Observed values for Ci/Co (mean +/- S.E. of mean) were [3H]nicotine, 6.17 +/- 0.12; [14C]morphine, 6.08 +/- 0.14 [3H]atropine, 7.10 +/- 0.16; [14C]trimethylamine, 6.73 +/- 0.13; [14C]procaine, 10.13 +/- 0.26. If Ci/Co were determined by the transmembrane pH gradient, the intracellular pH (pHi) appropriate to these concentration gradients lay between 6.4 and 6.6 at an extracellular pH (pHo) of 7.4. 3. the steady-state value of Ci/Co for the weak acid 5,5-dimethyl-2,4-oxazolidinedione (DMO) was 0.87 +/- 0.007. The appropriate pHi was 7.31 +/- 0.003. 4. The difference between the values of pHi calculated from the distribution of the weak bases and of DMO could not be attributed to (i) experimental error, (ii) partial permeation of protonated base, (iii) intracellular binding or carrier-mediated transport of base, (iv) lipid uptake of base or (v) different pK'a inside and outside cells. 5. The difference between the measurements of pHi made with DMO and nicotine (pHDMO-pHnic) was reduced or abolished by uncoupling agents, which act as transmembrane proton carriers. This effect was not reproduced by respiratory inhibitors or by exposure to lactate. 6. pHDMO-pHnic was small (less than 0.1 units) in human erythrocytes, which contain no intracellular organelles, and was exaggerated (1.0 unit) in slices of lipid-depleted brown adipose tissue which contained an abundance of mitochondria. 7. It is concluded that the different values of pHi determined using weak acids and bases arise from the presence of membrane-bound intracellular compartments of differing pH, and that where the use of pH-sensitive micro-electrodes is impracticable, it is desirable to measure pHi with both a weak acid and a weak base unless these can be shown equal over a wide range of pHi values.
Full text
PDF







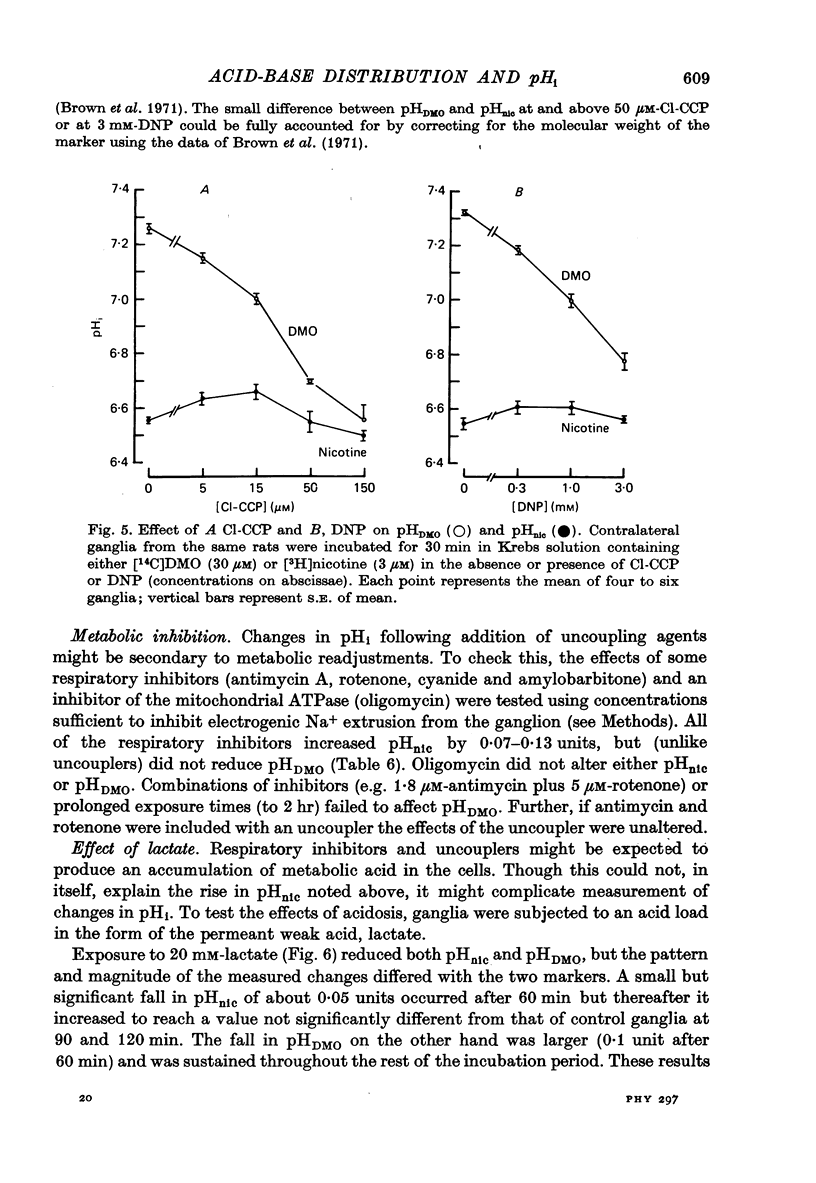
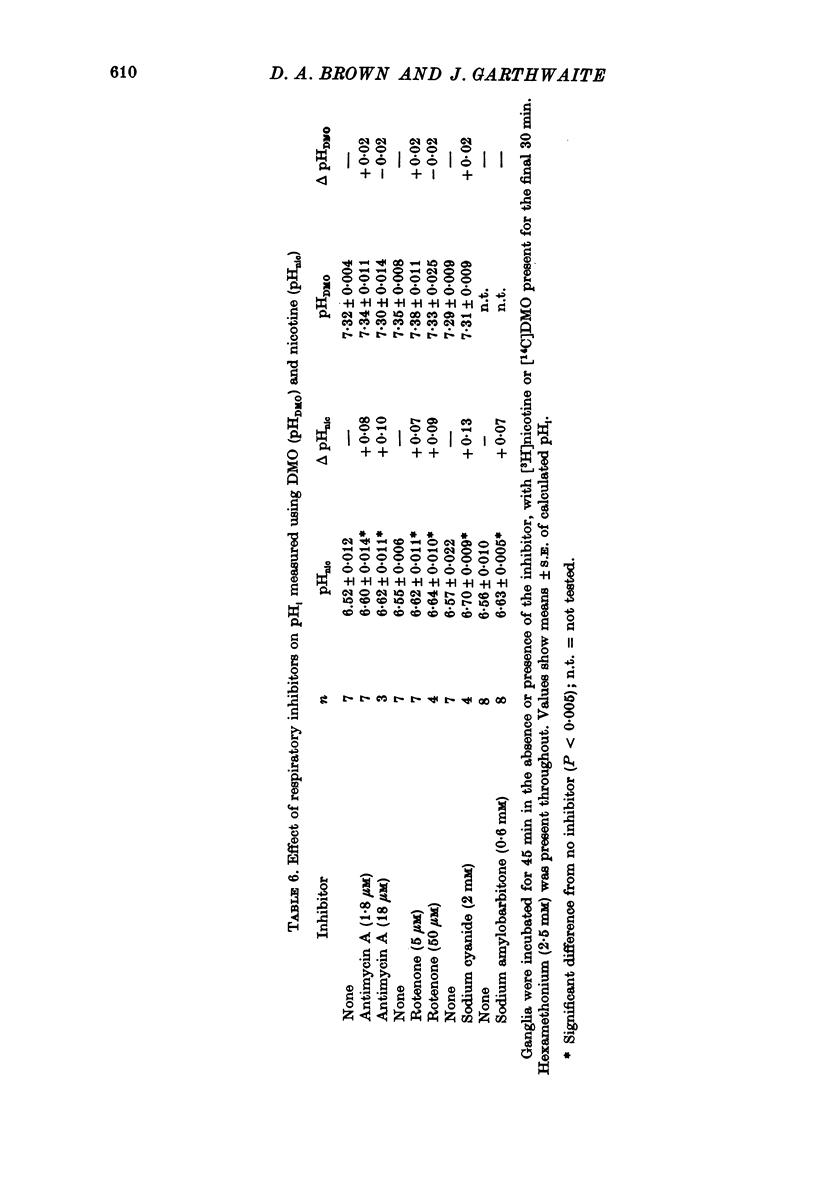
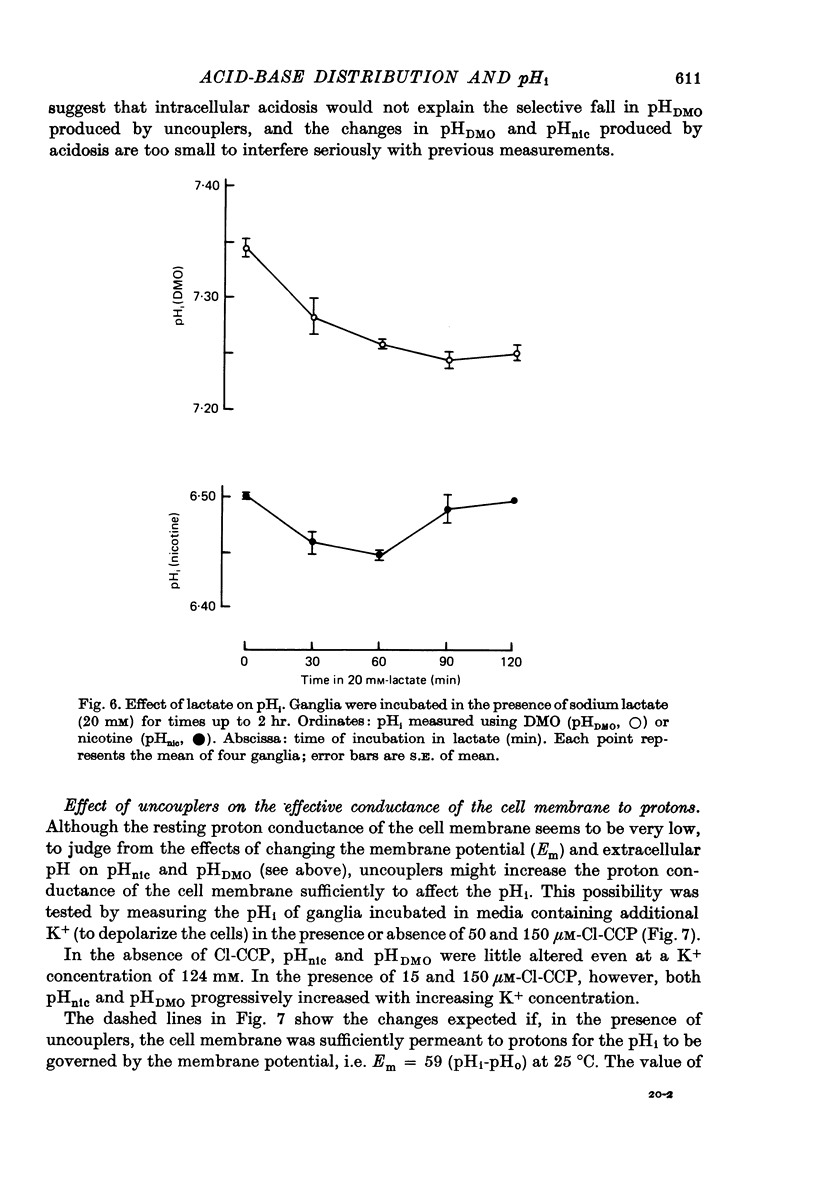
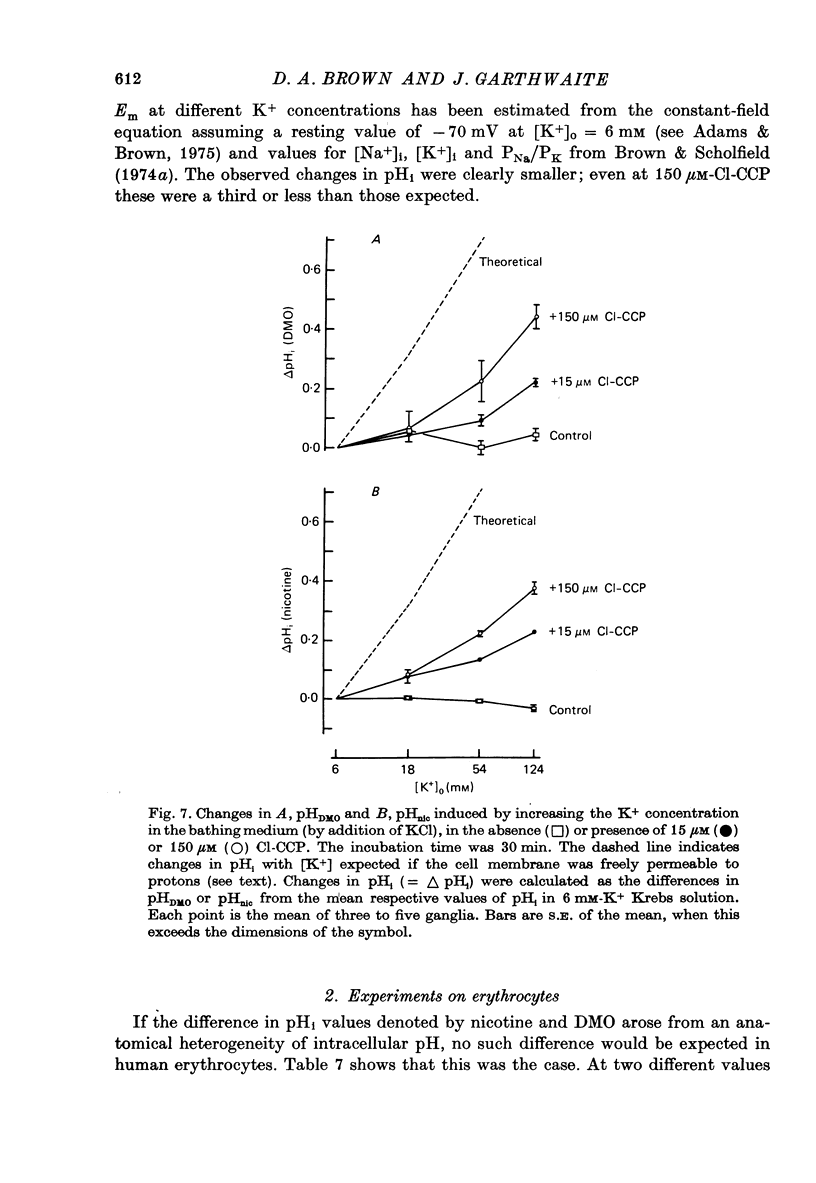







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELGREN L. E., HANSSON E., SCHMITERLOEW C. G. LOCALIZATION OF RADIOACTIVITY IN THE SUPERIOR CERVICAL GANGLION OF CATS FOLLOWING INJECTION OF C14-LABELLED NICOTINE. Acta Physiol Scand. 1963 Dec;59:330–336. doi: 10.1111/j.1748-1716.1963.tb02748.x. [DOI] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler S. The simultaneous determination of muscle cell pH using a weak acid and weak base. J Clin Invest. 1972 Feb;51(2):256–265. doi: 10.1172/JCI106810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Thomas R. C. Micro-electrode measurement of the internal pH of crab muscle fibres. J Physiol. 1975 Nov;252(3):803–815. doi: 10.1113/jphysiol.1975.sp011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone J. M., Verth A., Lambie A. T. Intracellular acid-base heterogeneity in nucleated avian erythrocytes. Clin Sci Mol Med. 1976 Aug;51(2):189–196. doi: 10.1042/cs0510189. [DOI] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Roos A. Comparison of microelectrode, DMO, and methylamine methods for measuring intracellular pH. Am J Physiol. 1976 Sep;231(3):799–809. doi: 10.1152/ajplegacy.1976.231.3.799. [DOI] [PubMed] [Google Scholar]
- Brauser B., Bücher Th, Dolivo M. Redox transitions of cytochromes and pyridine nucleotides upon stimulation of an isolated rat ganglion. FEBS Lett. 1970 Jun 27;8(5):297–300. doi: 10.1016/0014-5793(70)80291-5. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Brownstein M. J., Scholfield C. N. Origin of the after-hyperpolarization that follows removal of depolarizing agents from the isolated superior cervical ganglion of the rat. Br J Pharmacol. 1972 Apr;44(4):651–671. doi: 10.1111/j.1476-5381.1972.tb07305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Halliwell J. V. Intracellular pH in rat isolated superior cervical ganglia in relation to nicotine-depolarization and nicotine-uptake. Br J Pharmacol. 1972 Jun;45(2):349–359. doi: 10.1111/j.1476-5381.1972.tb08088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Halliwell J. V., Scholfield C. N. Uptake of nicotine and extracellular space markers by isolated rat ganglia in relation to receptor activation. Br J Pharmacol. 1971 May;42(1):100–113. doi: 10.1111/j.1476-5381.1971.tb07090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Hoffmann P. C., Roth L. J. 3H-Nicotine in cat superior cervical and nodose ganglia after close-arterial injection in vivo. Br J Pharmacol. 1969 Mar;35(3):406–417. doi: 10.1111/j.1476-5381.1969.tb08282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Marsh S. A very simple method for recording ganglion depolarization. J Physiol. 1975 Mar;246(2):24P–26P. [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Changes of intracellular sodium and potassium ion concentrations in isolated rat superior cervical ganglia induced by depolarizing agents. J Physiol. 1974 Oct;242(2):307–319. doi: 10.1113/jphysiol.1974.sp010709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Movements of labelled sodium ions in isolated rat superior cervical ganglia. J Physiol. 1974 Oct;242(2):321–351. doi: 10.1113/jphysiol.1974.sp010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. An investigation of the intracellular pH of crab muscle fibres by means of micro-glass and micro-tungsten electrodes. J Physiol. 1954 Oct 28;126(1):169–180. doi: 10.1113/jphysiol.1954.sp005201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. Studies on the internal pH of large muscle and nerve fibres. J Physiol. 1958 Jun 18;142(1):22–62. doi: 10.1113/jphysiol.1958.sp005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardasis C. A., Blanc W. A., Sinclair J. C. The effects of ambient temperature on the fasted newborn rabbit. II. Gross and microscopic changes in cervical and interscapular brown adipose tissue. Biol Neonate. 1972;21(5):347–360. doi: 10.1159/000240523. [DOI] [PubMed] [Google Scholar]
- Carter N. M. Symposium on acid-base homeostasis. Intracellular pH. Kidney Int. 1972 May;1(5):341–346. doi: 10.1038/ki.1972.45. [DOI] [PubMed] [Google Scholar]
- Effros R. M., Chinard F. P. The in vivo pH of the extravascular space of the lung. J Clin Invest. 1969 Nov;48(11):1983–1996. doi: 10.1172/JCI106164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatmark T., Pedersen J. I. Brown adipose tissue mitochondria. Biochim Biophys Acta. 1975 Mar 31;416(1):53–103. doi: 10.1016/0304-4173(75)90013-0. [DOI] [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. Metabolic studies on the hyperpolarization following activity in mammalian non-myelinated nerve fibres. J Physiol. 1962 May;161:414–423. doi: 10.1113/jphysiol.1962.sp006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J., Brown D. A. Proceedings: The uptake of weak acids and bases into isolated rat superior cervical ganglia in relation to intracellular pH. Br J Pharmacol. 1976 Mar;56(3):353P–353P. [PMC free article] [PubMed] [Google Scholar]
- HEYTLER P. G., PRICHARD W. W. A new class of uncoupling agents--carbonyl cyanide phenylhydrazones. Biochem Biophys Res Commun. 1962 May 4;7:272–275. doi: 10.1016/0006-291x(62)90189-4. [DOI] [PubMed] [Google Scholar]
- HILPERT P., FLEISCHMANN R. G., KEMPE D., BARTELS H. THE BOHR EFFECT RELATED TO BLOOD AND ERYTHROCYTE PH. Am J Physiol. 1963 Aug;205:337–340. doi: 10.1152/ajplegacy.1963.205.2.337. [DOI] [PubMed] [Google Scholar]
- Heilbronn E. The effect of phospholipases on the uptake of atropine and acetylcholine by slices of mouse brain cortex. J Neurochem. 1969 Apr;16(4):627–635. doi: 10.1111/j.1471-4159.1969.tb06862.x. [DOI] [PubMed] [Google Scholar]
- Hinke J. A., Menard M. R. Intracellular pH of single crustacean muscle fibres by the DMO and electrode methods during acid and alkaline conditions. J Physiol. 1976 Nov;262(3):533–552. doi: 10.1113/jphysiol.1976.sp011609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgins P. M., Putney J. W., Jr Distribution of local anesthetics and the intracellular pH in vascular smooth muscle. J Pharmacol Exp Ther. 1972 Jun;181(3):538–546. [PubMed] [Google Scholar]
- Hull D., Segall M. M. Distinction of brown from white adipose tissue. Nature. 1966 Oct 29;212(5061):469–472. doi: 10.1038/212469a0. [DOI] [PubMed] [Google Scholar]
- IRVINE R. O., SAUNDERS S. J., MILNE M. D., CRAWFORD M. A. Gradients of potassium and hydrogen ion in potassiumdeficient voluntary muscle. Clin Sci. 1961 Feb;20:1–18. [PubMed] [Google Scholar]
- Joel C. D. Stimulation of metabolism of rat brown adipose tissue by addition of lipolytic hormones in vitro. J Biol Chem. 1966 Feb 25;241(4):814–821. [PubMed] [Google Scholar]
- Lindgren G., Barnard T. Changes in interscapular brown adipose tissue of rat during perinatal and early postnatal development and after cold acclimation. IV. Morphometric investigation of mitochondrial membrane alterations. Exp Cell Res. 1972 Jan;70(1):81–90. doi: 10.1016/0014-4827(72)90184-x. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Micro-electrode measurement of the intracellular pH and buffering power of mouse soleus muscle fibres. J Physiol. 1977 Jun;267(3):791–810. doi: 10.1113/jphysiol.1977.sp011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- PATON W. D., RANG H. P. THE UPTAKE OF ATROPINE AND RELATED DRUGS BY INTESTINAL SMOOTH MUSCLE OF THE GUINEA-PIG IN RELATION TO ACETYLCHOLINE RECEPTORS. Proc R Soc Lond B Biol Sci. 1965 Aug 24;163:1–44. doi: 10.1098/rspb.1965.0058. [DOI] [PubMed] [Google Scholar]
- Reijngoud D. J., Tager J. M. The permeability properties of the lysosomal membrane. Biochim Biophys Acta. 1977 Nov 14;472(3-4):419–449. doi: 10.1016/0304-4157(77)90005-3. [DOI] [PubMed] [Google Scholar]
- Robson J. S., Bone J. M., Lambie A. T. Intracellular pH. Adv Clin Chem. 1968;11:213–275. doi: 10.1016/s0065-2423(08)60060-8. [DOI] [PubMed] [Google Scholar]
- Roos A. Intracellular pH and intracellular buffering power of the cat brain. Am J Physiol. 1965 Dec;209(6):1233–1246. doi: 10.1152/ajplegacy.1965.209.6.1233. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Horwitz B. A. Brown fat and thermogenesis. Physiol Rev. 1969 Apr;49(2):330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- Suter E. R. The fine structure of brown adipose tissue. I. Cold-induced changes in the rat. J Ultrastruct Res. 1969 Feb;26(3):216–241. doi: 10.1016/s0022-5320(69)80003-1. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Chipperfield A. R. The reaction mechanism of the sodium pump. Biochim Biophys Acta. 1975 Jun 30;415(2):149–171. doi: 10.1016/0304-4157(75)90001-5. [DOI] [PubMed] [Google Scholar]


