Abstract
In this report, we provide direct demonstration that the neurotrophin nerve growth factor (NGF) is released in the extracellular space in an activity-dependent manner in its precursor form (proNGF) and that it is in this compartment that its maturation and degradation takes place because of the coordinated release and the action of proenzymes and enzyme regulators. This converting protease cascade and its endogenous regulators (including tissue plasminogen activator, plasminogen, neuroserpin, precursor matrix metalloproteinase 9, and tissue inhibitor metalloproteinase 1) are colocalized in neurons of the cerebral cortex and released upon neuronal stimulation. We also provide evidence that this mechanism operates in in vivo conditions, as the CNS application of inhibitors of converting and degrading enzymes lead to dramatic alterations in the tissue levels of either precursor NGF or mature NGF. Pathological alterations of this cascade in the CNS might cause or contribute to a lack of proper neuronal trophic support in conditions such as cerebral ischemia, seizure and Alzheimer’s disease or, conversely, to excessive local production of neurotrophins as reported in inflammatory arthritis pain.
Keywords: matrix metalloproteinase 9, neuroserpin, plasminogen, tissue plasminogen activator, plasmin
The neurotrophin family of growth factors plays a critical role in neuronal survival and differentiation (1, 2). They are produced and liberated in an activity-dependent manner (3) and are responsible for maintaining neuronal phenotype in the adult CNS (4), including the regulation of the steady-state number of synapses (5). These actions are normally attributed to mature neurotrophins, although recently, a biological role for precursor neurotrophin molecules also has been proposed (6, 7). Despite this wealth of knowledge, it is not clear whether these neurotrophins convert to their mature and biologically active form intracellularly or extracellularly, nor is it clear whether, upon their activity-dependent release into the CNS, they are in their mature or precursor form. For example, nerve growth factor (NGF) has been proposed to be released in its mature form (8–10), whereas in the case of BDNF, recent experimental data suggests that the precursor form of BDNF was released and then was processed extracellularly to elicit long-term potentiation (11).
These issues are of functional significance because recent in vitro studies with cells transfected with furin-resistant mutated forms of precursor NGF (proNGF) have shown that unprocessed proNGF interacts preferentially with p75 neurotrophin receptor instead of the high-affinity NGF receptor, TrkA, facilitating an apoptotic mechanism in embryonic cells of the peripheral nervous system (7). Moreover, it has been proposed that, in the adult CNS, proNGF expression is up-regulated after CNS lesions, probably contributing to cell death through p75 neurotrophin receptor and sortilin (12, 13). However, other authors have provided evidence suggesting a neurotrophic role for proNGF, albeit to a lesser degree than that of the mature NGF (mNGF) (6).
The realization that proNGF might play a biological role in the CNS raised questions regarding the regulatory mechanisms leading to its release and the control of the proNGF to mNGF ratio and, ultimately, the degradation of the mNGF molecule. To answer these questions, we embarked on a series of in vitro and in vivo studies aimed at elucidating the preferential NGF form released from the cerebral cortex and the pathway leading to NGF maturation and degradation. These studies have revealed that proNGF is the main releasable form of the neurotrophin and that the maturation and degradation of mNGF largely occurs in the extracellular space with the involvement of a complex protease cascade.
Results
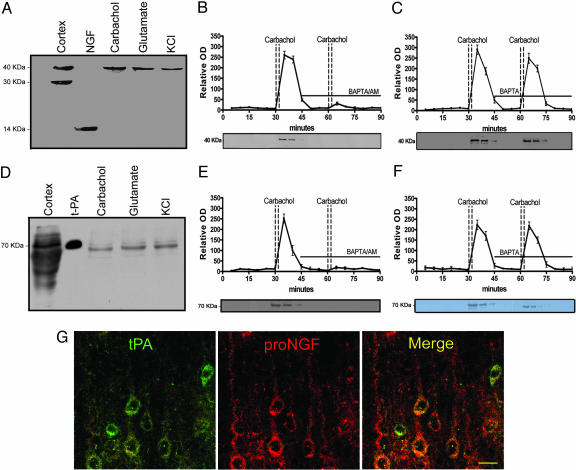
Previous studies have demonstrated two different neuronal NGF release mechanism, the constitutive and the activity-mediated secretion (9). To determine whether proNGF or mNGF, or a combination of the two, is released from mature CNS neurons, we superfused small slices of the frontal and parietal cerebral cortex of young Fisher 344 rats. To test the constitutive secretion mechanism, the tissue was superfused at 37°C for 60 min without stimulation, and collecting samples were analyzed by Western blot. Neither proNGF nor NGF was detectable under these conditions in our experiments. To test the activity-mediated neuronal secretion, after 30 min of tissue stabilization, the cortical tissue was stimulated with carbachol (100 nM), glutamate (60 μM) or KCl (50 mM), and superfusates were analyzed by Western blot. Contrary to the results obtained under nonstimulation conditions, our results consistently demonstrate the presence of a single immunoreactive 40-kDa band, after KCl or transmitter receptor stimulation, corresponding to proNGF. Contrary to our expectations, mNGF (14 kDa) was undetectable in these conditions (Fig. 1A). These results indicate that the precursor proNGF, instead of mNGF, is the molecular form preferentially released by neurons in an activity-dependent manner.
Fig. 1.
Neuronal colocalization and stimulus-coupled release of proNGF and tPA. Western blots demonstrating proNGF (A) and tPA (D) released from cerebral cortex after stimulation (see Materials and Methods). First lane illustrates immunoreactive bands from cortical homogenates. In the second lane, 5 ng mNGF (A) or 3 ng tPA (D) are loaded as control. Time course of proNGF (B) and tPA (E) released from cerebral cortex tissue. Activity-dependent release of neuroactive proteins was induced by two consecutive carbachol stimulations. The presence of the intracellular calcium chelator, BAPTA/AM (10 μM), in the superfusion buffer inhibited the release of proNGF (B) and of tPA (E) but the presence of the extracellular calcium chelator, BAPTA (10 μM), did not affect proNGF (C) or tPA (F) release (mean ± SEM). (G) Localization of tPA (green) and proNGF (red) in cortical pyramidal neurons; colocalization illustrated with merged images (yellow). (Scale bar: 20 μm.)
To determine whether this activity-dependent mechanism of proNGF release was reliant on intracellular or extracellular calcium, we applied at the second carbachol (100 nM) stimulation, plasma membrane permeable (BAPTA/AM), or plasma membrane impermeable (BAPTA) calcium chelators. The neutralization of intracellular calcium before the second carbachol stimulation with BAPTA/AM resulted in the total blockage of the second proNGF peak (Fig. 1B). On the other hand, when the nonpermeable calcium chelator, BAPTA, was included in the perfusion buffer, the second stimulus-coupled proNGF secretion was unimpeded (Fig. 1C). These results strongly indicate that the stimulus-coupled release of proNGF is independent of extracellular calcium but tightly dependent on intracellular calcium stores.
We next investigated how proNGF is converted to mNGF in the extracellular milieu upon its stimulus-coupled neuronal release. A mechanism involving plasminogen and the tissue plasminogen activator (tPA) was suspected because it has been shown that tPA plays a key role in synaptic plasticity (14) and, more recently, that tPA activation of plasmin for the cleavage of precursor BDNF into mature BDNF is essential for the formation of hippocampal long-term potentiation (11). To establish whether tPA and plasminogen also are released from the cerebral cortex in an activity-dependent manner, we stimulated cortical tissue samples with carbachol, glutamate, or KCl, as above. In these experiments, we consistently found tPA immunoreactive material in the superfusate after neuronal stimulation with a secretion pattern similar to that of proNGF (Fig. 1 D–F). We also found that the stimulus-coupled tPA release also depended on intracellular calcium (Fig. 1E) and independent of the presence of extracellular calcium (Fig. 1F). To localize the cell sites containing proNGF and tPA, we used confocal microscopy to investigate both proNGF and tPA immunoreactive sites by using highly specific antibodies. A close colocalization of the proneurotrophin with tPA in numerous pyramidal neurons of the rat cerebral cortex was revealed (Fig. 1G), indicating that both the substrate proNGF and the enzymatic cleavage complex would be liberated on demand from the same or similar neuronal stores and by analogous release mechanisms. Such a system would require the extracellular availability of the tPA substrate and an inhibitory loop to terminate the convertase reaction. The obvious tPA substrate is the inactive zymogen plasminogen. tPA converts plasminogen into the active protease plasmin, which ultimately cleaves proNGF into mNGF. A likely endogenous negative regulator for this system would be neuroserpin, a member of the serine proteinase inhibitor family, which is known to be secreted from axonal growth cones of the CNS, where it is thought to control tPA activity (15–17). We further investigated whether plasminogen and neuroserpin also could be released from the cerebral cortex upon neuronal activation. We found that plasminogen and neuroserpin (the endogenous brain inhibitor of tPA) both were released upon neuronal stimulation with carbachol (Fig. 2A and D) in an intracellular calcium dependent-manner (Fig. 8 A and B, which is published as supporting information on the PNAS web site).
Fig. 2.
Plasminogen and neuroserpin release conditions. Western blots of plasminogen (A) and neuroserpin (B) released after carbachol, glutamate, and KCl stimulation. The first lane illustrates immunoreactive bands from cortical homogenates; the second lane shows positive controls for mouse plasminogen (10 ng) and neuroserpin (6 ng), respectively.
In agreement with previous reports (16, 18), immunocytochemical investigations by using confocal microscopy revealed that plasminogen and neuroserpin also were colocalized in pyramidal neurons (Fig. 3A and B). Taken together, these results indicate the presence of a storage pool of proNGF, plasminogen, tPA, and neuroserpin in CNS cortical neurons, which is ready to be liberated to the extracellular space in response to neuronal activity. Thus, our investigations provide direct evidence for the activity-dependent release of the neurotrophin precursor protein (proNGF) and that of the protease cascade complex required for its maturation in the extracellular space. The rate of proNGF to mNGF conversion appears to be regulated by the endogenous tPA inhibitor neuroserpin.
Fig. 3.
Colocalization of tPA with plasminogen, neuroserpin, proMMP-9, and TIMP-1 at pyramidal neurons. (Left) tPA immunolocalization is shown in green. (Center) Immunolocalization of plasminogen, neuroserpin, proMMP9, and TIP-1 are shown in red. (Right) Merged images with tPA on left column; yellow indicates co-localization in the same neuron. (Scale bar: 20 μm.)
Both plasmin and some members of the matrix metalloproteinase (MMP) family have been suspected of being responsible for the conversion of proNGF into mNGF (7). In neurons, several components of the MMP family are expressed. Their proteolytic activity is controlled by endogenous tissue inhibitors (TIMP). The major targets of the TIMP/MMP system are proteins of the extracellular matrix. The MMPs are produced and released by cells in an inactive MMP (proMMP) form, and its activation is controlled by a cascade of steps involving other MMPs and the plasmin system (19). To confirm the presence of these proteins in the cerebral cortex and to ascertain whether MMP-9 and TIMP-1 follow the same release pattern as proNGF and that of the tPA/plasminogen protease cascade members, the same immunocytochemical and neurochemical protocols described above were applied, showing that MMP-9 and TIMP-1 colocalized in neocortical pyramidal neurons (Fig. 3 C and D). As we found for the tPA/plasminogen protease cascade, proMMP–9 and TIMP-1 were identified in the stimulated superfusate samples, indicating that both are released by neurons (Fig. 4A and B) in a manner dependent on intracellular, but independent of extracellular, calcium upon carbachol stimulation (Fig. 8 E–H).
Fig. 4.
Activity-dependant release of proMMP-9 and TIMP-1. Representative Western blots of proMMP-9 (A) and TIMP-1 (B) released by cerebral cortical tissue upon stimulation with carbachol (100 nM), glutamate (60 μM), or KCl (50 mM).
ProNGF, tPA, plasminogen, neuroserpin, proMMP-9, and TIMP-1 were released in an intracellular calcium-dependent manner after stimulation with either glutamate (60 μM) or KCl (50 mM) (data not shown). It is interesting that all these proteins shared these unusual stimulus-coupled release properties in contrast to the extracellular calcium regulated release of conventional transmitters and neuroactive peptides, such as substance P. In our tissue, superfusion model cortical substance P release followed the “canonical” extracellular dependency reported in previous studies (20). Thus, evoked substance P release was completely abolished in the absence of calcium in the superfusion buffer or by including the extracellular calcium chelator BAPTA (Fig. 9, which is published as supporting information on the PNAS web site).
To clarify which of these proteases is capable of converting the endogenously produced and released proNGF into mNGF, we incubated superfusate samples from stimulated cortical tissues with diverse convertase candidates. Under these conditions, only plasmin was capable of converting endogenous proNGF to mNGF (see Fig. 5A). The application of the inhibitor α2-antiplasmin to plasmin containing samples blocked the conversion of proNGF to mNGF confirming the specificity of such reaction (see Fig. 5A, lane 7). A further demonstration of the specificity of this metabolic network (the convertase tPA, the tPA substrate plasminogen, the inhibitor neuroserpin) is provided by our finding that none of them, on their own, were capable of activating the conversion of proNGF to its mature form (see Fig. 5B). This conversion only took place in the presence of tPA plus plasminogen (Fig. 5B, lane 5), and it was completely blocked when the tPA/plasminogen complex was incubated in the presence of neuroserpin (Fig. 5B, lane 6).
Fig. 5.
Maturation of released proNGF and degradation of mature NGF. (A) Plasmin-induced conversion of endogenously released proNGF into mNGF. The plasmin protease activity on proNGF was inhibited by α2-antiplasmin. MMP-2, MMP-7, and MMP-9 failed to convert proNGF into mNGF. (B) tPA, plasminogen, or neuroserpin alone (lanes 3, 4 and 7, respectively) were not able to convert proNGF on their own; it was only when both plasminogen and tPA were present that the conversion took place (lane 5). The convertase activity was inhibited by neuroserpin (lane 6). NGF (A, B, and D, lane 1) and proNGF (A and B, lane 2) are reference controls (C). MMP-9 alone was not able to degrade mNGF (B, lane 2) whereas NGF degradation occurred in the presence of either MMP-9, tPA, and plasminogen (lane 5) or with MMP-9 plus plasmin (lane 6). (D) Neither plasmin nor proMMP-9 alone degraded mNGF (lane 2 and 3, respectively). The plasmin activated MMP-9 degradation of mNGF (lane 4) was blocked by the metalloproteinase inhibitor GM6001 (lane 5) but not by the negative control GM6001 (lane 6).
During the course of our investigations, we found that the newly generated mNGF disappears promptly from the extracellular space. To determine whether the enzymatic components actively released upon neuronal stimulation could degrade NGF, we examined their effects on mNGF alone and in combination with putative proteases (Fig. 5C). In these experiments, neither latent inactive MMP-9, plasmin, plasminogen, nor tPA alone were capable of provoking the degradation of mNGF. However, our results showed that when MMP-9 is activated by plasmin, NGF degradation takes place (Fig. 5C, lanes 5 and 6). To determine whether the NGF degradation was mediated by the activated MMP-9, we incubated mNGF with plasmin-activated MMP-9 in the presence of the broad-spectrum metalloproteinase inhibitor, GM6001, or in the presence of the GM6001 negative control. The results obtained (Fig. 5D) indicate that the degradation of mNGF is blocked by the MMP inhibitor, GM6001, but not by the inactive congener. These observations support the notion that plasmin-activated MMP-9 is the most likely protease responsible for the rapid enzymatic inactivation of remnant, unused mNGF.
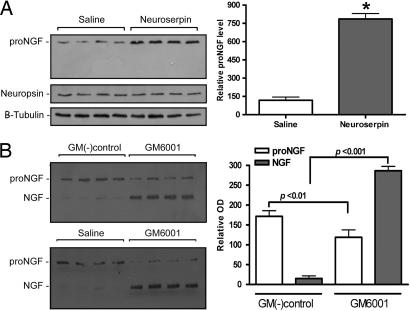
An obvious question emerged from these results: Do these proposed mechanisms have an in vivo relevance? To test the in vivo impact on the proNGF/mNGF extracellular conversion to mNGF and its eventual degradation by the tPA/plasmin/MMP-9 proteolytic cascade, we decided to disrupt this cascade locally in the cerebral cortex. Thus, we intervened at two levels of the proposed cascade. First, we blocked plasmin formation by inhibiting tPA action by infusing the tPA endogenous inhibitor, neuroserpin, and, second, by inhibiting the activated MMP-9 by infusing the broad-spectrum MMP inhibitor, GM6001. The first approach should define, in an in vivo situation, the relevance of tPA/plasminogen activation in the conversion of endogenous proNGF into its mature form, whereas the second should define whether endogenous MMP-9 is able to provoke the degradation of mNGF. The continuous, unilateral infusion of neuroserpin into the cerebral cortex of young rats for 72 h provoked a severalfold increment in proNGF tissue levels when compared with the contralateral, vehicle-injected, side (see Fig. 6A). In these experiments, no change was observed in cytoskeletal β-tubulin or neuropsin, a well characterized secretory serine protease present in pyramidal neurons and released in an activity-dependent manner for the cleavage of extracellular matrix proteins (21). The levels of proNGF returned to basal level 48 h after the neuroserpin infusion was stopped (data not shown). On the other hand, the unilateral infusion of the MMP-9 inhibitor, GM6001 in the cerebral cortex of young rats for 72 h caused a dramatic rise of mNGF content with a significant decrease in proNGF levels when compared with values from the contralateral side, which received either the GM6001 negative control or saline (see Fig. 6B). To investigate whether GM6001 could have a direct effect on proNGF maturation, released proNGF was incubated at 37°C. The MMP-9 inhibitor did not show any effect on proNGF levels or in the rate of conversion of proNGF into NGF (data not shown). Therefore, the marked change in the proNGF/NGF ratio in vivo caused by the MMP-9 inhibition of the NGF degradation suggests that the NGF accumulation might unleash a negative feedback mechanism on the proNGF production. The GM6001 effect on mNGF degradation disappeared 48 h after discontinuation of treatment, whereas the proNGF/NGF levels returned to basal levels (data not shown).
Fig. 6.
The cortical proNGF/matureNGF ratio is changed by the application of neuroserpin or MMP-9 inhibitors. (A) Increased amount of cortical proNGF in neuroserpin-treated animals (mean ± SEM; P < 0.001; t test). (B) The inhibitor of matrix metalloproteinase GM6001 significantly increased the cortical mNGF (P < 0.001) and decreased proNGF (P < 0.01) when compared with the GM6001 negative or saline control-treated (mean ± SEM). The levels of neuropsin, a serine protease secretory protein present in pyramidal neurons and β-tubulin were not altered in these experiments.
Discussion
In human, mouse and rat brain tissue, little or no mature NGF is detected (22). The lack of mNGF signals can be explained, in part, by the rapid internalization and retrograde transport in the form of NGF–TrkA complexes within signaling endosomes, as elegantly demonstrated by Grimes and coworkers (23). However, our results strongly indicate that newly generated NGF, which is not membrane-bound to TrkA receptors nor rapidly internalized, is promptly removed from the extracellular space by activated MMP-9 via the enzymatic degradation of mNGF.
NGF is generated from a precursor molecule, proNGF, which undergoes processing (conversion) to generate mNGF. For more than two decades, it has been assumed that the mature NGF form accounts for the neurotrophin’s biological activity, including cell survival, neurite outgrowth, and neuronal differentiation. Likewise, it has been assumed that proNGF had little or no biological action. However, Fahnestock et al. (22) have recently shown that proNGF is abundant in CNS tissue, whereas mNGF is undetectable, suggesting that proNGF either may have a function distinct from its role as a precursor or that the precursor is processed to mNGF intracellularly before secretion to the extracellular milieu. Hempstead et al. (7) generated a cDNA construct of a mutated, furin cleavage-resistant form of proNGF to “impair intracellular proteolysis.” This artificial, recombinant, transgene form of proNGF was shown to bind preferably p75 neurotrophin receptor and promote apoptosis in primary superior cervical ganglion neurons and smooth muscle cells, in vitro. In contrast, Fahnestock et al. (6), by using a different recombinant cleavage-resistant form of proNGF, showed that this precursor exhibits neurotrophic activity similar to mNGF, but is ≈5-fold less active. This artificial proNGF binds to TrkA but is less active in promoting phosphorylation of TrkA and its downstream signaling effectors, Erk1/2, in PC12 and NIH 3T3 cells.
Our study provides direct evidence for the differential activity-dependent release of endogenous precursors of mature neurotrophin in the fully differentiated and adult CNS. A similar activity-mediated secretion of NGF was found by Thoenen et al. (8, 9), where the analysis was performed under similar release conditions in native hippocampal slices and from NGF-cDNA transfected hippocampal neurons. However, in those experiments, NGF was quantified by highly sensitive ELISA, which does not discriminate mature NGF from the proNGF forms. Although the less-sensitive Western blot analysis does not allow to accurately investigate the so-called constitutive (spontaneous) release, it allows us to define with precision the molecular NGF forms involved in the activity-dependent release of this neuroptrophic peptide.
This work reports direct evidence of an activity-dependent release of the components of the proteolytic cascade responsible for the extracellular conversion of proNGF to mNGF. It also demonstrates the mechanism ultimately leading to in vivo enzymatic inactivation (degradation) of mNGF. These findings may be of great importance if pathological alterations of this cascade in the CNS cause or contribute to a lack of proper neuronal trophic support in conditions such as cerebral ischemia, seizure, and Alzheimer’s disease.
All of the members of the cascade reported here are known to be highly expressed in the CNS (16, 18). Thus, the gelatinase MMP-9 protein is highly expressed, predominantly in neurons of the hippocampus and cerebral cortex, where in line with our observation, it is suspected of playing a role in synaptic plasticity (24) and spatial learning (25). Its inhibitor, TIMP-1, also is present in cortical (26) and hippocampal neurons (27). These observations are consistent with the present report of a coordinated and simultaneous release of neurotrophin precursors and corresponding proteases.
Our studies reveal that, once converted, mature NGF is enzymatically degraded by activated MMP-9. We demonstrate that neuronal stimulation releases the required inactive proMMP-9, which is activated extracellularly by plasmin. The activated MMP-9, in consequence, causes the proteolytic degradation of mature NGF. Furthermore, we were able to demonstrate that such a NGF-degradative mechanism is operative in the adult CNS in vivo. The combined in vivo experiments illustrates that it is possible, at least experimentally, to manipulate the ratio of proNGF to mature NGF in the adult CNS. This observation suggests previously undescribed therapeutic opportunities for conditions in which the up-regulation of endogenous mature NGF would be desirable.
In summary, the present results indicates the existence of a CNS protease cascade responsible for the conversion of proNGF into mNGF and, ultimately, for its degradation within extracellular space. These events are schematically illustrated in Fig. 7A and B). Our findings support the view that the protease cascade, as well as the neurotrophin precursor, is stored in neuronal comportments from which it is simultaneously liberated with the NGF precursor upon functional demand after neuronal stimulation. These observations are consistent with the idea that metabolic networks do not interact at random but instead in a tightly coordinated manner (28).
Fig. 7.
Schematic representations of events leading to proNGF conversion into mNGF and its degradation. Neuronally stored proNGF, plasminogen, tPA, neuroserpin, proMMP-9, and TIMP-1 would be released into the extracellular space upon neuronal stimulation. Released tPA would induce the conversion of plasminogen to plasmin, where its activity is tightly regulated by secreted neuroserpin. The generated plasmin would convert proNGF into mature NGF and activate proMMP-9 into active MMP-9. Mature NGF would interact with its cognate receptors (TrkA and p75 neurotrophin receptor) or suffer degradation by activated MMP-9.
The deregulation of the protease cascade controlling proNGF conversion and NGF degradation should open new vistas on trophic responses in the adult CNS and in pathological circumstances, such as seizure, cerebral ischemia, and Alzheimer’s disease, where an up-regulation of proNGF is well documented (22). This pathway might be also of relevance in chronic arthritic pain, where NGF production is a prominent proinflammatory factor (29).
Materials and Methods
Animals.
Six-month-old Fischer-344 rats were used in this study. Efforts were made to minimize the number of animal used and their suffering. All procedures were approved beforehand by the Animal Care Committee of McGill University, and the guidelines of the Canadian Council on Animal Care were followed.
Perfusion System and Release Experiments.
Animals were decapitated, the cerebral cortex was rapidly dissected out at 4°C, cut in small blocks by using a TC-2 Tissue Sectioner (Sorvall), and placed into a perfusion system (chamber containing a 8.0 μM pore size membrane, Falcon). The tissue was constantly superfused with modified Hanks’ buffer equilibrated with 95% O2 and 5% CO2 with a flow rate of 0.25 ml/min at 37°C as described in ref. 10. The tissue was equilibrated for 30 min before stimulation. Tissue stimulation was induced either by carbachol (100 nM), glutamate (60 μM), or KCl (50 mM) applied over a 5-min period. Samples were collected at 5-min intervals. Specific calcium chelators (10 μM BAPTA and 10 μM BAPTA/AM; Calbiochem) were added in the perfusion buffer 45 min after the beginning of the perfusion and maintained during the second collection period until the end of the experiment (n = 6 for every condition).
Western Blots and ELISA.
ProNGF, tPA, plasminogen, neuroserpin, proMMP-9, and TIMP-1 were identified by SDS/PAGE and Western blotting from fractional release samples. For NGF and proNGF Western blot analysis, a well characterized rabbit polyclonal anti-NGF H-20 (1:750; Santa Cruz Biotechnology) was applied. Mature mouse NGF (5 ng; Cedarlane Laboratories) was used as a standard protein. For tPA Western blots, monoclonal anti-tPA (1:1,000) and single-chain tPA (3 ng) as a control were used (American Diagnostica). For neuroserpin Western blotting, rabbit polyclonal anti-neuroserpin (1:1,000; kindly provided by Daniel A. Lawrence, University of Maryland School of Medicine, Rockville) and neuroserpin (6 ng; kindly provided by David A. Lomas, University of Cambridge, Cambridge, U.K.) were used. For plasminogen Western blots, rabbit anti-mouse plasminogen 1:500 (Molecular Innovations, Southfield, MI) was used. ProMMP-9 was detected with rabbit anti-MMP-9 (1:1,000, H-129; Santa Cruz Biotechnology) and TIMP-1 with goat anti-TIMP-1 (1:500, R-18; Santa Cruz Biotechnology). Rat cerebral cortex homogenates containing 30 μg protein per well were loaded as controls.
Substance P was measured by an ELISA-type immunoassay parameter kit by following manufacturer’s instructions (Substance P Immnunoassay, catalog no. KGE007; R & D Systems).
Enzymatic Assays.
For proNGF maturation assays, superfusate samples of carbachol-stimulated tissue were rapidly centrifuged at 4°C. Superfusates were treated for 1 h at 37°C for maturation assays with the following enzymes: mouse MMP-2 (2 μg/μl; R & D Systems), MMP-7 (2.1 μg/μl; Calbiochem), mouse MMP-9 (2 μg/μl; R & D Systems), proMMP-9 (2 μg/μl; Oncogene), mouse plasmin (Molecular Innovations), and α2-antiplasmin (5 μg/μl; Sigma). The cleaved products were proved by Western blotting with an antibody to NGF (H-20; Santa Cruz Biotechnology). Every condition was repeated three times. NGF degradation assays used purified mature mouse NGF (Cedarlane Laboratories). ProMMP-9 or MMP-9 were preincubated for 1 h at 37°C with either 2 μg/μl plasmin or 1.6 μg/μl of mouse plasminogen (Molecular Innovations) and 2 μg/μl of mouse tPA (American Diagnostica) to induce their activation. NGF degradation reactions were performed for 1 h at 37°C. GM6001 and the GM6001 negative control were both applied at 1 μg/μl (Calbiochem). The results of the enzymatic reaction were proved by Western blotting with anti-NGF H-20.
Immunostaining and Confocal Microscopy.
Brain slices were processed as described by us in ref. 30. Briefly, rats were anesthetized and perfused trancardially, and the brain tissue was removed and postfixed. Thirty-five μm-thick free-floating sections were incubated for 1 h with the corresponding blocking sera at 10% in PBS-Triton X-100 (0.1%) and incubated overnight at 4°C with a monoclonal antibody against tPA (1:50; American Diagnostica), rabbit anti-proNGF (1:100; Alomone Labs, Jerusalem), rabbit anti-mouse-plasminogen (1:100; Molecular Innovations), rabbit anti-neuroserpin (1:100; Daniel A. Lawrence, American Red Cross), rabbit anti-MMP-9 (1:100 Santa Cruz Biotechnology), and goat anti-TIMP-1 (Santa Cruz Biotechnology). After washing, the sections were incubated for 2 h at room temperature with the corresponding secondary antibodies: goat anti-mouse biotinylated IgG (1:200; Vector Laboratories) followed by streptoavidin Alexa 488 conjugate (1:200; Molecular Probes) and donkey anti-rabbit coupled with rhodamine red (1:100; Jackson ImmunoResearch). The immunoreactive sites were examined with an LSM 510 two-photon laser-scanning confocal microscope (Zeiss).
Surgical Procedures and Intracortical Administration.
Rats were anesthetized and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Cannulae (Alzet 2001, 24 μl/day) were implanted into the parietal cerebral cortex bilaterally, one side receiving the experimental compound, whereas the other received the control solution for 72 h, by using the following coordinates from Bregma: anteroposterior, −1.3 mm; lateral, ± 4.0 mm; vertical, 1.6 mm. The cannulae were connected to sterile coiled polyethylene tubing filled with an air-oil spacer at the pump end. Rats receiving vehicle (n = 4) had pumps filled with phosphate-buffered artificial cerebrospinal fluid (CSF) (150 mM NaCl/1.8 mM CaCl2/1.2 mM MgSO4/2 mM K2HPO4/10 mM glucose/0.001% rat serum, pH 7.4). Some rats (n = 4) received a total dose of 36 μg of neuroserpin with 0.5 μg/μl diluted in saline vehicle. The same protocol was followed for GM6001-treated rats. Both, GM6001 and the GM6001 negative control (100 mg/kg) (31) were diluted in artificial CSF.
Supplementary Material
Acknowledgments
We thank Dr. Alfredo Ribeiro-da-Silva for advice on confocal microscopy; Adriana Ducatenzeiler and Vanessa Partridge for technical support; Sid Parkinson for editorial assistance; Drs. Elena Miranda and David Lomas (University of Cambridge, Cambridge, U.K.) for the generous provision of neuroserpin; and Dr. Daniel A. Lawrence for providing the anti-neuroserpin antibody. This work was supported by Canadian Institute of Health Research Grant M.O.P. 62735. M.A.B. received a fellowship from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina, and A.C.C. is the holder of the Charles E. Frosst Merck Chair of Pharmacology, McGill University.
Abbreviations
- MMP
matrix metalloproteinase
- mNGF
mature nerve growth factor
- NGF
nerve growth factor
- proMMP
inactive matrix metalloproteinase
- proNGF
precursor nerve growth factor
- tPA
tissue plasminogen activator
- TIMP
tissue inhibitor metalloproteinase.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bibel M., Barde Y. A. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 2.Levi-Montalcini R. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 3.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 4.Sofroniew M. V., Galletly N. P., Isacson O., Svendsen C. N. Science. 1990;247:338–342. doi: 10.1126/science.1688664. [DOI] [PubMed] [Google Scholar]
- 5.Debeir T., Saragovi H. U., Cuello A. C. Proc. Natl. Acad. Sci. USA. 1999;96:4067–4072. doi: 10.1073/pnas.96.7.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahnestock M., Yu G., Michalski B., Mathew S., Colquhoun A., Ross G. M., Coughlin M. D. J. Neurochem. 2004;89:581–592. doi: 10.1111/j.1471-4159.2004.02360.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee R., Kermani P., Teng K. K., Hempstead B. L. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 8.Blochl A., Thoenen H. Eur. J. Neurosci. 1995;7:1220–1228. doi: 10.1111/j.1460-9568.1995.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 9.Blochl A., Thoenen H. Mol. Cell. Neurosci. 1996;7:173–190. doi: 10.1006/mcne.1996.0014. [DOI] [PubMed] [Google Scholar]
- 10.Canossa M., Griesbeck O., Berninger B., Campana G., Kolbeck R., Thoenen H. Proc. Natl. Acad. Sci. USA. 1997;94:13279–13286. doi: 10.1073/pnas.94.24.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang P. T., Teng H. K., Zaitsev E., Woo N. T., Sakata K., Zhen S., Teng K. K., Yung W. H., Hempstead B. L., Lu B. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 12.Harrington A. W., Leiner B., Blechschmitt C., Arevalo J. C., Lee R., Morl K., Meyer M., Hempstead B. L., Yoon S. O., Giehl K. M. Proc. Natl. Acad. Sci. USA. 2004;101:6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nykjaer A., Lee R., Teng K. K., Jansen P., Madsen P., Nielsen M. S., Jacobsen C., Kliemannel M., Schwarz E., Willnow T. E., et al. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 14.Qian Z., Gilbert M. E., Colicos M. A., Kandel E. R., Kuhl D. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- 15.Barker-Carlson K., Lawrence D. A., Schwartz B. S. J. Biol. Chem. 2002;277:46852–46857. doi: 10.1074/jbc.M207740200. [DOI] [PubMed] [Google Scholar]
- 16.Hastings G. A., Coleman T. A., Haudenschild C. C., Stefansson S., Smith E. P., Barthlow R., Cherry S., Sandkvist M., Lawrence D. A. J. Biol. Chem. 1997;272:33062–33067. doi: 10.1074/jbc.272.52.33062. [DOI] [PubMed] [Google Scholar]
- 17.Osterwalder T., Cinelli P., Baici A., Pennella A., Krueger S. R., Schrimpf S. P., Meins M., Sonderegger P. J. Biol. Chem. 1998;273:2312–2321. doi: 10.1074/jbc.273.4.2312. [DOI] [PubMed] [Google Scholar]
- 18.Tsirka S. E., Rogove A. D., Bugge T. H., Degen J. L., Strickland S. J. Neurosci. 1997;17:543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzwonek J., Rylski M., Kaczmarek L. FEBS Lett. 2004;567:129–135. doi: 10.1016/j.febslet.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 20.Iversen L. L., Jessell T., Kanazawa I. Nature. 1976;264:81–83. doi: 10.1038/264081a0. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu C., Yoshida S., Shibata M., Kato K., Momota Y., Matsumoto K., Shiosaka T., Midorikawa R., Kamachi T., Kawabe A., et al. J. Biol. Chem. 1998;273:11189–11196. doi: 10.1074/jbc.273.18.11189. [DOI] [PubMed] [Google Scholar]
- 22.Fahnestock M., Michalski B., Xu B., Coughlin M. D. Mol. Cell Neurosci. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- 23.Grimes M. L., Zhou J., Beattie E. C., Yuen E. C., Hall D. E., Valletta J. S., Topp K. S., LaVail J. H., Bunnett N. W., Mobley W. C. J. Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szklarczyk A., Lapinska J., Rylski M., McKay R. D., Kaczmarek L. J. Neurosci. 2002;22:920–930. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright J. W., Masino A. J., Reichert J. R., Turner G. D., Meighan S. E., Meighan P. C., Harding J. W. Brain Res. 2003;963:252–261. doi: 10.1016/s0006-8993(02)04036-2. [DOI] [PubMed] [Google Scholar]
- 26.Newton S. S., Collier E. F., Hunsberger J., Adams D., Terwilliger R., Selvanayagam E., Duman R. S. J. Neurosci. 2003;23:10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivera S., Tremblay E., Timsit S., Canals O., Ben Ari Y., Khrestchatisky M. J. Neurosci. 1997;17:4223–4235. doi: 10.1523/JNEUROSCI.17-11-04223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong H., Tombor B., Albert R., Oltvai Z. N., Barabasi A. L. Nature. 2000;407:651–654. doi: 10.1038/35036627. [DOI] [PubMed] [Google Scholar]
- 29.Iannone F., Lapadula G. Aging Clin. Exp. Res. 2003;15:364–372. doi: 10.1007/BF03327357. [DOI] [PubMed] [Google Scholar]
- 30.Bruno M. A., Clarke P. B., Seltzer A., Quirion R., Burgess K., Cuello A. C., Saragovi H. U. J. Neurosci. 2004;24:8009–8018. doi: 10.1523/JNEUROSCI.1508-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J., Tsirka S. E. Brain. 2005;128:1622–1633. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.