Abstract
Background
It has been shown that the contractile state of airway smooth muscle cells (SMCs) in response to agonists is determined by the frequency of Ca2+ oscillations occurring within the SMCs. Therefore, we hypothesized that the relaxation of airway SMCs induced by agents that increase cAMP results from the down-regulation or slowing of the frequency of the Ca2+ oscillations.
Methods
The effects of isoproterenol (ISO), forskolin (FSK) and 8-bromo-cAMP on the relaxation and Ca2+ signaling of airway SMCs contracted with methacholine (MCh) was investigated in murine lung slices with phase-contrast and laser scanning microscopy.
Results
All three cAMP-elevating agents simultaneously induced a reduction in the frequency of Ca2+ oscillations within the SMCs and the relaxation of contracted airways. The decrease in the Ca2+ oscillation frequency correlated with the extent of airway relaxation and was concentration-dependent. The mechanism by which cAMP reduced the frequency of the Ca2+ oscillations was investigated. Elevated cAMP did not affect the re-filling rate of the internal Ca2+ stores after emptying by repetitive exposure to 20 mM caffeine. Neither did elevated cAMP limit the Ca2+ available to stimulate contraction because an elevation of intracellular Ca2+ concentration induced by exposure to a Ca2+ ionophore (ionomycin) or by photolysis of caged-Ca2+ did not reverse the effect of cAMP. Similar results were obtained with iberiotoxin, a blocker of Ca2+-activated K+ channels, which would be expected to increase Ca2+ influx and contraction. By contrast, the photolysis of caged-IP3 in the presence of agonist, to further elevate the intracellular IP3 concentration, reversed the slowing of the frequency of the Ca2+ oscillations and relaxation of the airway induced by FSK. This result implied that the sensitivity of the IP3R to IP3 was reduced by FSK and this was supported by the reduced ability of IP3 to release Ca2+ in SMCs in the presence of FSK.
Conclusion
These results indicate that the relaxant effect of cAMP-elevating agents on airway SMCs is achieved by decreasing the Ca2+ oscillation frequency by reducing internal Ca2+ release through IP3 receptors.
Introduction
A major symptom of asthma is an excessive contraction of airway smooth muscle cells (SMCs) which results in airway hyper-reactivity. To alleviate this acute and chronic airway constriction, β-adrenergic agonists, that relax SMCs, are commonly administered [1]. Yet, despite their efficacy and wide-spread application, the signaling pathways underlying the relaxing effect of β-agonists are not fully understood.
It is generally accepted that stimulation of β-adrenergic receptors activates adenylyl cyclase (AC), via receptor associated G proteins, to increase cAMP levels to mediate SMC relaxation [2]. The role of cAMP in relaxation has been confirmed with other compounds that increase intracellular cAMP concentration ([cAMP]i), such as forskolin (FSK) that directly activates AC, theophylline that inhibits phosphodiesterase and 8-bromo-cAMP that is an analog of cAMP [3-5]. The traditional mechanism for cAMP action is via the stimulation of protein kinase A (PKA) to phosphorylate a variety of target proteins to induce airway SMCs relaxation. Alternatively, cAMP may act independently of PKA by interacting with exchange proteins (EPACs) [6]. EPACs have been found to activate PCLε to enhance IP3 induced Ca2+ release in non-smooth muscle cells [7]. However, before the specific details of cAMP-mediated signaling can be explored, it is essential to initially identify the fundamental action of cAMP on the mechanisms that regulate SMC contraction.
Airway SMC contraction is determined by the balance between phosphorylation and de-phosphorylation of the regulatory light chain of myosin (rMLC). Phosphorylation of rMLC is induced by Ca2+-calmodulin activated myosin light-chain kinase (MLCK). De-phosphorylation of rMLC is believed to be mediated by myosin light chain phosphatase (MLCP) [8]. As a result, SMC relaxation may be the result of different cellular pathways that culminate in either or both a reduction in MLCK activity and an increase in MLCP activity.
Perhaps the most direct method of relaxation is a reversal of the Ca2+ response that stimulates MLCK activity and contraction. Ca2+ signaling in airway SMCs is frequently induced by agonists such as ACh, 5-HT and ATP and consists of Ca2+ oscillations [9-11]. In our recent studies with lung slices, we found that the frequency of these Ca2+ oscillations correlated with the contractile state of the airways, a relationship that indicates the SMC tone is regulated in a frequency-modulated manner [12-14].
In previous studies with isolated tracheal SMCs, cAMP was found to modulate the frequency of Ca2+ oscillations [15,16]. However, its effect on airway contraction or the mechanism of action was not determined. In other studies with different SMC types, it has been suggested that cAMP interacts with intracellular Ca2+ signaling pathways at multiple sites [17]. These include a decrease in Ca2+ influx [3,15,16,18], particularly by the activation of large conductance Ca2+-activated K+ channels (BKCa) to hyperpolarize the membrane [19-21], an increase in Ca2+ efflux [22] or uptake by the sarcoplasmic reticulum [23,24], or an inhibition of agonist-induced IP3 formation [5,25]. All of these effects of cAMP would be expected to decrease the available intracellular Ca2+ concentration ([Ca2+]i). However, it is unknown how any of these effects would influence the frequency of Ca2+ oscillations of SMCs.
To explore the hypothesis that cAMP induces intrapulmonary airway relaxation by down-regulating Ca2+ oscillations, we examined the effect of isoproterenol (ISO) and other cAMP elevating agents (FSK and 8-bromo-cAMP) on methacholine (MCh)-induced contractility and Ca2+ signaling of airway SMCs in murine lung slices. The importance of using lung slices, instead of cultured or isolated cells, is that Ca2+ signaling and airway contractility can be measured simultaneously. We found that cAMP induced relaxation of MCh-contracted airways and reduced the frequency of the Ca2+ oscillations. In addition, we confirmed that cAMP did not affect Ca2+ influx or the refilling of depleted Ca2+ stores. By contrast, we found that an elevation of intracellular IP3 concentration ([IP3]i) reversed the effect of FSK by increasing the frequency of the Ca2+ oscillations to induce contraction. In addition, we also show that cAMP inhibits the Ca2+ release via the IP3 receptor. From these data, we suggest that airway relaxation induced by cAMP is achieved by decreasing the Ca2+ oscillation frequency of airway SMCs by inhibiting IP3-induced Ca2+ mobilization.
Materials and methods
Materials
Cell culture reagents were obtained from GIBCO/Invitrogen Corp. Oregon Green 488 BAPTA-1 AM and O-nitrophenyl EGTA AM (NP-EGTA AM) were obtained from Molecular Probes (Eugene, OR). Caged-iso-Ins(1,4,5)P3/PM was obtained from Alexis Biochemicals (San Diego, CA) and dissolved in DMSO, aliquoted and frozen at -80°C. Other reagents were obtained from Sigma-Aldrich or Calbiochem. Hanks' balanced salt solution was supplemented with 20 mM HEPES buffer (sHBSS) and adjusted to pH 7.4.
Lung slices
The preparation of lung slices has been previously described in detail [14]. Briefly, male Balb/C inbred strain mice (Charles River Breeding Labs, Needham, MA, 7–10 weeks old) were killed by intra-peritoneal injection of 0.3 ml of pentobarbital sodium (Nembutal) as approved by the IACUC of the University of Massachusetts Medical School. The trachea was exposed and cannulated. After the thoracic cavity was opened, the lung lobes were slowly inflated with a warm agarose solution (2%, 37°C, ~1.3 ml). Subsequently ~0.2 ml of air was injected to flush the agarose out of the airway into the distal alveoli. The lungs were cooled at 4°C to gel the agarose. A single lung lobe was removed and sectioned with a vibratome (model EMS-4000; Electron Microscope Sciences, EMS) into slices of ~140 μm thick starting at the lung periphery. Airways were identified by their lining of epithelial cells with beating cilia. Serial sections were collected and transferred to culture solution containing DMEM supplemented with antibiotics and anti-mycotics and NaHCO3. Slices were maintained in culture media at 37°C and 10% CO2 for up to 3 days.
Measurement of airway contraction and relaxation
Lung slices were observed in a custom-made perfusion chamber constructed from 2 cover-glasses sealed with thin strips of silicone grease (Valve lubricant and sealant, Dow Corning, Midland, MI) on a Plexiglas support. The slices were held in place with a small sheet of nylon mesh (CMN-300-B, Small Parts Inc, Miami Lakes, FL) with a small hole to view the selected airway and avoid the mesh from influencing the contraction of the airway. A gravity-driven perfusion system with a multi-tube manifold with a single output (Warner Instruments, Inc.) was used, in conjunction with valves (LFVA, Lee Company, CT) under TTL control, to exchange solutions in the chamber. The chamber volume was about 100 μl. The perfusion rate was 800 μl/min.
The lung slices were observed with an inverted microscope (IX71, Olympus) with a 10× objective and a zoom adapter. Phase-contrast images were recorded using a CCD camera (model LCL-902C, Watec America Corp, USA), a camera interface ("Picolo", Eurosys Inc., Belgium) and image acquisition software ("Video Savant"; IO industries, Inc, London, Ontario, Canada). Digital images were recorded in time-lapse (0.5 Hz) and analyzed with "NIH /Scion Image" software (Scion Corp.). The lumen area of the airway was measured by summing the number of pixels below a selected threshold grey level with respect to time. All experiments were performed at room temperature (RT, 20 ± 2°C).
Measurements of [Ca2+]i
Slices were loaded for 30 minutes at 30°C with 20 μM Oregon Green 488 BAPTA-1 AM in sHBSS containing 0.1% pluronic (Pluronic F-127; Calbiochem) and 100 μM sulfobromophthalein. Lung slices were de-esterified for another 30 minutes at 30°C in sHBSS containing 100 μM sulfobromophthalein before being placed in the perfusion chamber as described above.
Fluorescence imaging was performed using a custom-built video-rate confocal or 2-photon scanning laser microscope. Either a 488 nm beam from a diode laser or an 800 nm beam from a Ti-sapphire laser (Tsunami, Spectra-Physics, Mountain View, CA) pumped with a 5 W 525 nm diode laser (Millennia, Spectra-Physics) was scanned across the specimen with two oscillating mirrors (X- and Y-scan) through an inverted microscope (Nikon DIAPHOT 200 for confocal microscopy; Olympus IX71FVSF-2 for 2-photon microscopy). For confocal microscopy, the emitted fluorescence (>510 nm) was separated from the excitation light by a dichroic mirror, a long pass filter and a confocal aperture [26]. For 2-photon microscopy, the emitted fluorescence was separated with a dichroic mirror (670uvdclp, Chroma Technology Corp, Rockingham, VT) and a long pass filter (E700SP or E600SP-2P, Chroma Technology Corp) positioned immediately below the objective. Emitted fluorescence was detected with a photomultiplier tube (PMT, R5929, Hamamatsu USA, Bridgewater, NJ). Grayscale images were recorded at 1, 15 or 30 Hz with a frame-grabber (Raven; Bit Flow, Inc). Changes in fluorescence intensity were obtained, frame-by-frame, from selected regions of interest (ROI, ~5 × 5 pixel). A line-scan analysis was made by extracting a row of pixels from each frame and aligning them in a time sequence.
Flash photolysis of caged-Ca2+ or caged-IP3
Flash photolysis of caged-Ca2+ (NP-EGTA) or caged-IP3 was used to experimentally increase the [Ca2+]i or [IP3]i. Slices were initially loaded with Oregon Green 488 BAPTA-1 AM as described above. Subsequently, slices were incubated, at RT, with either 1 μM NP-EGTA AM (for 15 min) or 2 μM Caged-iso-Ins(1,4,5)P3/PM (for 1 hr) in sHBSS containing 0.1% pluronic and 100 μM sulfobromophthalein followed by de-esterification for 30 min in the sHBSS containing 100 μM sulfobromophthalein. The details of the flash photolysis setup have been previously described [27]. Briefly, a flash of UV light was produced from a mercury arc lamp with a mechanical shutter and focused into the microscope with a biconvex lens (focal distance 200 mm) through a bandpass filter (330 nm). The flash duration was determined by electronic control and the image acquisition software. The intensity of the flash was regulated by a neutral density filter (Optical Density, OD 0.5 ~ 1.0, transmission = log [1/OD]). The size of the area illuminated was adjusted with an iris diaphragm and measured by illuminating and monitoring the emitted fluorescence from a thin uniform layer of fura-2 salt solution (1 μM) between 2 cover glasses.
Data analysis
Results are expressed as means ± S.E. Concentration-response curves of MCh, ISO and FSK were fitted using a non-linear interactive fitting program (Microcal Origin 7.0, Microcal Software Inc, MA, USA). Statistical significance was determined by using paired student's t-test.
Results
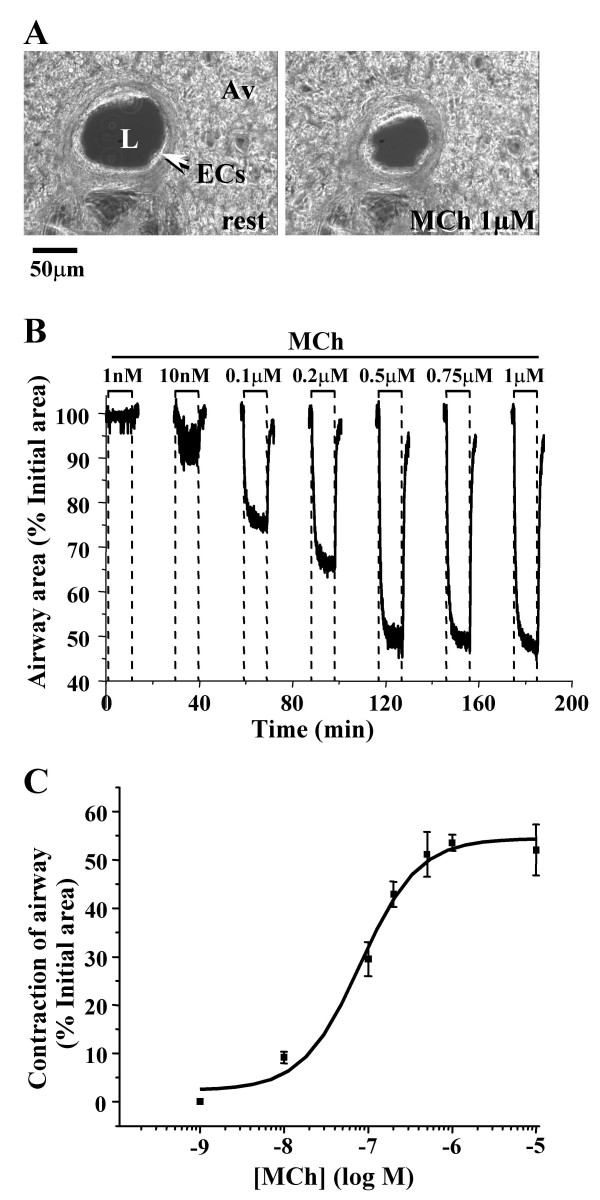
Experiments were only performed on airways that were lined with epithelial cells (ECs) showing active ciliary beating and had a lumen that was free of agarose (Fig. 1A). To assess the contractile activity of the airway SMCs, the changes in the airway area were measured with time-lapse video microscopy and image-analysis. Upon exposure to MCh, at concentrations ≥ 100 nM, the airway quickly contracted. The luminal area was reduced to, and maintained at, a smaller stable size. Although lower concentrations of MCh (10 nM) induced a reduction in the airway lumen, this change was the result of twitching rather than a sustained contraction of the SMCs (Fig. 1B). The MCh-induced airway contraction was concentration-dependent over the range of 10 to 500 nM (Fig. 1C). A maximal contraction of about 52 % was achieved when the concentration of MCh was >0.5 μM. The EC50 was 78 nM.
Figure 1.
The contractile response of airways in mouse lung slices to MCh. (A) Low magnification phase-contrast images (scale bar = 50 μm) of the same airway before and 5 minutes after stimulation with 1 μM MCh. L, airway lumen; ECs, epithelial cells; Av, alveolar tissue. (B) The relative change in the area of the airway lumen (% of the initial area) in response to MCh from 1 nM to 1μM. During the interval between each application of MCh (15 min) the airway area was not recorded but the slice was continually washed with sHBSS. (C) The concentration-dependent contractile response of airways to MCh. Each point represents 5 to 7 experiments (mean ± S.E.) in different airways from at least 3 mice. The data were fitted with a logistic function.
The relaxing effect of ISO on airways contracted by MCh
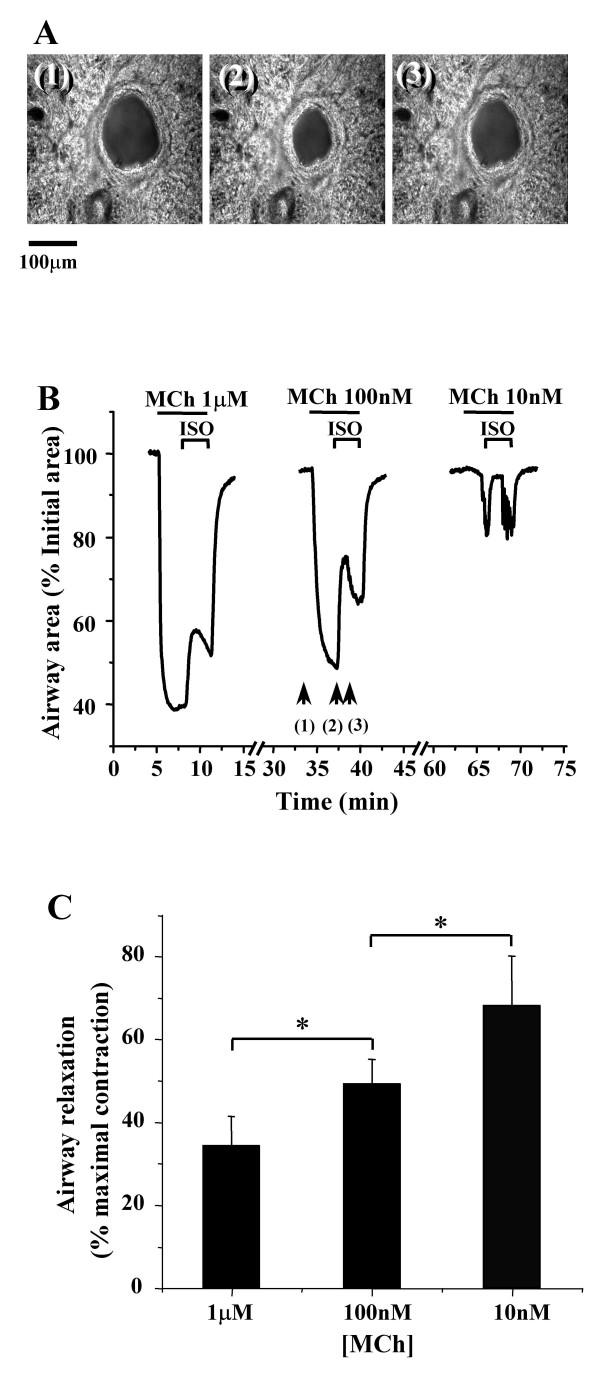
The relaxing effect of ISO on airways was established by initially contracting the airways with MCh for 3 minutes and subsequently exposing the airways to ISO in the presence of MCh. In the first set of experiments, the airways were contracted with a range of MCh concentrations (10 nM to 1 μM) and relaxed with a single concentration of ISO (10 μM). The increasing concentrations of MCh induced an increasing airway contraction and under each condition, addition of ISO induced airway relaxation during the first 2 minutes (Fig. 2A and 2B). This relaxation effect was not sustained even though the airways were continually perfused with MCh and ISO. After 2 minutes, the airways gradually re-contracted until the lung slice was washed with sHBSS at which time the airway fully relaxed. The magnitude of the initial relaxation, measured after 60 to 90 seconds of ISO exposure, decreased from ~68% to ~34% (% maximal contraction pre-exposed to ISO) as the concentrations of MCh increased from 10 nM to 1 μM (Fig. 2B and 2C). The relaxation response was significant different between MCh concentrations (n = 4 slices from 3 mice, p < 0.05). These results indicated that the efficacy of ISO at relaxing the airway is reduced by an increasing contractile stimulus.
Figure 2.
Relaxation of contracted airways by ISO. (A) A series of phase-contrast images of the same airway (scale bar = 100 μm) at times indicated by arrows in B (middle trace) showing (1) the initial state, (2) the contracted state induced by MCh (100 nM) and (3) the relaxed state induced by ISO (10 μM). (B) The changes in the area of an airway in response to ISO (10 μM) following contraction with MCh. The airway was contracted with decreasing concentrations of MCh (1μM to 10 nM) for 3 min and exposed to the same concentration of ISO (10 μM) for another 3 min. The slices were washed with sHBSS 15 minutes between each concentration of MCh. (C) Summary of the ISO-induced (10 μM) relaxation of airways contracted with different concentrations of MCh (n = 4 slices from 3 mice). The magnitude of the airway relaxation induced by ISO increased as the concentration of MCh was decreased (*, p < 0.05, comparison between two different MCh concentrations).
Because MCh can stimulate the M2 receptor and this could possibly lead to the inhibition of adenylyl cyclase (AC) to enhance the contractile response, we examined the effects of the M2 receptor antagonist, methoctramine (0.2 μM) on ISO-induced relaxation. In the presence of the M2 antagonist, airway contraction induced by MCh (200 nM) was decreased by about ~5%. However, the transient relaxation induced by ISO (10 μM) was unchanged in the presence of methoctramine. A similar transient relaxation was also observed in response to ISO (10 μM), when the airway was pre-contracted with another agonist, serotonin (5-HT, 1 μM).
In a second set of experiments, the airways were contracted with a single concentration of MCh and relaxed with different ISO concentrations. The previous data indicated that it was necessary to use a low MCh concentration in order that the relaxation responses could be differentiated. We determined that 200 nM MCh satisfied these requirements. In airways contracted with MCh (200 nM), ISO induced relaxation with a typical pattern of an initial relaxation followed by a slow re-contraction (Fig. 3D solid line). The magnitude of the initial relaxation was concentration-dependent from 1 nM to 1 μM ISO, ranging from 11 ± 1% (n = 5 slices from 3 mice) to 53 ± 2% (n = 6 slices from 4 mice) (Fig. 3E solid line). No further relaxation was induced by ISO at concentrations higher than 1μM (Fig. 3E).
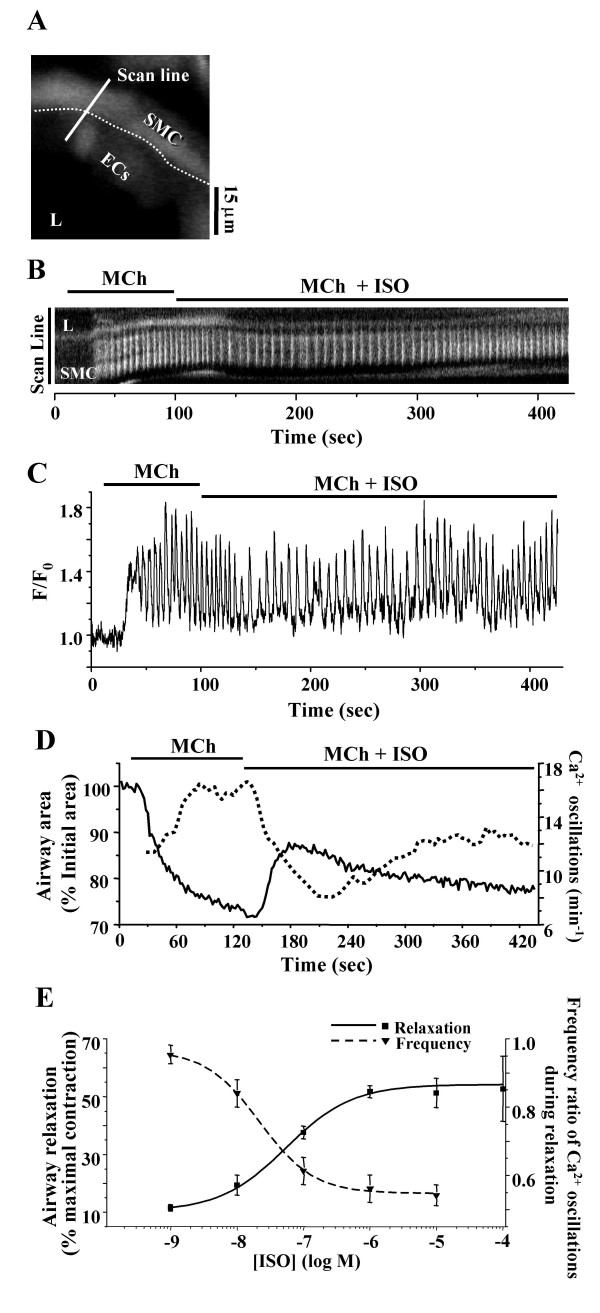
Figure 3.
The effect of ISO on the Ca2+signaling of airway SMCs. (A) A fluorescence image of part of an airway obtained by two-photon microscopy under resting conditions. L, lumen; ECs, epithelial cells; SMC, smooth muscle cell. Dotted line indicates the interface between ECs and SMCs. Scale bar = 15 μm. The intracellular Ca2+ signaling represented by (B) a line-scan plot, constructed from the white line across the SMC as indicated in A, and the sequence of recorded images and (C) a small ROI in the same airway SMC as shown in B in response to the MCh (200 nM) and ISO (1 μM). (D) The correlation of the Ca2+ oscillation frequency (dotted line) and airway area (solid line) in a representative experiment with contraction induced by MCh (200 nM) and relaxation induced by ISO (10 μM). The Ca2+ oscillation frequency was determined by the time interval between 2 adjacent peaks. The frequency of the Ca2+ oscillations was inversely coupled to the contraction of the airway. (E) The mean concentration-dependent relaxation (solid line, ■) and the change in the frequency of the Ca2+ oscillations (dash line, ▼) induced by ISO in airways contracted with MCh (200 nM). The mean Ca2+ oscillation frequency after ISO exposure was expressed as a ratio to the Ca2+ oscillation frequency during MCh exposure. As the concentration of ISO increased, the Ca2+ oscillation frequency decreased. Each point represents 5 to 8 experiments (mean ± S.E.) in different airways from at least 3 mice for the contraction data and 5 – 6 experiments in different cells from at least 3 mice for the Ca2+ oscillation data. Data were fitted with a logistic function.
Ca2+ events underlying airway contraction and relaxation
In response to 200 nM MCh, the airway SMCs demonstrated an initial increase of intracellular Ca2+ which was followed by the onset of Ca2+ oscillations (Figs. 3B and 3C). These Ca2+ oscillations were superimposed on a slightly elevated baseline. The frequency of the Ca2+ oscillations increased to a stable rate within 1 minute. Upon exposure to 1 μM ISO, the frequency of the Ca2+ oscillations slowed, although the baseline of the Ca2+ signal did not substantially change. In the continued exposure to ISO with MCh and after about 150 seconds, the frequency of Ca2+ oscillations began to increase and approached a new steady rate; this rate was less than the initial rate induced by MCh alone.
The frequency of the Ca2+ oscillations was determined from the period of each oscillation (time interval between 2 adjacent peaks of the Ca2+ signal). As a result a correlation between the dynamic changes in the frequency of the Ca2+ oscillations with the changes in the cross-sectional area of the airway could be made (Fig. 3D). In response to MCh, the airway contracted to a smaller stable size and this correlated with the initiation and increase in the frequency of the Ca2+ oscillations to a stable rate. Similarly, the ISO-induced decrease in the frequency of the Ca2+ oscillations correlated with the ISO-induced initial relaxation of the airway. The subsequent re-contraction of the airway correlated with the late increase in the frequency of the Ca2+ oscillations.
The effect of ISO on the frequency of the Ca2+ oscillations was expressed as the ratio of the frequency of the Ca2+ oscillations after ISO exposure (60 to 90 seconds) to the frequency of the Ca2+ oscillations before ISO exposure. In the range from 1 nM to 10 μM, ISO induced a concentration-dependent decrease in the frequency ratio from 0.93 ± 0.03 (n = 5 slices from 3 mice) to 0.56 ± 0.03 (n = 6 slices from 4 mice). The decline in the frequency ratio was inversely proportional to the change in airway size (Fig. 3E).
Relaxation of airways by repeated exposure to ISO
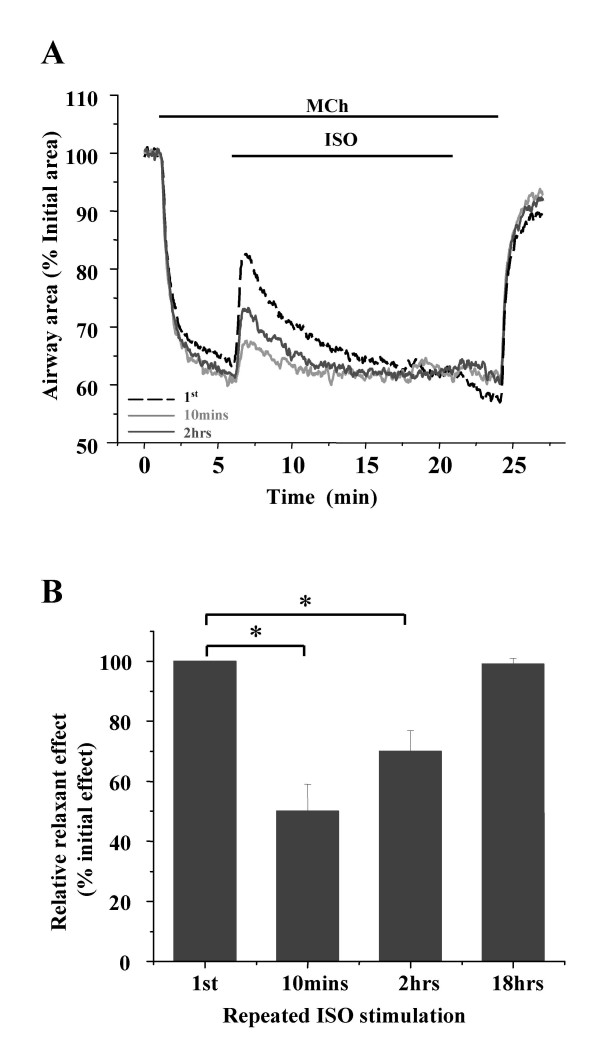
Although we found that ISO induced a concentration-dependant relaxation of pre-contracted airway, this relaxation response did not persist in the presence of ISO. A possible explanation for this response is receptor accommodation or desensitization to the continual presence of agonists. To address this possibility, we examined the relaxation response of the same contracted airway (with MCh, 200 nM) to multiple exposures of ISO (1 μM) (Fig. 4). The interval between each ISO exposure was increased from 10 minutes to 2 hours and 18 hours. In comparison with the initial relaxation response to ISO, the subsequent relaxation of the airway decreased to 50 ± 9 % (n = 5 slices from 4 mice, p < 0.01) in response to ISO exposure after 10 minutes (Fig 4A and 4B). However, the efficacy of ISO was substantially improved when the interval between exposures was lengthened to 2 hours. After 18 hours, the ISO induced-relaxation was found to have recovered to 97 ± 3 % (n = 2 slices from 2 mice, P = 0.36 compared to initial effect) (Fig. 4A and 4B).
Figure 4.
The ability of ISO to relax an airway is influenced by repetitive stimulation. (A) Relaxation responses of the same contacted airway (with MCh, 200 nM) in response to the first (black dash line) and subsequent 15 minute-exposures of ISO (1 μM) with the time intervals of 10 minutes (light grey line) and 2 hours (dark grey line). The slices were washed with sHBSS between experiments. (B) The summary of the relative relaxation responses induced by repeated ISO stimulation after different intervals. ISO-induced relaxation declined to about half after 10 minutes but was fully recovered after 18 hours. Relaxation was expressed as a percentage of the initial relaxation response. Data from experiments with 5 different airways from 4 mice (*, p < 0.05 compared to the 1st relaxation).
Relaxing effect of FSK and 8-bromo-cAMP on contracted airways
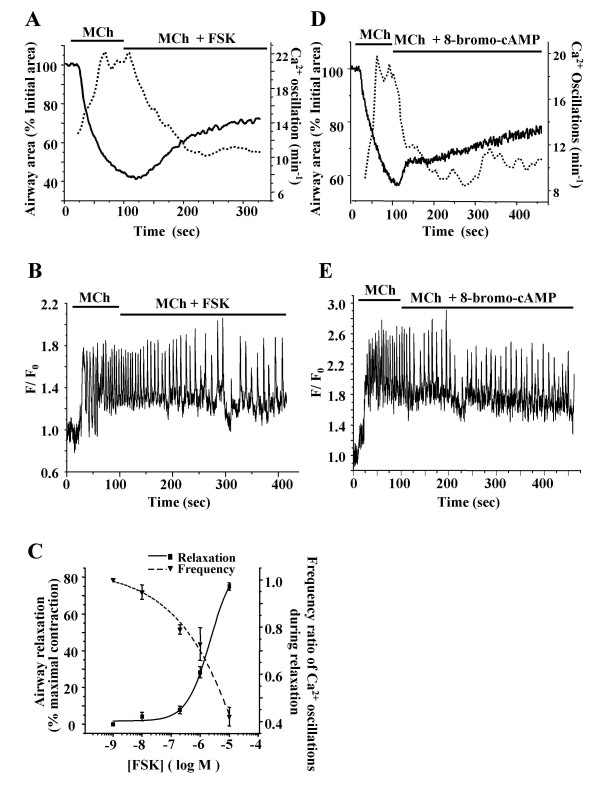
To investigate the airway relaxation response to the elevation of [cAMP]i, we examined the effects of FSK, an activator of AC, and 8-bromo-cAMP, an active membrane permeable analogue of cAMP. Both FSK (10 μM) and 8-bromo-cAMP (500 μM) induced an initial fast relaxation followed by a slow progressive relaxation of MCh contracted airways (Fig. 5A and 5D solid line). The increase in the lumen area induced by 500 μM 8-bromo-cAMP was 49 ± 9 % (n = 3 slices from 2 mice). The effect of FSK was concentration-dependent (Fig. 5C); increasing concentrations of FSK (10 nM to 10 μM) induced an increasing relaxation (measured after 5 minutes exposure) from 4 ± 1 % to 75 ± 2 % (n = 4 slices from 3 mice).
Figure 5.
The effect of FSK and 8-bromo-cAMP on contraction and Ca2+ oscillations of airway SMCs. (A) FSK (10 μM, solid line) and (D) 8-bromo-cAMP (500 μM, solid line) induced an initial quick relaxation followed by slower relaxation. (B) FSK and (E) 8-bromo-cAMP decreased the Ca2+ oscillation frequency initiated by MCh to a slower rate. The changes in the frequency of the Ca2+ oscillations induced by each compound (dotted line in A and D) correspond with opposite changes in airway area (solid line in A and D). (C) With increasing FSK concentration, the relaxation (solid line, ■) increased while the frequency of the Ca2+ oscillations (dotted line, ▼) decreased; each point is the mean ± S.E. from at least 4 different airways from 3 mice. Data points were fitted with a logistic function.
Recordings of intracellular Ca2+ in airway SMCs revealed that both FSK and 8-bromo-cAMP reduced the frequency of the Ca2+ oscillations initiated by MCh (Fig. 5B and 5E). In contrast to the effects of ISO, the frequency of the Ca2+ oscillations declined throughout the experiment. In range from 10 nM to 10 μM, FSK induced a concentration-dependent decrease in the frequency of the Ca2+ oscillations (Fig. 5C). The frequency of the Ca2+ oscillations after 5 minutes of FSK exposure, expressed as the ratio to the Ca2+ oscillation frequency induced by MCh exposure, decreased from 0.95 ± 0.03 to 0.42 ± 0.04 (n = 4 slices from 3 mice) as the FSK concentration increased. The changes in frequency of the Ca2+ oscillations induced by FSK or 8-bromo-cAMP correlated with a progressive relaxation of the airway (Fig. 5A and 5D).
A decrease in Ca2+ oscillation frequency correlates with airway relaxation
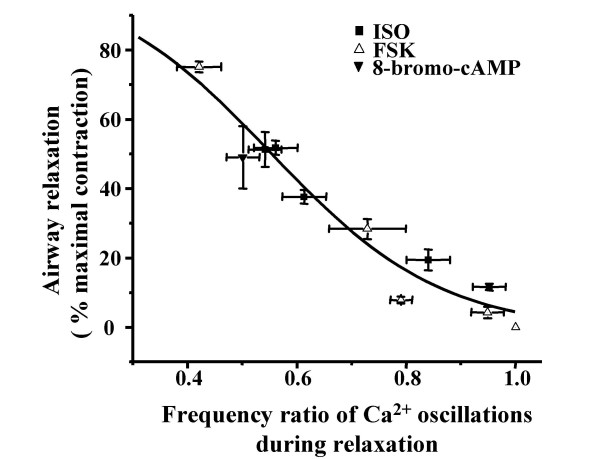
During all of the relaxation responses to ISO, FSK or 8-bromo-cAMP, the frequency of the Ca2+ oscillations initiated by MCh was reduced (Fig. 3D, Fig. 5A and 5D). A summary plot of these data (from Fig. 3E, Fig. 5C and 5D) is presented in figure 6. This analysis suggests that, irrespective of the agonist, the frequency of the Ca2+ oscillations determines the extent of airway relaxation: the lower the frequency of the Ca2+ oscillations in airway SMCs, the greater the relaxation of the airway (Fig. 6).
Figure 6.
Relationship between the reduction in Ca2+ oscillation frequency in SMCs and airway relaxation. The relaxation state (percentage of the MCh-induced contraction) and corresponding Ca2+ oscillation frequency (ratio to the frequency of MCh-induced Ca2+ oscillations) upon exposure to ISO, FSK and 8-bromo-cAMP (at various concentrations) were plotted and fitted with a logistic function. The data indicates that a greater airway relaxation correlated with a larger reduction in the Ca2+ oscillation frequency.
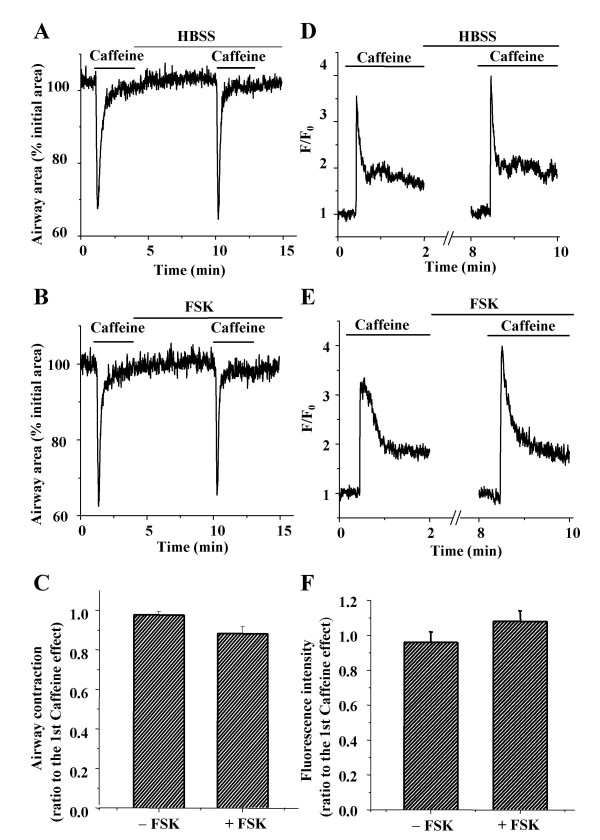
cAMP does not affect caffeine-induced Ca2+ release
To explore the mechanisms by which cAMP reduced the frequency of the Ca2+ oscillations, we investigated the effect of cAMP on caffeine-induced Ca2+ release and refilling of intracellular Ca2+ stores (Fig. 7). In view of the transient nature of ISO-induced relaxation, FSK was used to elevate cAMP because of its direct and stable action on AC. Upon exposure to 20 mM caffeine, the airways responded with a transient contraction (Fig 7A). This treatment also induced in the SMCs a transient increase in [Ca2+]i (peak fluorescence intensity ratio, F/F0 = ~3.5) followed by a sustained plateau of [Ca2+]i (fluorescence ratio = ~1.8, Fig. 7D). The incubation of the same airway with FSK (10 μM for 5 min) had no significant effect on caffeine-induced airway contraction (Fig. 7B) or [Ca2+]i elevation (Fig. 7E). The ratio of contraction induced by the second exposure to caffeine as compared to the first exposure was 0.96 ± 0.02 in the absence of FSK and 0.88 ± 0.04 in the presence of FSK (Fig. 7C). The ratios of the peak fluorescence intensities were 0.96± 0.06 in the absence of FSK and 1.0 ± 0.06 in the presence of FSK (n = 4 slices from 3 mice, Fig. 7F). The similarity of the results suggests that cAMP does not affect caffeine-induced Ca2+ release (i.e. via the ryanodine receptor) from the SR or the subsequent refilling of the depleted internal Ca2+ store.
Figure 7.
Effect of FSK on caffeine-induced Ca2+ release. Representative experiments demonstrating (A) contraction and (D) Ca2+ signaling of airway SMCs induced by repetitive exposure to caffeine (20 mM) in the absence of FSK. FSK (10 μM) had no effect on caffeine-induced (B) contraction or (E) Ca2+ signaling. (C) Airway contraction and (F) the peak fluorescence intensity of Ca2+ signaling (ratio of second to first exposure) induced by caffeine (20 mM) in the slices incubated with or without FSK (10 μM). Data represents the mean ± S.E. from 4 different slices from 3 mice.
Supplementation of intracellular Ca2+ does not block the relaxing effect of cAMP
Because a reduction of [Ca2+]i, brought about by decreasing Ca2+ influx and/or increasing Ca2+ efflux or reuptake, has been proposed as a mechanism by which cAMP relaxed airway SMCs, we examined the effect of elevating [Ca2+]i during the response of SMCs to cAMP. The [Ca2+]i was increased with 3 different agents: 1) NP-EGTA, a caged-Ca2+ compound, which upon UV photolysis releases Ca2+, 2) ionomycin, a Ca2+ selective ionophore, and 3) iberiotoxin (IbTX), a specific inhibitor of BKCa channels, which enhances Ca2+ influx by blocking K+ efflux to induce hyper-polarization.
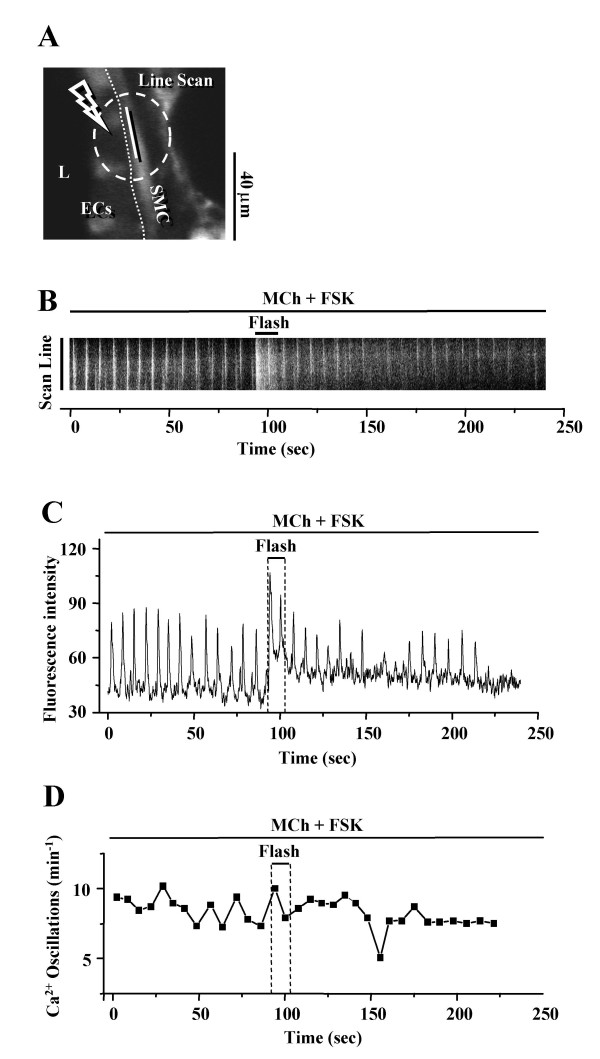
In airway SMCs pre-treated with MCh (200 nM) and FSK (10 μM), flash photolysis of caged-Ca2+ within the SMC increased the fluorescence intensity of intracellular Ca2+ signaling (Fig. 8B). However this served only to elevate the baseline, but not the frequency, of the Ca2+ oscillations (Fig. 8C and 8D, 6 airways from 4 mice). No change in the airway luminal area or an increase in contraction of the illuminated SMCs was observed.
Figure 8.
Effect of flash photolysis of caged-Ca2+ on SMCs treated with MCh and FSK. (A) A fluorescence image of part of an airway obtained by confocal microscopy. L, lumen; ECs, epithelial cells; SMC, smooth muscle cell. The dashed white oval indicates the position and size of the zone of UV illumination (10 s, OD 0.6). Dotted line indicates the interface between ECs and SMCs. Scale bar = 40 μm. The intracellular Ca2+ signaling represented by(B) a line-scan plot, constructed by sequentially aligning the pixels along the length of the SMC (white line indicated in A) and from each recorded fluorescence image and (C) a selected ROI within the SMC and (D) the frequency of Ca2+ oscillations of SMCs in response to flash photolysis of caged-Ca2+ during stimulation with MCh (200 nM) and FSK (10 μM). Flash photolysis induced a temporary rise in [Ca2+]i but did not increase the slow frequency of the Ca2+ oscillations. Representative traces of 6 different slices from 4 mice.
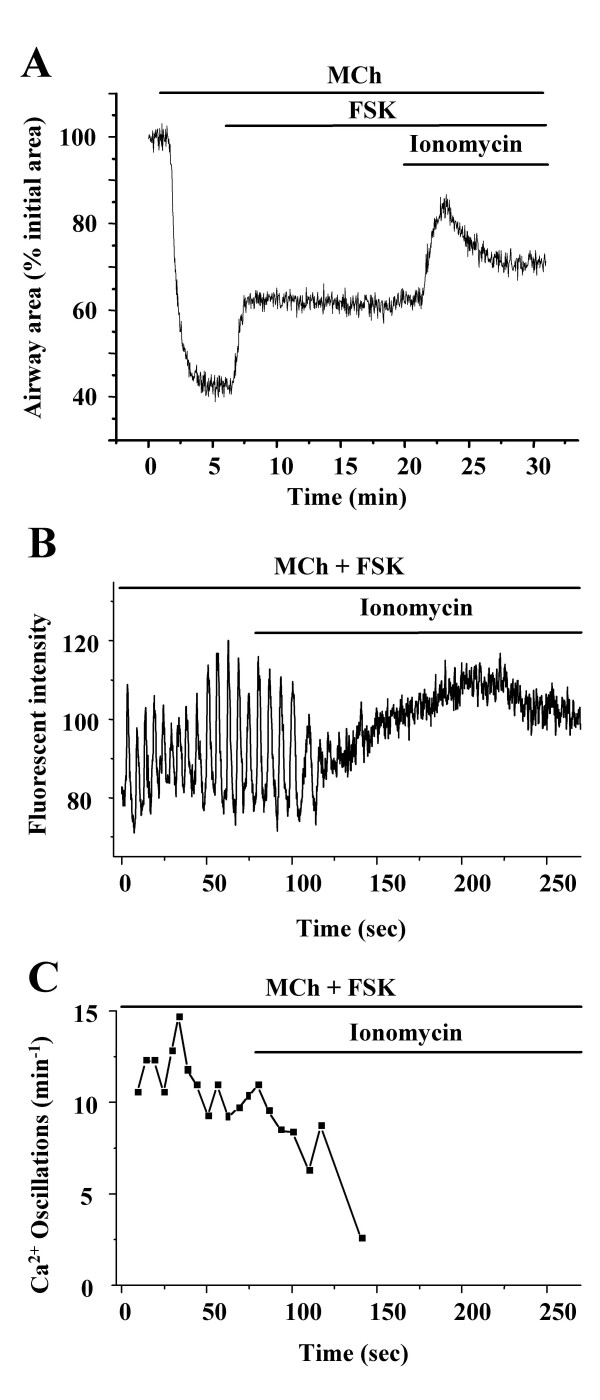
Upon exposure to ionomycin (5 μM), the relaxation induced by FSK (10 μM) in MCh (200 nM) contracted airway SMCs was initially enhanced, reaching a maximal relaxation after 2 min (Fig. 9A). This additional relaxation correlated with the cessation of the Ca2+ oscillations (Fig. 9B and 9C). After continued ionomycin exposure, the airway began to re-contract and this correlated with an steady increase in the [Ca2+]i (Fig 9A and 9B) (5 airways from 4 mice).
Figure 9.
Effect of ionomycin on FSK-induced airway relaxation. (A) Airway contraction in response to MCh (200 nM), FSK (10 μM) and ionomycin (5 μM). Ionomycin initially enhanced the relaxation induced by FSK but subsequently re-contracted the airway. Representative traces from 4 experiments from 3 mice. (B) Changes in intracellular Ca2+ and (C) the frequency of the Ca2+ oscillations induced by MCh (200 nM) and FSK (10 μM) followed by exposure to ionomycin. Ionomycin stopped the Ca2+ oscillation and subsequently elevated the [Ca2+]i. Representative traces of five experiments from 4 mice.
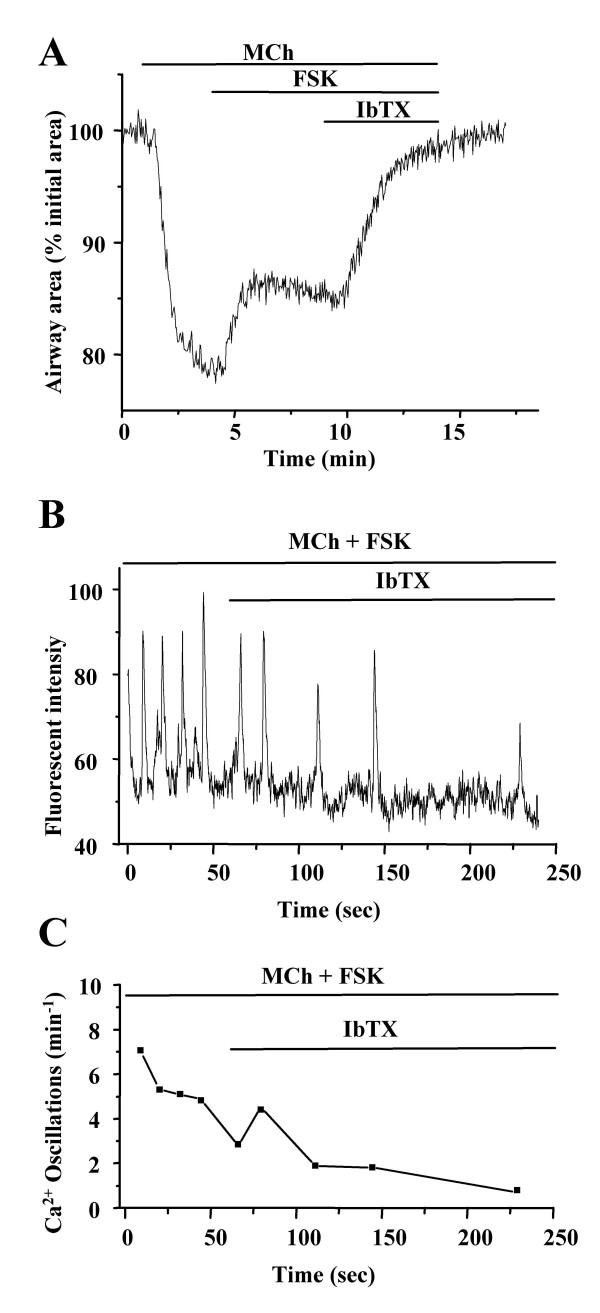
A similar change in the contraction and [Ca2+]i of airway SMCs was observed when the lung slices treated with MCh (200 nM) and FSK (10 μM) were subsequently exposed to IbTX (50 nM). The airway responded with a quick and full relaxation within 3 mins (Fig. 10A). Again, the accompanying change in the [Ca2+]i revealed a rapid slowing of the frequency of the Ca2+ oscillations to a full stop. In contrast to the effect of ionomycin, the [Ca2+]i of SMCs remained near baseline after the Ca2+ oscillations ceased (Fig. 10B, 6 airways from 5 mice).
Figure 10.
Effect of iberiotoxin on FSK-induced airway relaxation. (A) Airway contraction in response to MCh (200 nM), FSK (10 μM) and IbTX (50 nM). IbTX greatly enhanced the relaxation induced by FSK to fully relax the airway. Representative traces of 6 experiments from 3 mice. (B) Changes in intracellular Ca2+ signaling and (C) the frequency of Ca2+ oscillations induced by MCh (200 nM) and FSK (10 μM) followed by exposure to IbTX (50 nM). The frequency of the Ca2+ oscillations was further slowed by IbTX. Representative traces of 8 experiments from 5 mice.
In summary, we used three different methods to increase the [Ca2+]i during FSK- induced relaxation but the frequency of the Ca2+ oscillation could not be increased to reverse the FSK effect. Moreover, the increase of [Ca2+]i induced by ionomycin inhibited the Ca2+ oscillations to induce further relaxation. These data suggest that a reduction in the availability of Ca2+ is not the cause of cAMP-induced slowing of Ca2+ oscillations.
cAMP exerts a relaxant effect by inhibiting IP3-induced Ca2+ release
Because Ca2+ release through the IP3 receptor of internal Ca2+ stores is a major mechanism contributing to the formation of Ca2+ oscillations, we investigated whether cAMP inhibited IP3-induced Ca2+ release. When airway SMCs loaded with caged-IP3 were illuminated with UV light for about 1 s, several Ca2+ oscillations were observed to propagate as Ca2+ waves from the illuminated zone through the rest of the SMC (data not shown). When the intensity and exposure time of UV light was optimized, repetitive UV flashes could induce similar repetitive Ca2+ waves in the same SMC.
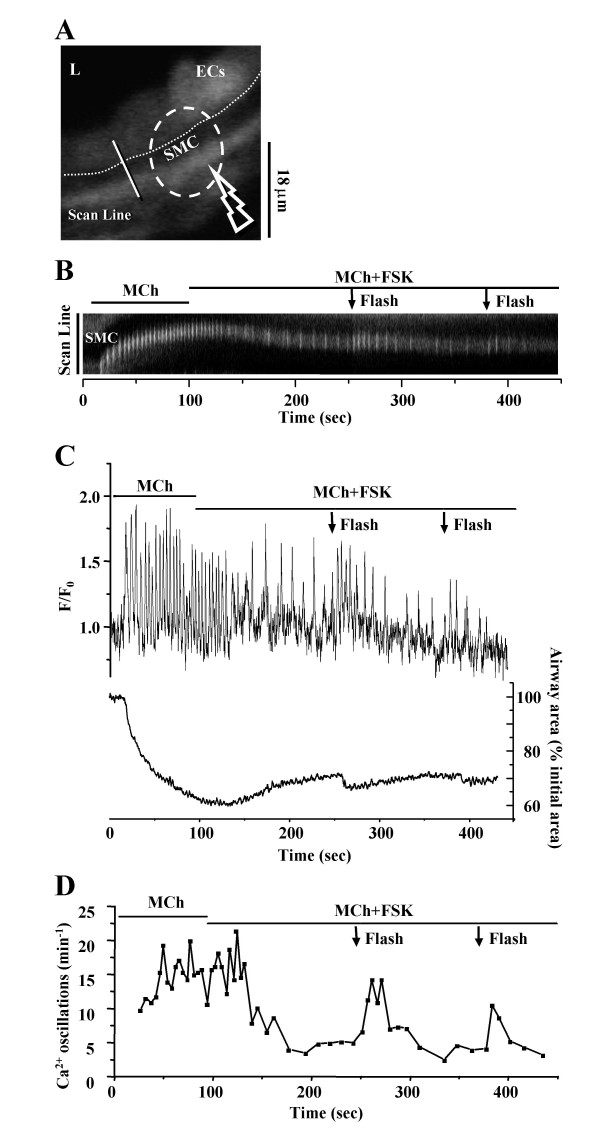
A similar set of experiments was then performed in the presence of MCh and FSK. The inhibition of the frequency of the Ca2+ oscillations and the accompanying relaxation of SMCs were observed when the slices were exposed to MCh (200 nM) and FSK (10 μM). But, in response to UV illumination (0.5s, OD 0.5) and photolysis of caged IP3, the frequency of the Ca2+ oscillations increased significantly for a short period (Fig. 11B, C, D). A second flash again increased the oscillation frequency, although to a lesser extent. Simultaneous recording of changes in the frequency of Ca2+ oscillations and contraction of airway SMCs revealed that the airway re-contracted after each UV flash (Fig. 11C). These results suggest that FSK exerts its relaxant effect by inhibiting the IP3 induced Ca2+ release from internal Ca2+ stores.
Figure 11.
Effect of flash photolysis of caged-IP3 on SMCs treated with MCh and FSK. (A) A fluorescence image of part of an airway obtained by confocal microscopy. The dashed white oval indicates the position and size of the zone of UV illumination (0.5 s, OD 0.5). L, lumen; ECs, epithelial cells; SMC, smooth muscle cell. Dotted line indicates the interface between ECs and SMCs. Scale bar = 18 μm. Intracellular Ca2+ signaling represented by (B) a line-scan plot, constructed by sequentially aligning the pixels along the line across the SMCs and ECs (white line indicated in A) from each recorded fluorescence image. (C) The simultaneous recording of intracellular Ca2+ signaling of SMC (upper trace, and B), and the changes in airway lumen area (lower trace) and (D) frequency of the Ca2+ oscillations in response to the flash photolysis of caged-IP3 during stimulation with MCh (200 nM) and FSK (10 μM). Repetitive release of IP3 by flash photolysis (0.5 s, OD 0.5, indicated by arrows) increased the frequency of the Ca2+ oscillations induced by MCh and FSK and re-contracted the airway. Representative traces of 6 different slices from 4 mice.
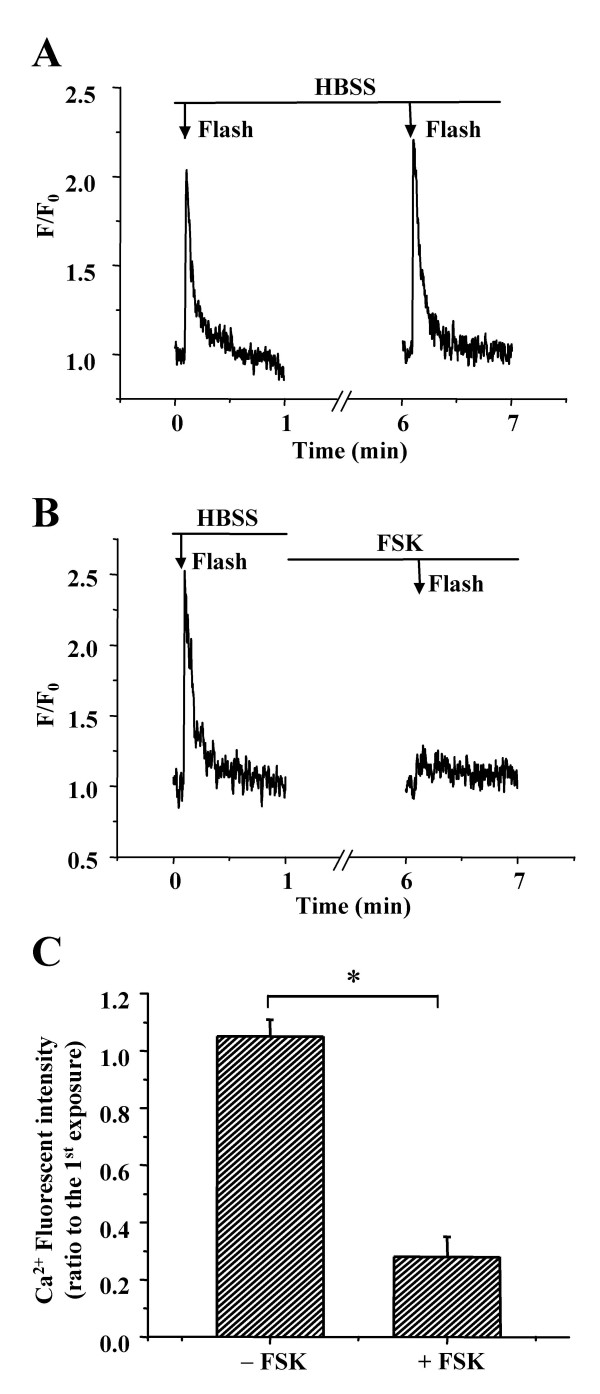
If the IP3 receptor is inhibited by cAMP, it should be possible to block Ca2+ release induced by IP3 in cAMP treated SMCs. In control experiments, a lower level of UV illumination (reduced to 1/3, by using a neutral density filter with OD 1.0) induced a single spike of elevated [Ca2+]i in untreated airway SMCs (Fig. 12). A second flash gave a similar response. However, after the SMCs were treated with FSK (10 μM) for 5 minutes, the increase in [Ca2+]i was virtually abolished in response to the UV flash (Fig. 12B and 12C).
Figure 12.
Effect of FSK on the intracellular Ca2+ signaling induced by IP3. The Ca2+ signaling of SMCs induced by repetitive flash-photolysis (0.5 s, OD 1.0, arrows) of caged-IP3 in lung slices incubated (A) without or (B) with FSK for 5 min. Exposure to FSK almost completely abolished the Ca2+ response induced by flash photolysis of IP3. (C) Summary of the changes in Ca2+ signaling (peak fluorescence intensity) induced by IP3 in the slices incubated without or with FSK. The Ca2+ signal after exposure to the sHBSS without or with FSK was expressed as a ratio to the Ca2+ response before exposure. Data represents the mean ± S.E. from at least 5 different slices from 4 mice. *, p < 0.05.
CICR through the ryanodine receptor is not required for Ca2+ oscillations
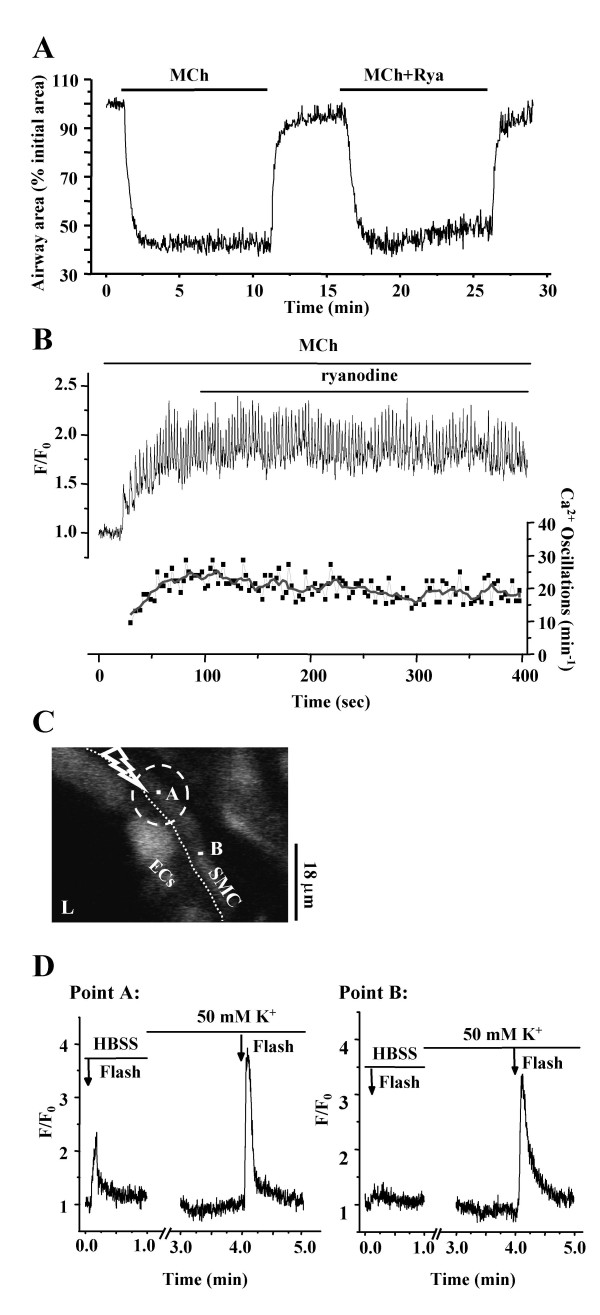
Because the ryanodine receptor (RyR) is involved in Ca2+-induced Ca2+ release (CICR) in a variety of cell types, we examined the effect of CICR via RyR in airway SMCs. We found that exposure to ryanodine (50 μM, sufficient to inhibit the RyR) induced only a small and slow decrease in the MCh-induced airway contraction (~5%, after 5 minutes, Fig. 13A) and frequency of Ca2+ oscillation in the SMCs (~4 min-1, after 5 minutes, Fig. 13B). The amplitude of the Ca2+ oscillations was unaffected. These result suggest that the increases in the [Ca2+]i associated with the Ca2+ oscillations are not mediated by RyR or are sufficient to invoke CICR via the RyR.
Figure 13.
Effect of the ryanodine on contraction and Ca2+ signaling. (A) MCh (200 nM) induced contraction of the same airway in the absence or presence of ryanodine (50 μM, Rya). (B) Changes in fluorescence (upper trace) and frequency of oscillatory Ca2+ signaling (lower trace) of a single airway SMC treated with 200 nM MCh and 50 μM ryanodine. Representative traces of 6 different slices from 4 mice.(C) A fluorescence image of part of the airway obtained by confocal microscopy. The dashed white oval indicates the position and size of the zone of UV illumination (5 s, OD 0.6). L, lumen; ECs, epithelial cells; SMC, smooth muscle cell. Dotted line indicates the interface between ECs and SMCs. Scale bar = 18 μm. Point A was located at the center of the zone of illumination on a single SMC and Point B was located on the same SMC outside the zone of illumination. (D) The local Ca2+ signals at point A and B induced by flash photolysis of caged-Ca2+ in the absence or presence of 50 mM K+ (incubation time of 3 min). Representative traces of 5 different slices from 3 mice.
To examine the Ca2+ conditions required for the induction of CICR, we released Ca2+ by flash photolysis of caged Ca2+. When a restricted area within a single SMC was exposed to UV light (Fig 13C), only a brief localized increase in [Ca2+]i was detected (Fig. 13D). The increase in Ca2+, as determined by the fluorescence change, was similar to that induced by a Ca2+ oscillation, but this Ca2+ change did not spread to the rest of the cell. By contrast, when the same airway SMCs was treated with sHBSS containing 50 mM K+ for ~3 minutes, a second identical UV flash induced a larger Ca2+ release in the zone of illumination and induced a Ca2+ wave that propagated throughout the cell (Fig 13D, 5 airways from 3 mice). Treatment with high K+ was used because it has previously been reported that high K+ initiates membrane-depolarization that results in excessive Ca2+ influx, overfilling of internal Ca2+ store and sensitization of the RyR [14]. These results indicate that CICR through RyR only occurs in airway SMCs under certain circumstances or "stressed conditions" and implies that RyRs do not play a significant role in the normal maintenance of agonist-induced Ca2+ oscillations.
Discussion
In this study, thin lung slices were used to explore the changes in intracellular Ca2+ that occur in airway SMCs during relaxation induced by ISO and other cAMP elevating compounds. The airway SMCs were initially contracted with MCh, an analogue of ACh, which is more slowly metabolized and widely used in clinical lung function tests. We found that MCh induced both a concentration-dependent contraction of the airways and increase in the frequency of Ca2+ oscillations within airway SMCs. These results are similar to our previous findings obtained with ACh in lung slices [12,14]. Upon exposure to ISO, the MCh-contracted airways initially displayed a transient relaxation but subsequently re-contracted. The ability of ISO to relax the airway was dependent both on the ISO concentration and the MCh concentration used to contract the airway. If the airways were contracted with high concentration of MCh (1 μM), ISO, even at high concentrations (i.e.10 μM) was ineffective at relaxing the airway. Only at low MCh concentrations was ISO able to induce substantial relaxation. A similar relaxation and subsequent re-contraction in response to ISO (10 nM ~10 μM) was also observed in MCh-contracted (1 μM) rat lung slices (data not shown). These results suggest that the relaxing action of ISO is relatively weak in comparison to the contractile action of MCh and that the airway becomes de-sensitized to ISO-induced relaxation to allow re-contraction.
In addition to being activated by β-adrenoceptor associated G-stimulatory proteins (Gs), AC can also be inhibited by receptor-coupled G-inhibitory proteins (Gi). Therefore, a potential mechanism for the down-regulation of the ISO response is the inhibition of AC by activation of M2 muscarinic receptors and their associated Gi by MCh [28,29]. However, in the presence of the M2 receptor antagonist, methoctramine, MCh only induced a slightly smaller airway contraction (about 95%), but more importantly, ISO induced a similar transient relaxation. This transient response to a high concentration of ISO also persisted across a wide range of MCh concentrations, which would be expected to differentially activate the M2 receptors. Moreover, a similar transient response to ISO was also observed in the airways contracted by 5-HT, an agonist with no effect on the AC. In other studies [30,31], the pretreatment of cultured SMCs or bronchial smooth muscle strips with ISO (30 min to 24 hours) reduced subsequent ISO-mediated cAMP generation and relaxation. Similarly, we found that repetitive exposure of the airway to ISO (1 μM, 10 min to 2 hours) significantly decreased the relaxation response (by 30 to 50%). These results indicate that the M2 receptor has a minimal effect on AC activity at mid-range MCh concentrations and that the down-regulation of the ISO response is mediated by rapid de-sensitization and slow re-sensitization at the β-adrenoceptor. Desensitization of the β-adrenoceptor can be mediated by phosphorylation of the receptor by cAMP-independent β-adrenoceptor kinase or other G protein-coupled receptor kinases or by receptor internalization – a process that takes a longer time to reverse (hours) [2,32].
Because β-adrenoceptor agonists primarily relax SMCs by increasing [cAMP]i, an implication of receptor desensitization is that the [cAMP]i within the SMCs initially increases but subsequently declines. The idea that increases in [cAMP]i correlate with relaxation was addressed by circumventing the β-adrenergic receptor by either activating adenylyl cyclase with FSK or by increasing the [cAMP]i directly with 8-bromo-cAMP. A major characteristic of FSK or 8-bromo-cAMP-induced relaxation was that the relaxation was sustained and that re-contraction never occurred. This response is consistent with the idea that the [cAMP]i must be maintained or increased if relaxation is to be sustained and that some form of desensitization of β-adrenoceptor occurs allowing re-contraction.
The correlation of increases in [cAMP]i with airway relaxation has been reported in mice [33], rats [23,34], guinea pigs [35], rabbits [36,37], dogs [3], cows [4,24,38], and humans [24,37]. However, these studies were all conducted on isolated airway muscle strips or tracheal rings by measuring changes in isometric tension. Consequently, to explore the cellular mechanisms by which ISO and other agents acting via cAMP to relax SMCs, we examined their effects on intracellular Ca2+. As previously described, MCh, as well as ACh, initiates Ca2+ oscillations in SMCs and the frequency of these Ca2+ oscillations determines the extent of the SMC contraction [9,11,12,14]. During the initial response to ISO, the frequency of the MCh-induced Ca2+ oscillations was quickly reduced and this was accompanied by fast relaxation of the airway. However, after a short time, the Ca2+ oscillation frequency began to slowly increase and this was accompanied by the slow re-contraction of the airway. A similar slowing of the frequency of the Ca2+ oscillations and airway relaxation was induced by FSK and 8-bromo-cAMP. But, in contrast to ISO, the frequency of the Ca2+ oscillations continued to slow to lower frequencies; a result that correlates with the greater airway relaxation. Although the relationship to contraction could not be measured, Salbutamol, a β2-adrenoceptor agonist, has also been found to slow and reduce the baseline of ACh-induced Ca2+ oscillation in dissociated porcine tracheal SMCs [16]. In our studies, we found that the baseline of the Ca2+ oscillations was relatively stable. Consequently, we hypothesized, especially in view of the fact that the peak [Ca2+]i of the Ca2+ oscillations is substantially high, that increases in [cAMP]i induce airway relaxation by reducing the frequency of Ca2+ oscillations in SMCs rather than by lowering the baseline of global [Ca2+]i.
We [12-14] and others [10,39] have shown that agonist-induced Ca2+ oscillations in airway SMCs are generated by cycles of Ca2+ release and uptake by the sarcoplasmic reticulum (SR). Because extracellular Ca2+ is required to replenish internal stores, it has been proposed that cAMP might exert its action on Ca2+ oscillations by 1) the inhibition of Ca2+ replenishment of the internal stores by inhibiting Ca2+ pumps and/or 2) blocking the Ca2+ influx through voltage-dependent Ca2+ channels (VOC) or store-operated Ca2+ channels (SOCC), or 3) by inhibiting the Ca2+ release via IP3 and/or ryanodine receptors.
To test the first hypothesis, that the internal Ca2+ stores were depleted, we investigated the effects of caffeine on cAMP-treated lung slices. We found that elevated [cAMP]i did not significantly affect the Ca2+ signaling or contractile response induced by repetitive exposures to caffeine. These results demonstrate that the Ca2+ capacity of the internal store was fully restored to normal in the presence of cAMP and imply that Ca2+ loading of the SR by Ca2+ pumps was not significantly altered.
To investigate the second possibility that cAMP decreased Ca2+ influx to limit the Ca2+ available to replenish internal stores and maintain the ongoing Ca2+ oscillation, we used flash photolysis of caged-Ca2+ or exposure to ionomycin, a Ca2+ ionophore, to directly increase the available Ca2+. We found that while a transient global increase in [Ca2+]i resulted from the photolysis of caged-Ca2+, this had little effect on the slow frequency of the Ca2+ oscillations in the presence of FSK. Furthermore, the application of ionomycin initially inhibited the FSK-reduced Ca2+ oscillation to relax the airway. However, the gradual increase in [Ca2+]i induced by continued exposure to ionomycin subsequently induced re-contraction of the airway. Because in both experiments, an increase in the frequency of the Ca2+ oscillations was not observed, these results indicate that a lowered Ca2+ influx is not a limiting factor. It is possible that ionomycin might have emptied the Ca2+ of SR to inhibit the Ca2+ oscillations; however considering the higher concentration of Ca2+ in the extracellular environment it is unlikely that the internal store would have been emptied before an influx of Ca2+ had occurred.
Another mechanism inhibiting SMC Ca2+ influx appears to involve membrane hyper-polarization resulting from cAMP-activation of BKCa channels to down-regulate voltage-dependent Ca2+ influx [19-21]. Specific blockers of the BKCa channels such as IbTX or charybdotoxin have been shown to antagonize cAMP-induced relaxation in isolated trachea or bronchi from guinea-pigs [21], pig [40], horse [41]. However, in keeping with the effects of ionomycin, we found that in lung slices treated with MCh and FSK, IbTX further inhibited the Ca2+ oscillations to enhance airway relaxation. This effect suggests IbTX (and ionomycin) may actually inhibit Ca2+ oscillation by enhancing Ca2+ influx. The failure to observe a significant elevation of baseline Ca2+ suggests either that the influx of Ca2+ is sufficiently low and quickly sequestered into internal stores or that an alternative, non-specific action of IbTX must be considered. The failure of IbTX to induce re-contraction is consistent with the fact that voltage-dependent Ca2+ influx does not appear to play a prominent role in maintaining the agonist-induced Ca2+ oscillations in airway SMCs and that airway contraction and Ca2+signaling induced by membrane depolarization (with KCl) were completely different to that induced by agonists [42]. Furthermore, there are conflicting reports regarding the contribution of BKCa channels based on differences amongst species and regions of the airway as well as experimental conditions [43,44]. Consequently, it appears unlikely that the BKCa channel is a major mechanism mediating the effects of cAMP in mouse airway SMCs. Taken together, these data led us to believe that decreased [Ca2+]i is not the primary cause of the modulation of the frequency of Ca2+ oscillations.
Therefore, we examined the third idea that cAMP altered the Ca2+ release from SR. Although the RyR has an important role in CICR in many other cell types [45], Ca2+ oscillations and airway contraction induced by MCh were minimally affected in the short term by the presence of ryanodine. This suggests that the Ca2+ oscillations do not require RyRs and therefore it is unlikely that any effects of cAMP on RyR will be significant. The slight decline in the Ca2+ oscillation frequency may be explained by an increased leak of Ca2+ through RyR locked in the open state by ryanodine. However, this process must be occurring independently of the Ca2+ oscillations otherwise the Ca2+ oscillations would quickly stop as a result of empty Ca2+ stores. These results are also consistent with the fact that the release of caged-Ca2+ only initiated CICR when the cell was previously depolarized with KCl to overload the internal Ca2+ stores and sensitize the RyR.
The IP3R is another important Ca2+ release channel of the SR and we found that the slow frequency of Ca2+ oscillations induced by FSK could be increased when the [IP3]i was increased by flash photolysis. This response was accompanied by the re-contraction of airway SMCs. The idea that the sensitivity of the IP3R to IP3 is down-regulated by FSK is further supported by the finding that photolysis of caged IP3 induced a propagating Ca2+ wave in the absence, but not in the presence of FSK. A similar effect of cAMP on Ca2+ release has also been reported in other types of SMCs or non-excitable cells [46-49]. The underlying mechanism may involve cAMP-dependent PKA to phosphorylate the IP3R. However, the outcome of phosphorylation differs with the preparation and either an enhancement or inhibition of Ca2+ release can result [46-49]. Alternatively, cAMP may influence the IP3R via EPACs.
Although the molecular details mediating the action of cAMP will require further investigation, we propose that the cAMP has an inhibitory effect on the IP3R and reduces the open probability of the receptor. Moreover, cAMP may also change the Ca2+ sensitivity of the IP3R. Normally, the open probability of the IP3R is a bell-shaped function related to the [Ca2+]i; the receptor is closed by high Ca2+ concentrations at a constant IP3 concentration [50]. With a decrease in [IP3]i, the bell-shaped response curve is shifted to the left, with the result that the IP3R closes at a lower [Ca2+]i [51,52]. In view of this change in sensitivity, we hypothesize that the inhibition of IP3R by cAMP results in a similar leftward and downward shift of the bell-shaped curve. This idea is consistent with our observation that in the presence of cAMP, an increase in Ca2+ influx, induced by IbTX or ionomycin, leads to the closure of the IP3R to mediate the cessation of Ca2+ oscillations and relaxation of the airway SMCs.
An additional mechanism that would augment the inhibitory action of cAMP on the IP3R is a reduction in the production of IP3. This idea is based on the evidence that G protein-activated PLCβ activity can be inhibited by PKA-induced phosphorylation [4,5,53,54]. This requires further investigation but is beyond the scope of this study.
An alternative mechanism for cAMP-induced SMC relaxation is the desensitization of contractile apparatus to Ca2+ [8,24,40,55]. Phosphorylation of MLCK can lead to decreased affinity of MLCK for Ca2+/calmodulin while phosphorylation of Rho-kinase or protein kinase C can increase the activity of MLCP. We have also found that cAMP can down-regulate the Ca2+-sensitivity of agonist-induced contraction in lung slices [56]. However, it is not yet clear if such changes occur in parallel with the changes in Ca2+ oscillation frequency.
In summary, we conclude that the frequency of Ca2+ oscillations controls the contractile state of airway SMCs and that compounds that elevate cAMP slow the frequency of Ca2+ oscillations within SMCs to proportionally induce airway relaxation. Furthermore, cAMP appears to regulate the Ca2+ oscillation frequency by inhibiting Ca2+ release through IP3R rather than influencing Ca2+ influx or the refilling of internal stores. In addition, we find that, under normal conditions, the RyR is not essential for Ca2+ oscillations of airway SMCs.
Acknowledgments
Acknowledgements
This work was supported by the NIH grant HL 71930 to MJS
Contributor Information
Yan Bai, Email: Yan.Bai@umassmed.edu.
Michael J Sanderson, Email: Michael.Sanderson@umassmed.edu.
References
- Global Initiative for Asthma. Global Strategy for asthma management and prevention. , National Institute of Health 2002, NIH publication no. 02-3659; [Google Scholar]
- Benovic JL. Novel β2-adrenergic receptor signaling pathways. J Allergy Clin Immunol. 2002;110:S229–35. doi: 10.1067/mai.2002.129370. [DOI] [PubMed] [Google Scholar]
- McGrogan I, Lu S, Hipworth S, Sormaz L, Eng R, Preocanin D, Daniel EE. Mechanisms of cyclic nucleotide-induced relaxation in canine tracheal smooth muscle. Am J Physiol. 1995;268:L407–13. doi: 10.1152/ajplung.1995.268.3.L407. [DOI] [PubMed] [Google Scholar]
- Hoiting BH, Meurs H, Schuiling M, Kuipers R, Elzinga CR, Zaagsma J. Modulation of agonist-induced phosphoinositide metabolism, Ca2+ signalling and contraction of airway smooth muscle by cyclic AMP-dependent mechanisms. Br J Pharmacol. 1996;117:419–426. doi: 10.1111/j.1476-5381.1996.tb15207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CM, Hsu MC, Tsao HL, Chiu CT, Ong R, Hsieh JT, Fan LW. Effect of cAMP elevating agents on carbachol-induced phosphoinositide hydrolysis and calcium mobilization in cultured canine tracheal smooth muscle cells. Cell Calcium. 1996;19:243–254. doi: 10.1016/S0143-4160(96)90025-1. [DOI] [PubMed] [Google Scholar]
- Springett GM, Kawasaki H, Spriggs DR. Non-kinase second-messenger signaling: new pathways with new promise. Bioessays. 2004;26:730–738. doi: 10.1002/bies.20057. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, Jakobs KH. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- Pfitzer G. Invited review: regulation of myosin phosphorylation in smooth muscle. J Appl Physiol. 2001;91:497–503. doi: 10.1152/jappl.2001.91.1.497. [DOI] [PubMed] [Google Scholar]
- Kuo KH, Dai J, Seow CY, Lee CH, van Breemen C. Relationship between asynchronous Ca2+ waves and force development in intact smooth muscle bundles of the porcine trachea. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1345–53. doi: 10.1152/ajplung.00043.2003. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Kannan MS, Sieck GC. Regulation of intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am J Physiol. 1997;272:C966–75. doi: 10.1152/ajpcell.1997.272.3.C966. [DOI] [PubMed] [Google Scholar]
- Roux E, Guibert C, Savineau JP, Marthan R. [Ca2+]i oscillations induced by muscarinic stimulation in airway smooth muscle cells: receptor subtypes and correlation with the mechanical activity. Br J Pharmacol. 1997;120:1294–1301. doi: 10.1038/sj.bjp.0701061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergner A, Sanderson MJ. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J Gen Physiol. 2002;119:187–198. doi: 10.1085/jgp.119.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergner A, Sanderson MJ. ATP stimulates Ca2+ oscillations and contraction in airway smooth muscle cells of mouse lung slices. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1271–9. doi: 10.1152/ajplung.00139.2002. [DOI] [PubMed] [Google Scholar]
- Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol. 2005;125:535–553. doi: 10.1085/jgp.200409216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttle LC, Farley JM. Frequency modulation of acetylcholine-induced oscillations in Ca2+ and Ca2+-activated Cl- current by cAMP in tracheal smooth muscle. J Pharmacol Exp Ther. 1996;277:753–760. [PubMed] [Google Scholar]
- Prakash YS, van der Heijden HF, Kannan MS, Sieck GC. Effects of salbutamol on intracellular calcium oscillations in porcine airway smooth muscle. J Appl Physiol. 1997;82:1836–1843. doi: 10.1152/jappl.1997.82.6.1836. [DOI] [PubMed] [Google Scholar]
- Hall IP. Second messengers, ion channels and pharmacology of airway smooth muscle. Eur Respir J. 2000;15:1120–1127. doi: 10.1034/j.1399-3003.2000.01523.x. [DOI] [PubMed] [Google Scholar]
- Ay B, Iyanoye A, Sieck GC, Prakash YS, Pabelick CM. Cyclic nucleotide regulation of store-operated Ca2+ influx in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2005. [DOI] [PubMed]
- Kotlikoff MI, Kamm KE. Molecular mechanisms of b-adrenergic relaxation of airway smooth muscle. Annu Rev Physiol. 1996;58:115–141. doi: 10.1146/annurev.ph.58.030196.000555. [DOI] [PubMed] [Google Scholar]
- Zhou XB, Arntz C, Kamm S, Motejlek K, Sausbier U, Wang GX, Ruth P, Korth M. A molecular switch for specific stimulation of the BKCa channel by cGMP and cAMP kinase. J Biol Chem. 2001;276:43239–43245. doi: 10.1074/jbc.M104202200. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Yamashita Y, Yamaki F, Horinouchi T, Shigenobu K, Koike K. MaxiK channel mediates β2-adrenoceptor-activated relaxation to isoprenaline through cAMP-dependent and -independent mechanisms in guinea-pig tracheal smooth muscle. J Smooth Muscle Res. 2003;39:205–219. doi: 10.1540/jsmr.39.205. [DOI] [PubMed] [Google Scholar]
- Yamanaka J, Nishimura J, Hirano K, Kanaide H. An important role for the Na+-Ca2+ exchanger in the decrease in cytosolic Ca2+ concentration induced by isoprenaline in the porcine coronary artery. J Physiol. 2003;549:553–562. doi: 10.1113/jphysiol.2002.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolloczko B, Jia YL, Martin JG. Effects of cAMP on serotonin evoked calcium transients in cultured rat airway smooth muscle cells. Am J Physiol. 1997;272:L865–71. doi: 10.1152/ajplung.1997.272.5.L865. [DOI] [PubMed] [Google Scholar]
- Janssen LJ, Tazzeo T, Zuo J. Enhanced myosin phosphatase and Ca2+-uptake mediate adrenergic relaxation of airway smooth muscle. Am J Respir Cell Mol Biol. 2004;30:548–554. doi: 10.1165/rcmb.2003-0212OC. [DOI] [PubMed] [Google Scholar]
- Ding KH, Husain S, Akhtar RA, Isales CM, Abdel-Latif AA. Inhibition of muscarinic-stimulated polyphosphoinositide hydrolysis and Ca2+ mobilization in cat iris sphincter smooth muscle cells by cAMP-elevating agents. Cell Signal. 1997;9:411–421. doi: 10.1016/S0898-6568(97)00018-1. [DOI] [PubMed] [Google Scholar]
- Sanderson MJ, Parker I. Video-rate confocal microscopy. Methods Enzymol. 2003;360:447–481. doi: 10.1016/s0076-6879(03)60123-0. [DOI] [PubMed] [Google Scholar]
- Leybaert L, Sanderson MJ. Intercellular calcium signaling and flash photolysis of caged compounds. A sensitive method to evaluate gap junctional coupling. Methods Mol Biol. 2001;154:407–430. doi: 10.1385/1-59259-043-8:407. [DOI] [PubMed] [Google Scholar]
- Ehlert FJ. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal, airway and urinary bladder smooth muscle. Life Sci. 2003;74:355–366. doi: 10.1016/j.lfs.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Matsui M, Griffin MT, Shehnaz D, Taketo MM, Ehlert FJ. Increased relaxant action of forskolin and isoproterenol against muscarinic agonist-induced contractions in smooth muscle from M2 receptor knockout mice. J Pharmacol Exp Ther. 2003;305:106–113. doi: 10.1124/jpet.102.044701. [DOI] [PubMed] [Google Scholar]
- Hall IP, Daykin K, Widdop S. β2-adrenoceptor desensitization in cultured human airway smooth muscle. Clin Sci (Lond) 1993;84:151–157. doi: 10.1042/cs0840151. [DOI] [PubMed] [Google Scholar]
- Penn RB, Panettieri RAJ, Benovic JL. Mechanisms of acute desensitization of the β2AR-adenylyl cyclase pathway in human airway smooth muscle. Am J Respir Cell Mol Biol. 1998;19:338–348. doi: 10.1165/ajrcmb.19.2.3025. [DOI] [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- McGraw DW, Forbes SL, Kramer LA, Witte DP, Fortner CN, Paul RJ, Liggett SB. Transgenic overexpression of β2-adrenergic receptors in airway smooth muscle alters myocyte function and ablates bronchial hyperreactivity. J Biol Chem. 1999;274:32241–32247. doi: 10.1074/jbc.274.45.32241. [DOI] [PubMed] [Google Scholar]
- Koto H, Mak JC, Haddad EB, Xu WB, Salmon M, Barnes PJ, Chung KF. Mechanisms of impaired β-adrenoceptor-induced airway relaxation by interleukin-1beta in vivo in the rat. J Clin Invest. 1996;98:1780–1787. doi: 10.1172/JCI118977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif MH, Thulesius O. Forskolin reverses tachyphylaxis to the bronchodilator effects of salbutamol: an in-vitro study on isolated guinea-pig trachea. J Pharm Pharmacol. 1999;51:181–186. doi: 10.1211/0022357991772114. [DOI] [PubMed] [Google Scholar]
- Schramm CM. Beta-adrenergic relaxation of rabbit tracheal smooth muscle: a receptor deficit that improves with corticosteroid administration. J Pharmacol Exp Ther. 2000;292:280–287. [PubMed] [Google Scholar]
- Endou K, Iizuka K, Yoshii A, Tsukagoshi H, Ishizuka T, Dobashi K, Nakazawa T, Mori M. 8-Bromo-cAMP decreases the Ca2+ sensitivity of airway smooth muscle contraction through a mechanism distinct from inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;30:30. doi: 10.1152/ajplung.00287.2003. [DOI] [PubMed] [Google Scholar]
- Takuwa Y, Takuwa N, Rasmussen H. The effects of isoproterenol on intracellular calcium concentration. J Biol Chem. 1988;263:762–768. [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Ise S, Nishimura J, Hirano K, Hara N, Kanaide H. Theophylline attenuates Ca2+ sensitivity and modulates BK channels in porcine tracheal smooth muscle. Br J Pharmacol. 2003;140:939–947. doi: 10.1038/sj.bjp.0705508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H, Hall IP, Washabau RJ, Takagi K, Kotlikoff MI. β-adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J Clin Invest. 1994;93:371–379. doi: 10.1172/JCI116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen LJ. Ionic mechanisms and Ca2+ regulation in airway smooth muscle contraction: do the data contradict dogma? Am J Physiol Lung Cell Mol Physiol. 2002;282:L1161–78. doi: 10.1152/ajplung.00452.2001. [DOI] [PubMed] [Google Scholar]
- Ito Y, Takagi K, Tomita T. Relaxant actions of isoprenaline on guinea-pig isolated tracheal smooth muscle. Br J Pharmacol. 1995;116:2738–2742. doi: 10.1111/j.1476-5381.1995.tb17235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corompt E, Bessard G, Lantuejoul S, Naline E, Advenier C, Devillier P. Inhibitory effects of large Ca2+-activated K+ channel blockers on b-adrenergic- and NO-donor-mediated relaxations of human and guinea-pig airway smooth muscles. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:77–86. doi: 10.1007/PL00005141. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Elementary and global aspects of calcium signalling. J Physiol. 1997;499 ( Pt 2):291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tertyshnikova S, Fein A. Inhibition of inositol 1,4,5-trisphosphate-induced Ca2+ release by cAMP-dependent protein kinase in a living cell. Proc Natl Acad Sci U S A. 1998;95:1613–1617. doi: 10.1073/pnas.95.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule DI, Straub SV, Bruce JI. Modulation of Ca2+ oscillations by phosphorylation of Ins(1,4,5)P3 receptors. Biochem Soc Trans. 2003;31:954–957. doi: 10.1042/bst0310954. [DOI] [PubMed] [Google Scholar]
- Abdel-Latif AA. Cross talk between cyclic nucleotides and polyphosphoinositide hydrolysis, protein kinases, and contraction in smooth muscle. Exp Biol Med (Maywood) 2001;226:153–163. doi: 10.1177/153537020122600302. [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszodi A, Andersson KE, Krombach F, Mayerhofer A, Ruth P, Fassler R, Hofmann F. Defective smooth muscle regulation in cGMP kinase I-deficient mice. Embo J. 1998;17:3045–3051. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Kaftan EJ, Ehrlich BE, Watras J. Inositol 1,4,5-trisphosphate (InsP3) and calcium interact to increase the dynamic range of InsP3 receptor-dependent calcium signaling. J Gen Physiol. 1997;110:529–538. doi: 10.1085/jgp.110.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak DO, McBride S, Foskett JK. Inositol 1,4,5-trisphosphate activation of inositol trisphosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci U S A. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy KS, Severi C, Grider JR, Makhlouf GM. Inhibition of IP3 and IP3-dependent Ca2+ mobilization by cyclic nucleotides in isolated gastric muscle cells. Am J Physiol. 1993;264:G967–74. doi: 10.1152/ajpgi.1993.264.5.G967. [DOI] [PubMed] [Google Scholar]
- Yue C, Dodge KL, Weber G, Sanborn BM. Phosphorylation of serine 1105 by protein kinase A inhibits phospholipase Cb3 stimulation by Galphaq. J Biol Chem. 1998;273:18023–18027. doi: 10.1074/jbc.273.29.18023. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Bai Y, Sanderson MJ. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am J Physiol Lung Cell Mol Physiol, in submission. 2006. [DOI] [PubMed]