Abstract
Several lines of evidence have indicated that the establishment of long-term memory requires protein synthesis, including the synthesis of immediate-early gene products. Although the anatomical expression patterns of the c-fos gene, a transcription factor-encoding immediate-early gene, in conditioned taste aversion (CTA) are well documented, the functional roles of c-fos gene expression and Fos-mediated transcription remain to be clarified. Using the antisense oligodeoxynucleotide (AS-ODN) method in rats and gene-targeting knockout techniques in mice (c-fos−/− mice), we examined the roles of c-fos gene expression in the acquisition, retrieval, and retention of CTA. Preconditioning microinfusion of AS-ODN directed against c-fos mRNA (c-fos AS-ODN) into the parabrachial nucleus (PBN) impaired the acquisition, whereas infusion of control ODNs consisting of a randomized or inverted base order had no effect. Microinfusion of c-fos AS-ODN into either the amygdala or insular cortex did not impair the acquisition, whereas it attenuated the retention. Retrieval and subsequent retention of an acquired CTA were not disrupted by c-fos AS-ODN infusion into the PBN or amygdala. Microinfusion of another AS-ODN directed against zif268 (egr-1, krox-24, NGFI-A) mRNA into the PBN or amygdala did not affect the acquisition and retention. The genetic deficiency in c-fos−/− mice caused normal acquisition and retention. The present results suggest that the Fos-mediated gene transcription in the PBN, amygdala, or insular cortex plays critical roles in the acquisition and/or consolidation, but not the retrieval, of long-term taste memory; nevertheless, some other factors could compensate CTA mechanism when Fos-mediated transcription is not available.
Keywords: antisense oligodeoxynucleotide, c-fos knockout mice, amygdala, conditioned taste aversion, zif268
Several lines of evidence suggest that the formation of long-term memory requires up-regulation of various genes, i.e., immediate-early genes (IEGs), and synthesis of new proteins (1–4). The activated IEG products seem to be involved in the mechanism by which sensory stimuli trigger long-term changes in the control of genetic expression and modify the neuronal response to the subsequent events (5). The expression of one of the IEGs, c-fos, and the synthesis of the gene product, Fos protein, have been reported to increase in the rat brain in response to various stimuli, including stressful events (6, 7) and nonstressful manipulations (8–11). Fos regulates transcription of other genes as an AP-1 complex and is suggested to contribute to the long-term modifications in neuronal biochemistry and structure proposed to underlie learning (2, 12).
Conditioned taste aversion (CTA) is a form of learning in which a long-term gustatory memory is established whereby animals avoid ingesting a flavored substance (conditioned stimulus, CS) whose previous ingestion has been associated with visceral malaise (unconditioned stimulus, US) (13–15). In CTA, the patterns of the c-fos gene expression in the brainstem nuclei, including the parabrachial nucleus (PBN), amygdala (AMY), and insular cortex (IC) induced by a taste CS and/or a visceral US have been extensively reported (16–23). However, the functional significance of the up-regulation of c-fos gene expression and Fos protein synthesis in these brain sites remains unclear. Several studies have shown that reversible blockage of Fos protein production by the application of a specific antisense oligodeoxynucleotide (AS-ODN) against c-fos mRNA (c-fos AS-ODN) in the brain is useful for studying the function of the c-fos gene and Fos protein (7, 24–26). Here, we studied the potential roles of Fos protein in the PBN, AMY, and IC in CTA formation using the AS-ODN-mediated disruption of Fos production in vivo. Additionally, we examined the effects of the reduction of expression of another IEG, zif268 (egr-1, NGFI-A, krox-24) (27), by an AS-ODN directed against zif268 mRNA (zif AS-ODN) on the CTA formation in rats. To compare the effects with those of the acute and reversible blockage of Fos protein production, we also examined the effect of chronic deficiency of the c-fos gene on the CTA formation in mice.
Results
Effective Inhibition of c-fos Expression by Intracranial c-fos AS-ODN Infusion.
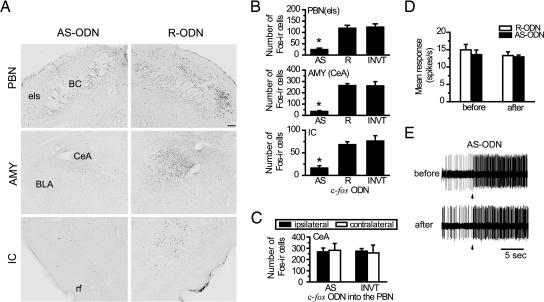
To verify that the c-fos AS-ODN used in the present study was effective in disrupting Fos synthesis, we performed immunohistochemical analysis by counting the number of Fos-like immunoreactivity (Fos-li)-positive cells in response to the US application, an i.p. injection of LiCl, in the PBN, AMY, or IC. At first, we infused c-fos AS-ODN into one side of these regions, and as controls we infused reverse (R)- or inverted (INVT)-ODN against c-fos into the other side 8 h before the US application. Fig. 1A shows representative images of US-induced Fos-li with infusion of c-fos AS- or R-ODN. A unilateral infusion of c-fos R-ODN did not reduce the Fos-li-positive cells in the external lateral subnucleus of the PBN (PBNels), central nucleus of the AMY (CeA), or caudal part of the insular and piriform cortex. In contrast, c-fos AS-ODN infused contralaterally remarkably reduced the number of Fos-li-positive cells.
Fig. 1.
Suppression of Fos protein synthesis by c-fos AS-ODN. (A) Representative photomicrographs of LiCl-induced Fos-li in the PBN, AMY, and IC after infusion of c-fos AS-ODN on one side and of control c-fos R-ODN on the other side. BC, brachium conjunctivum; BLA, basolateral nucleus of the AMY; CeA, central nucleus of the AMY; els, external lateral subnucleus of the PBN; rf, rhinal fissure. (Scale bar: 100 μm.) (B) Summary of the induction of Fos-li by i.p. injection of LiCl 8 h after infusion of c-fos ODNs. INVT, inverted ODN. ∗, P < 0.05 in comparison with groups infused with R- or INVT-ODN (n = 5–15 rats per group). (C) Induction of Fos-li in the CeA by LiCl injection after infusion of c-fos AS- or INVT-ODN into the ipsilateral PBN. The contralateral PBN received vehicle. The Fos-li in the CeA in the ipsilateral side of the AS-ODN-treated PBN was not significantly different from that in the contralateral control side or INVT-ODN-treated side (F1,12 = 0.082, P = 0.77). (D) Mean magnitudes of unit responses (n = 4) in the PBN before and after ODN infusion. (E) Unitary responses to 0.2 M NaCl in the PBN before and after c-fos AS-ODN infusion into the same PBN.
As shown in Fig. 1B, infusion of c-fos AS-ODN significantly (P < 0.05) reduced the Fos-li-positive cells in the PBNels, CeA, and IC in comparison with those in the regions infused with c-fos R- or INVT-ODN. Next, we examined whether c-fos AS-ODN caused a long-term reduction of Fos expression and found that no change in the number of Fos-li-positive cells was observed when c-fos AS-ODN was injected 24 h before the US administration (data not shown).
To examine the effects of c-fos AS-ODN on the synaptic transmission from the PBN to the AMY in response to the LiCl-induced visceral stimulation, we compared the number of Fos-li-positive cells in the CeA when c-fos AS- or INVT-ODN was infused into the PBN, which sends visceral signals to the ipsilateral CeA. As shown in Fig. 1C, the number of LiCl-induced Fos-li cells in the CeA was not affected when the c-fos AS- or INVT-ODN was injected into one side of the PBN; i.e., no differences in the Fos-li-positive cells were found in the ipsilateral nor contralateral AMY to the AS-ODN-infused PBN. The mean magnitudes of responses of PBN neurons recorded for 15 s after onset of stimulation with 0.2 M NaCl before and after c-fos AS- or R-ODN infusion in the PBN were essentially the same (Fig. 1D). Representative responses of two different PBN neurons to 0.2 M NaCl before and after c-fos AS-ODN infusion are shown in Fig. 1E.
Effects of c-fos AS-ODN Infusion into the PBN, AMY, and/or IC on the Acquisition and Retention of CTA.
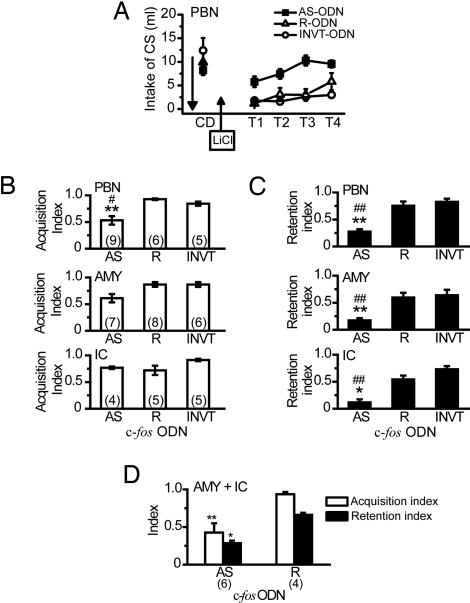
We examined the effects of c-fos AS-ODN on the acquisition and retention of CTA. Eight hours after bilateral infusion of c-fos AS-, R-, or INVT-ODN into the PBN, AMY, or IC, each rat received a pairing of the CS (saccharin) ingestion and US (LiCl) i.p. injection. Fig. 2A shows the daily intake of the CS during the CTA paradigm in rats with infusion of each c-fos ODN into the bilateral PBN before the conditioning. Concerning the amount of intake of the CS among three ODN groups, two-way ANOVA with repeated measure revealed significant main effects of ODN (F2,17 = 10.62, P < 0.001) and Trial (F4,68 = 17.35, P < 0.001), and a significant ODN × trial interaction (F8,68 = 5.13, P < 0.0001). Post hoc analysis revealed significant (P < 0.01) differences between intakes during tests of rats infused with c-fos AS-ODN and those infused with c-fos R- or INVT-ODN, implying that c-fos AS-ODN infused into the PBN disrupted CTA formation in comparison with either control ODN.
Fig. 2.
Assessment of the requirement for Fos protein in the acquisition and retention of CTA. (A) Mean volume of intake of the CS for 20 min in rats with infusion of c-fos AS-, R-, or INVT-ODN into the bilateral PBN on the conditioning day (CD) and during the subsequent 4 test days (from T1 to T4). Bilateral infusion of c-fos AS-ODN prevented the learned reduction of the CS intake during tests (ODN: F2,17 = 10.62, P < 0.001; two-way interaction of ODN and trial, F8,68 = 5.13, P < 0.0001). Intakes of the CS in rats infused with c-fos AS-ODN on test 1 (T1) were not significantly lower than those on CD (P = 0.89), whereas CS intakes on T1 in either control group were significantly reduced compared with those on CD (P < 0.001). Total intakes of the CS during tests in the c-fos AS-ODN group were significantly larger than those in either control group (Tukey’s test, P < 0.01). The downward arrow indicates intracranial microinfusion. (B and C) Quantification of the effects of infusion of ODNs into the PBN, AMY, or IC on acquisition and retention using the acquisition and retention indices. Overall three-way interaction (site × ODN × index) was not significant (F4,92 = 1.06, P = 0.38), but two-way interactions of ODN × index (F2,92 = 8.26, P < 0.001) and site × index (F2,92 = 3.62, P < 0.05) were significant. ANOVA and post hoc analysis revealed that significant deficits in retention (indicated by lower retention indices, C) with infusions of c-fos AS-ODN into the PBN, AMY, or IC, and significant impairment of acquisition (B) by infusion of c-fos AS-ODN into the PBN, but not into the AMY or IC, were observed. ∗, P < 0.05; ∗∗, P < 0.01 in comparison with the R-ODN group. #, P < 0.05, ##, P < 0.01 in comparison with the control INVT-ODN group. (D) Combined infusion of c-fos AS-ODN into both the AMY and IC. Significant deficits (ODN: F1,16 = 26.35, P < 0.001) in the formation of CTA were observed in the AS-ODN group, as indicated by both lower acquisition and retention indices, but not in the R-ODN group. ∗, P < 0.05, ∗∗, P < 0.01 vs. the R-ODN group, post hoc analysis between groups. All values are means + SEM. The numbers of animals used are shown in parentheses.
The rats that had previously been infused with c-fos AS-ODN into the PBN acquired a strong aversion [mean acquisition index = 0.86] to another CS (0.2 M NaCl) when they received no infusion of c-fos AS-ODN.
To compare the effectiveness of each ODN infused before conditioning on the acquisition of CTA, the acquisition indices (see ref. 28 and Materials and Methods) when different ODNs were infused into the bilateral PBN, AMY, or IC and the results are shown in Fig. 2B. Infusion of c-fos AS-ODN into the PBN significantly (P < 0.05, ANOVA) reduced the acquisition index or attenuated the acquisition, whereas that into the AMY or IC did not affect the acquisition indices.
To examine the effects of different ODNs infused before conditioning on the retention of CTA, the retention indices (see ref. 28 and Materials and Methods) were compared. As shown in Fig. 2C, intracranial c-fos AS-ODN infusion into the PBN, AMY, or IC significantly (P < 0.01) reduced the retention indices, whereas the corresponding control c-fos R- or INVT-ODN infusion did not. The data described above indicate that the acute reduction of Fos synthesis in the AMY, or IC attenuates the retention, but not acquisition, of CTA. However, as illustrated in Fig. 2D, the simultaneous infusions of c-fos AS-ODN into both the bilateral AMY and IC led to significant impairment of both the acquisition and retention of CTA compared with those obtained with c-fos R-ODN, as indicated by significantly (P < 0.01) smaller acquisition and retention indices.
Lack of Disruptive Effect of c-fos AS-ODN Infusion into the PBN or AMY on the Acquired CTA.
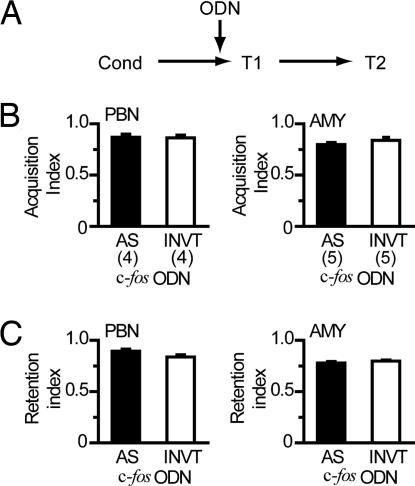
The effects of c-fos AS-ODN on the acquired CTA were examined. Rats were conditioned with a single pairing of the CS and US and were then divided into four groups with combinations of bilateral infusions of c-fos AS- or INVT-ODN into the PBN or AMY before T1 (Fig. 3A). As shown in Fig. 3B, bilateral infusions of c-fos AS-ODN into the PBN or AMY before the test did not disrupt the expression of learned aversion to the CS. In addition, as shown in Fig. 3C, there was no impairment of the subsequent retention on the second test (T2) day in these animals, suggesting that the retrieval and expression of CTA memory after its acquisition is not dependent on the Fos-mediated cellular process.
Fig. 3.
Assessment of the requirement of Fos protein for the retrieval and subsequent retention of CTA. (A) Experimental design. Each ODN was bilaterally infused into the PBN or AMY 8 h before the first test (T1) after conditioning (Cond). (B) Summary of CTA retrieval after infusion of ODNs into the PBN or AMY 8 h before the first test (day 3). No impairment of the retrieval was observed after infusion of c-fos AS-ODN, as well as INVT-ODN, as indicated by high values of acquisition index (ANOVA, P > 0.05). No significant main effects either of site (F1,14 = 0.07, P = 0.80) and ODN (F1,14 = 0.16, P = 0.69) or of interaction (F1,14 = 0.63, P = 0.44) were observed. (C) Infusion of c-fos AS-ODN into the PBN or AMY did not impair the subsequent retention on T2 (day 4), as indicated by high values of retention index (ANOVA, P > 0.05). The numbers of animals used are shown in parentheses. In this particular experiment, the retention index was calculated on the basis of CS intake only on T2.
Lack of Disruption of CTA Induced by AS-ODN for Another IEG, zif268.
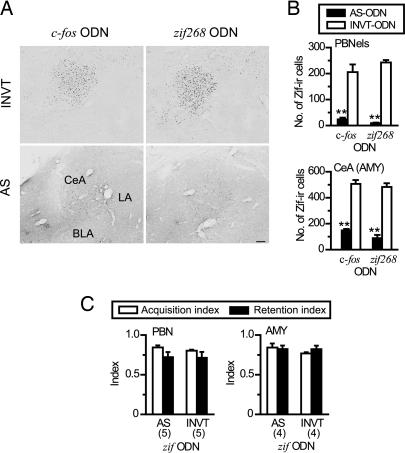
It is suggested that the expression of Zif is reduced by c-fos AS-ODN (29), and zif268 knockout (KO) mice show a deficit in acquiring CTA (27). The interaction of the expressions of c-fos and zif268 and the effect of disruption of zif268 expression on the acquisition of CTA, however, remain to be clarified. To address these issues, additional immunohistochemical and behavioral analyses were performed with in vivo antisense technology directed against zif268 mRNA. As expected, the Zif-immunoreactive cells in the CeA were significantly decreased by infusion of the AS-ODN directed against zif268 mRNA (zif AS-ODN) compared with those with zif INVT-ODN. Fig. 4A shows the effects of c-fos and zif AS-ODN on the expression of the Zif synthesis in response to an i.p. injection of LiCl. The number of Zif-immunoreactive-positive cells in the CeA was also remarkably reduced by the c-fos AS-ODN infusion into the unilateral AMY in comparison with that in the contralateral AMY infused with c-fos INVT-ODN as a control.
Fig. 4.
Suppression of Zif protein synthesis did not impair CTA formation. (A) INV-ODN against c-fos or zif268 did not block the expression of Zif synthesis induced by i.p. injection of LiCl in the AMY (Upper), whereas both c-fos AS-ODN and zif AS-ODN suppressed the Zif protein synthesis (Lower). (Scale bar: 100 μm.) (B) The expression of the zif268 gene in the PBN and AMY was significantly blocked by infusion of c-fos AS-ODN (filled bar) on one side, but not by INVT-ODN (open bar) on the other side (PBNels, ODN: F1,16 = 174.09, ∗∗, P < 0.001; CeA (AMY), ODN: F1,23 = 247.49, ∗∗, P < 0.001). PBNels, external lateral subnucleus of the PBN; CeA, central nucleus of the AMY. (C) Acute blockage of zif268 gene expression in the PBN or AMY by infusion of zif AS-ODN did not affect CTA memory formation, as indicated by high values of acquisition (open bar) and retention (filled bar) indices (PBN, ODN: F1,16 = 0.226, P = 0.64; AMY, ODN: F1,12 = 0.863, P = 0.371). ∗∗, P < 0.001 vs. corresponding INVT-ODN group, post hoc analysis between groups. The numbers of animals used are shown in parentheses.
As summarized in Fig. 4B, significant reduction of Zif immunoreactivity in the PBN (P < 0.05, ANOVA) and CeA (P < 0.01, ANOVA) was seen when the c-fos, as well as zif AS-ODNs, was infused, whereas the control INVT-ODNs induced no reduction. These results suggest the possibility that the disruptive effects of the c-fos AS-ODN on CTA were caused by suppression of zif268 expression alone or by combined suppression of both c-fos and zif268 expressions.
To clarify the contribution of zif268 gene expression and Zif protein to the CTA formation, we examined the effect of bilateral infusion of zif AS-ODN into the PBN or AMY on CTA memory. Unlike infusion of the c-fos AS-ODN, infusion of zif AS-ODN induced no disruptive effect on the formation of CTA, as indicated by the high scores of acquisition and retention indices (Fig. 4C), showing that suppression of Zif protein synthesis in the PBN and AMY does not impair the acquisition or retention of CTA.
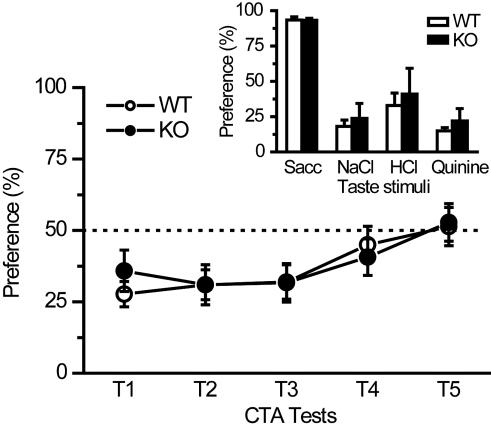
Unimpaired Acquisition of CTA in Chronic c-fos Gene-Deficient Mice.
Naive c-fos gene KO mice (30) showed normal preference and aversion behavior to the four basic tastes as compared with control WT mice (Fig. 5Inset). Fig. 5 shows the formation of CTA as seen by two-bottle preference ratios in KO and WT mice. The preference ratios in conditioned c-fos KO mice during 1–4 test days were <50%, which were similar to those in the control WT littermate mice. Two-way ANOVA with repeated measure did not reveal any significant main effect of genotype (WT, KO) (F1,21 = 0.022, P = 0.88), nor did two-way interaction of group × day (F4,84 = 0.43, P = 0.79). ANOVA revealed a main effect of time (F4,84 = 7.20, P = 0.000049 < 0.0001). These results suggest that there is no deficit of CTA formation in c-fos KO mice.
Fig. 5.
Chronic genetic deficiency of c-fos gene did not impair CTA formation in mice. Mutant mice with c-fos gene deficiency (c-fos KO mice, n = 10, filled circles) showed normal acquisition and retention of CTA compared with WT (n = 13, open circles) littermate mice. (Inset) KO mice (n = 2–6, filled bar) showed normal taste preference and aversion to four basic taste stimuli compared with WT mice (n = 3–8, open bar). Sacc, sodium saccharin.
Discussion
Neurons in the PBN, the second-order gustatory relay station in the rat central gustatory pathway, are known to induce c-fos expression in response to gustatory and visceral stimuli (16, 22, 23, 31, 32). The present study showed that PBN neuronal responses at least at the early phase (15 s) of taste stimulation were not altered after the acute blockage of c-fos mRNA translation (Fos protein synthesis) by AS-ODN within the PBN, and that the same treatment did not affect the expression of LiCl-induced Fos-li-positive cells in the CeA. Additionally, the infusion of c-fos AS- or INVT-ODN into the PBN or AMY before the test caused no disruption in the expression of CTA memory. These results suggest that the c-fos AS-ODN used in the present study had no apparent disruptive effects on synaptic transmission and cellular excitability in the PBN and AMY (33).
The PBN is suggested to be involved in the acquisition of CTA (14, 22, 34). The present results showed that the loss of Fos-mediated signaling in the PBN during the conditioning weakened the acquisition of CTA, resulting in the attenuated retention of CTA. This finding suggests that Fos-mediated gene transcription in the PBN plays an important role in cellular plasticity underlying the acquisition of taste memory. There remains a possibility, however, that neuronal responses of the PBN to taste as well as to LiCl might become less maintained; i.e., long-term, but not short-term, signal transmission might be disrupted after the AS-ODN administration, leading to weak CS–US association in the AMY and/or IC. c-fos AS-ODN-mediated disruption may be transient and reversible and may not induce long-term secondary effects because the rats that had been infused with c-fos AS-ODN into the PBN could acquire CTA to NaCl, another taste stimulus.
In contrast, the acute blockage of Fos synthesis by c-fos AS-ODN in either the AMY or IC was not sufficient to impair acquisition, whereas the simultaneous blockage of Fos synthesis in both these structures did impair the acquisition. These results indicate that Fos-mediated signaling in at least one of them is sufficient for the acquisition as far as the PBN is intact. Considering the implication (18, 19) that the AMY and IC independently affect the brainstem function to induce c-fos expression related with CTA, either one of the descending pathways from the AMY or IC to the brainstem, including the PBN, may be sufficient in the process of CTA formation. On the other hand, the retention of long-term CTA memory may be established by intact Fos-mediated signaling pathways in both the AMY and IC because infusion of c-fos AS-ODN into either or both of these areas impaired the retention. Lamprecht et al. (35) also showed that inhibition of cAMP response element-binding protein (CREB), one of the regulatory transcriptional factors for c-fos gene expression, in the AMY with in vivo AS-ODN technology disrupted the retention, but not the acquisition, of CTA. In line with these results, Bermudez-Rattoni and colleagues (36, 37) have suggested that positive interaction between the basolateral AMY-IC enhances the strength of long-term CTA memory.
In contrast to zif268 KO mice (27), the present study showed that c-fos KO mice did acquire CTA normally, indicating that there are some differences between the roles of c-fos and zif268 in CTA formation. It seems possible that there are redundant signaling pathways that compensate for chronic dysfunction of c-fos, but not zif268. For example, fosB or fra-1 (fos-l1), fos family genes, compensates for the function of c-fos gene in developmental, cellular, and/or behavioral function in c-fos KO mice (38–41). Similarly, stress-induced cellular responses have been suggested to be compensated by other fos family or jun family genes (42). Acute reduction of c-fos expression by in vivo AS-ODN is so rapid and transient that the possible compensatory mechanisms may not be fully prepared or activated to establish long-term cellular changes for acquisition and retention. Taken together, genetic c-fos deficiency might induce various changes in the molecular, cellular, anatomical, and physiological aspects of central nervous system, including CTA neural substrates. The possible changes might cause differences of neural mechanisms of CTA between mice lacking c-fos gene expression and rat infused with c-fos AS-ODN.
Concerning the cellular processes related to c-fos expression, previous studies have reported finding mutual regulation between c-fos and CREB gene expression (12, 43) and involvement of amygdaloid CREB protein synthesis in long-term but not short-term memory of CTA (35). Our data also showed that suppression of Fos synthesis in the AMY by c-fos AS-ODN impaired the long-term retention, but not the acquisition, of CTA. Mutual activation of the c-fos and CREB genes might mediate plasticity-related gene expression to induce the long-term cellular changes underlying CTA formation. In addition, there is a possibility that the CREB-dependent signal cascades are part of the compensatory pathways contributing to the normal acquisition of CTA in c-fos KO mice.
In conclusion, the expression of the c-fos, but not the zif268, gene in the gustatory and visceral neuroaxis may play an important role in the cellular plasticity for acquisition and/or consolidation, but not for retrieval, of the aversive long-term gustatory memory.
Materials and Methods
Animals and Surgery.
For the AS-ODN study, male Wistar rats (300–350 g at the beginning of the experiment) were used. Rats were housed individually in a temperature-controlled (22°C) and light-controlled (12-h light/12-h dark) room and were provided food and water ad libitum except when experimental requirements dictated otherwise. They were habituated to laboratory conditions for ≈1 week before surgery. Mice lacking the c-fos gene were used as homozygous c-fos −/− mice (c-fos KO, c-fos−/−) (30), and their WT littermate mice were used as controls. The study was approved by the animal welfare committee of our institute, and all animals were treated in accordance with the Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health, 1985) and Guiding Principles for the Care and Use of Animals in the Field of the Physiological Sciences (Physiological Society of Japan, 2003). All possible efforts were made to minimize the discomfort of the animals and the number of animals used.
ODN Infusion Procedure.
The sequences of c-fos AS-ODN, R-ODN, and INVT-ODN were as follows: AS-ODN, 5′-GAACATCATGGTCGT-3′; R-ODN, 5′-GTACCAATCGGGATT-3′; and INVT-ODN, 5′-TGCTGGTACTACAAG-3′ (44). The sequences of zif AS-ODN and INVT-ODN were as follows: AS-ODN, 5′-GGTAGTTGTCCATGGTGG-3′; and INVT-ODN, 5′-GGTGGTACCTGTTGATGG-3′ (45). Each ODN was chimeric phosphorothioate/phosphodiester “end-capped” ODN (EC-ODN) that had phosphorothioate linkages on the three terminal bases of both the 5′ and 3′ ends and phosphodiester internal linkages (46, 47). The 5′ end base was also biotinylated. HPLC-purified ODNs (Kurabou, Osaka) were dissolved in PBS (pH 7.4). Rats were anesthetized with Nembutal (50 mg/kg, i.p.) and immobilized stereotaxically for infusion into the brain. The AS-ODN directed against c-fos or zif268 mRNA loaded in a glass micropipette (tip outer diameter, 50–60 μm) was infused unilaterally into the PBN [anteroposterior (AP), −11.5 mm; mediolateral (ML), 1.4–1.7 mm; and dorsoventral (DV), 6.8–7.0 mm from bregma, angled 20° caudally in the parasagittal plane], AMY (the border between the CeA and BLA; AP, −2.8 mm; ML, 4.5–4.7 mm; and DV, 8.4 mm from bregma), or IC (AP, −1.0 mm; ML, 4.9–5.1 mm; and DV, 4.5–5.0 mm from bregma). The micropipette was connected to a 10-μl Hamilton microsyringe by using PE10 tubing filled with mineral oil. R-ODN and INVT-ODN sequences were infused as controls into the counterpart site of the contralateral side for c-fos-like immunohistochemistry. Each ODN solution (1.0, 2.5, or 5 nmol/μl) was infused at the rate of 0.1 μl/min until a total volume of 0.25 (PBN) or 0.5 μl (AMY or insular cortex) was reached. The pipette remained inserted for another 3 min before being withdrawn.
Verification of Effects of AS-ODN by Immunohistochemical and Electrophysiological Assays.
After 8 h of recovery from the surgery for the intracranial infusions of AS-, R-, or INVT-ODN against either c-fos or zif-268 mRNA, rats received an i.p. injection of 0.15 M LiCl (US, 2% volume of body weight) to examine the extent of suppression of the expression of each gene. Ninety minutes later, animals were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). For the study of the long-term effect of AS-ODN, other animals received a US administration 24 h after the microinfusion of ODN and were perfused in the same manner. The brain was removed, immersed in 30% sucrose in 0.1 M PB, and sectioned at a thickness of 50 μm on a freezing microtome.
Every third section was incubated with anti-Fos-like (catalog no. sc-253) or anti-Zif268 (Egr-1; catalog no. sc-189) rabbit antiserum (Santa Cruz Biotechnology) and processed with the labeled streptavidin–biotin (LSAB; DAKO) method by using diaminobenzidine-nickel staining. To study the effect of c-fos AS-ODN on zif-268 expression, every other section of the PBN or AMY in an animal was alternatively incubated with the same anti-Fos-like or -Zif268 rabbit antiserum. Photomicrographs of the Fos-li or Zif immunoreactivity were captured by using an Olympus light microscope equipped with a charge-coupled device (CCD) camera interfaced to a personal computer. Fos-li- or Zif-immunoreactive-positive cells were counted with the aid of image analysis software (winroof 3.5; Mitani Corp., Osaka, Japan). Five to seven sections for each site per animal were used to count these IEG-positive cells, and the average number of IEG-immunoreactive cells was calculated. The number of immunoreactive cells in each structure was subjected to one-way ANOVA among groups followed by post hoc Tukey’s honestly significant difference (HSD) test.
To examine the effect of c-fos AS-ODN on synaptic transmission in the gustatory neuroaxis, taste responses to 0.2 M NaCl in the PBN neurons (n = 4 each for each ODN and recording session) were compared before and after infusions of c-fos AS- or R-ODN into the ipsilateral PBN, using the unit recording technique (48).
Behavioral Study.
For the CTA study, the rats were placed on 20-h water-deprivation training. They were habituated to drink distilled water (DW) for 20 min of exposure each day at 1300, and their intake was measured until the intake level was stabilized. On the conditioning day (day 1), each rat was presented with 5 mM sodium-saccharin (saccharin) as the CS for 20 min and received an i.p. injection of 0.15 M LiCl (2% of body weight) as the US 10 min after the CS exposure. The rats were allowed to drink DW for ≈3 h after their recovery from sickness and deprived of water again for 20 h. On the next day (day 2), DW was presented to rats and was withdrawn at 1700. For all experiments, intakes of the CS were measured daily during 4 successive days (T1, T2, T3, and T4) under the same water-deprivation conditions from the first test day (T1 or day 3). To examine the effects of each ODN on the acquisition of CTA, rats received the intracranial infusions 8 h before the CS–US pairing on the conditioning day. Bilateral infusions of ODN (1.0, 2.5, or 5 nmol/μl) were made into the PBN, AMY, or IC with the above-mentioned surgical procedure by using the stereotaxic method.
To study the effects of each ODN on the retrieval and subsequent retention, the conditioned rats were infused with ODN into the PBN or AMY 8 h before the first reexposure to the CS on day 3 with the same surgical procedure, and their intake of the CS was measured on day 3 (T1) and subsequently on day 4 (T2). Animals that received infusions of c-fos AS-ODN into the PBN were examined for another CTA formation by using 0.2 M NaCl as a new CS after saccharin CTA tests.
On the basis of volumes of preconditioning water intake and of CS intake in the following 4 test days, we calculated the following two indices (28) as the parameters indicating how well the animals “acquired” taste aversions and how well the acquired aversions were “retained”: acquisition index = 1 − (intake of the CS on T1/mean intake of water during 4 preconditioning training days); and retention index = 1 − (total intake of the CS during T2, T3 and T4/3 × mean intake of water during 4 preconditioning training days). The larger the value of acquisition and retention indices, the better CTAs are acquired and retained, respectively.
The CS intake and data for each index were subjected to three-way ANOVA. When an ANOVA revealed a significant main effect or interaction, post hoc multiple comparison was conducted by using Tukey’s HSD test.
For the CTA study in mice lacking the c-fos gene (c-fos KO mice) (30), 10 KO and 13 WT mice were used. They were deprived of water for 18 h and were trained to two bottles during the daily 6 h. On the first test day (day 1), each animal was given access to 0.005 M saccharin as a CS and then given an i.p. injection of 0.15 M LiCl (2 ml/100 g body weight) as a US to induce gastrointestinal malaise. On the next day (day 2), each mouse received the same two-bottle drinking of DW. The two-bottle preference test for 6 h was performed for the subsequent 5 days from day 3. The preference ratio was calculated as percentage (= 100 × intake volume of test solution/sum of intake volume of test solution and DW). To examine the basic capacity of taste-guided ingestive behavior in KO mice, 48-h two-bottle preference tests using 5 mM sodium saccharin (Sacc), 0.15 M NaCl, 1 mM HCl, and 0.1 mM quinine hydrochloride were performed.
Acknowledgments
This research was supported by 21st Century Centers of Excellence (COE) Program, Scientific Research Grant-in-Aid 17390494 (to T.Y.) and Young Scientist (B) Grant-in-Aid 16700289 (to Y.Y.); by grants from the Japan Society for the Promotion of Science; and by Sumitomo Foundation Grant 990186 (to Y.Y.).
Abbreviations
- AMY
amygdala
- AS-ODN
antisense oligodeoxynucleotide
- CeA
central nucleus of the amygdala
- CS
conditioned stimulus
- CTA
conditioned taste aversion
- IC
insular cortex
- IEG
immediate-early gene
- INVT-ODN
inverted oligodeoxynucleotide
- PBN
parabrachial nucleus
- R-ODN
randomized oligodeoxynucleotide
- US
unconditioned stimulus
- Fos-li
Fos-like immunoreactivity
- KO
knockout
- CREB
cAMP response element-binding protein
- DW
distilled water
- PBNels
external lateral subnucleus of the PBN.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Guzowski J. F. Hippocampus. 2002;12:86–104. doi: 10.1002/hipo.10010. [DOI] [PubMed] [Google Scholar]
- 2.Dragunow M. Behav. Genet. 1996;26:293–299. doi: 10.1007/BF02359385. [DOI] [PubMed] [Google Scholar]
- 3.Pedreira M. E., Maldonado H. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- 4.McGaugh J. L. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 5.Bailey C. H., Bartsch D., Kandel E. R. Proc. Natl. Acad. Sci. USA. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melia K. R., Ryabinin A. E., Schroeder R., Bloom F. E., Wilson M. C. J. Neurosci. 1994;14:5929–5938. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrow B. A., Elsworth J. D., Inglis F. M., Roth R. H. J. Neurosci. 1999;19:5666–5673. doi: 10.1523/JNEUROSCI.19-13-05666.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campeau S., Hayward M. D., Hope B. T., Rosen J. B., Nestler E. J., Davis M. Brain Res. 1991;565:349–352. doi: 10.1016/0006-8993(91)91669-r. [DOI] [PubMed] [Google Scholar]
- 9.Graybiel A. M., Moratalla R., Robertson H. A. Proc. Natl. Acad. Sci. USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mack K. J., Mack P. A. Mol. Brain Res. 1992;12:141–147. doi: 10.1016/0169-328x(92)90077-o. [DOI] [PubMed] [Google Scholar]
- 11.Sharp F. R., Sagar S. M., Hicks K., Lowenstein D., Hisanaga K. J. Neurosci. 1991;11:2321–2331. doi: 10.1523/JNEUROSCI.11-08-02321.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanyal S., Sandstrom D. J., Hoeffer C. A., Ramaswami M. Nature. 2002;416:870–874. doi: 10.1038/416870a. [DOI] [PubMed] [Google Scholar]
- 13.Garcia J., Kimeldorf D. J., Koelling R. A. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- 14.Bures J., Bermudez-Rattoni F., Yamamoto T. Conditioned Taste Aversion: Memory of a Special Kind. Oxford: Oxford Univ. Press; 1998. [Google Scholar]
- 15.Bermudez-Rattoni F. Nat. Rev. Neurosci. 2004;5:209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- 16.Houpt T. A., Philopena J. M., Joh T. H., Smith G. P. Learn. Mem. 1996;3:25–30. doi: 10.1101/lm.3.1.25. [DOI] [PubMed] [Google Scholar]
- 17.Sakai N., Yamamoto T. NeuroReport. 1997;8:2215–2220. doi: 10.1097/00001756-199707070-00025. [DOI] [PubMed] [Google Scholar]
- 18.Schafe G. E., Bernstein I. L. Brain Res. 1996;741:109–116. doi: 10.1016/s0006-8993(96)00906-7. [DOI] [PubMed] [Google Scholar]
- 19.Schafe G. E., Bernstein I. L. Brain Res. 1998;800:40–47. doi: 10.1016/s0006-8993(98)00492-2. [DOI] [PubMed] [Google Scholar]
- 20.Swank M. W., Schafe G. E., Bernstein I. L. Brain Res. 1995;673:251–261. doi: 10.1016/0006-8993(94)01421-d. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto T., Sako N., Sakai N., Iwafune A. Neurosci. Lett. 1997;226:127–130. doi: 10.1016/s0304-3940(97)00265-6. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T., Shimura T., Sakai N., Ozaki N. Physiol. Behav. 1994;56:1197–1202. doi: 10.1016/0031-9384(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T., Sawa K. Brain Res. 2000;866:144–151. doi: 10.1016/s0006-8993(00)02242-3. [DOI] [PubMed] [Google Scholar]
- 24.He J., Yamada K., Nabeshima T. Neuropsychopharmacology. 2002;26:259–268. doi: 10.1016/S0893-133X(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 25.Lamprecht R., Dudai Y. Learn. Mem. 1996;3:31–41. doi: 10.1101/lm.3.1.31. [DOI] [PubMed] [Google Scholar]
- 26.Countryman R. A., Kaban N. L., Colombo P. J. Neurobiol. Learn. Mem. 2005;84:175–183. doi: 10.1016/j.nlm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Jones M. W., Errington M. L., French P. J., Fine A., Bliss T. V., Garel S., Charnay P., Bozon B., Laroche S., Davis S. Nat. Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto T., Fujimoto Y., Shimura T., Sakai N. Neurosci. Res. 1995;22:31–49. doi: 10.1016/0168-0102(95)00875-t. [DOI] [PubMed] [Google Scholar]
- 29.Dragunow M., Tse C., Glass M., Lawlor P. Cell. Mol. Neurobiol. 1994;14:395–405. doi: 10.1007/BF02088826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z. Q., Ovitt C., Grigoriadis A. E., Mohle-Steinlein U., Ruther U., Wagner E. F. Nature. 1992;360:741–745. doi: 10.1038/360741a0. [DOI] [PubMed] [Google Scholar]
- 31.Karimnamazi H., Travers S. P., Travers J. B. Brain Res. 2002;957:193–206. doi: 10.1016/s0006-8993(02)03438-8. [DOI] [PubMed] [Google Scholar]
- 32.King C. T., Deyrup L. D., Dodson S. E., Galvin K. E., Garcea M., Spector A. C. J. Comp. Neurol. 2003;465:296–308. doi: 10.1002/cne.10851. [DOI] [PubMed] [Google Scholar]
- 33.Abraham W. C., Logan B., Thompson V. L., Williams J. M., Tate W. P. Neuropharmacology. 1997;36:345–352. doi: 10.1016/s0028-3908(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 34.Ivanova S. F., Bures J. Physiol. Behav. 1990;48:543–549. doi: 10.1016/0031-9384(90)90297-h. [DOI] [PubMed] [Google Scholar]
- 35.Lamprecht R., Hazvi S., Dudai Y. J. Neurosci. 1997;17:8443–8450. doi: 10.1523/JNEUROSCI.17-21-08443.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bermudez-Rattoni F., Ramirez-Lugo L., Gutierrez R., Miranda M. I. Cell. Mol. Neurobiol. 2004;24:25–36. doi: 10.1023/B:CEMN.0000012722.45805.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferreira G., Miranda M. I., De la Cruz V., Rodriguez-Ortiz C. J., Bermudez-Rattoni F. Eur. J. Neurosci. 2005;22:2596–2604. doi: 10.1111/j.1460-9568.2005.04440.x. [DOI] [PubMed] [Google Scholar]
- 38.Gruda M. C., van Amsterdam J., Rizzo C. A., Durham S. K., Lira S., Bravo R. Oncogene. 1996;12:2177–2185. [PubMed] [Google Scholar]
- 39.Fleischmann A., Hafezi F., Elliott C., Reme C. E., Ruther U., Wagner E. F. Genes Dev. 2000;14:2695–2700. doi: 10.1101/gad.187900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleischmann A., Hvalby O., Jensen V., Strekalova T., Zacher C., Layer L. E., Kvello A., Reschke M., Spanagel R., Sprengel R., et al. J. Neurosci. 2003;23:9116–9122. doi: 10.1523/JNEUROSCI.23-27-09116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gass P., Fleischmann A., Hvalby O., Jensen V., Zacher C., Strekalova T., Kvello A., Wagner E. F., Sprengel R. Mol. Brain Res. 2004;130:16–22. doi: 10.1016/j.molbrainres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Senba E., Ueyama T. Neurosci. Res. 1997;29:183–207. doi: 10.1016/s0168-0102(97)00095-3. [DOI] [PubMed] [Google Scholar]
- 43.Sheng M., McFadden G., Greenberg M. E. Neuron. 1990;4:571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- 44.Katsumori H., Hashimoto K., Tomitaka S., Osawa M., Minabe Y. Neurosci. Lett. 1997;225:149–152. doi: 10.1016/s0304-3940(97)00203-6. [DOI] [PubMed] [Google Scholar]
- 45.Hebb M. O., Robertson H. A. Mol. Brain Res. 1997;48:97–106. doi: 10.1016/s0169-328x(97)00086-7. [DOI] [PubMed] [Google Scholar]
- 46.Hebb M. O., Robertson H. A. Mol. Brain Res. 1997;47:223–228. doi: 10.1016/s0169-328x(97)00048-x. [DOI] [PubMed] [Google Scholar]
- 47.Guzowski J. F., McGaugh J. L. Proc. Natl. Acad. Sci. USA. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tokita K., Karadi Z., Shimura T., Yamamoto T. J. Neurophysiol. 2004;92:265–279. doi: 10.1152/jn.01090.2003. [DOI] [PubMed] [Google Scholar]