Abstract
Background
Because of its dramatic public health impact, Plasmodium falciparum resistance to chloroquine (CQ) has been documented early on. Chloroquine-resistance (CQR) emerged in the late 1950's independently in South East Asia and South America and progressively spread over all malaria areas. CQR was reported in East Africa in the 1970's, and has since invaded the African continent. Many questions remain about the actual selection and spreading process of CQR parasites, and about the evolution of the ancestral mutant gene(s) during spreading.
Methods
Eleven clinical isolates of P. falciparum from Cambodia and 238 from Africa (Senegal, Ivory Coast, Bukina Faso, Mali, Guinea, Togo, Benin, Niger, Congo, Madagascar, Comoros Islands, Tanzania, Kenya, Mozambique, Cameroun, Gabon) were collected during active case detection surveys carried out between 1996 and 2001. Parasite DNA was extracted from frozen blood aliquots and amplification of the gene pfcrt exon 2 (codon 72–76), exon 4 and intron 4 (codon 220 and microsatellite marker) were performed. All fragments were sequenced.
Results
124 isolates with a sensitive (c76/c220:CVMNK/A) haplotype and 125 isolates with a resistant c76/c220:CVIET/S haplotype were found. The microsatellite showed 17 different types in the isolates carrying the c76/c220:CVMNK/A haplotype while all 125 isolates with a CVIET/S haplotype but two had a single microsatellite type, namely (TAAA)3(TA)15, whatever the location or time of collection.
Conclusion
Those results are consistent with the migration of a single ancestral pfcrt CQR allele from Asia to Africa. This is related to the importance of PFCRT in the fitness of P. falciparum point out this protein as a potential target for developments of new antimalarial drugs.
Background
The molecular basis of chloroquine resistance has recently been elucidated. Pfcrt (Plasmodium falciparum chloroquine resistance transporter), a single copy, 13-exon gene, localized on chromosome 7, codes for a parasite digestive vacuole trans-membrane protein, which plays a key role in CQR [1,2]. The pfcrt gene appears to be polymorphic and more than 20 different amino-acid sequences have been identified [3-5]. Recent genome wide microsatellite scanning has shown marked linkage disequilibrium around the pfcrt locus and pointed to four distinct founder events, with one ancestral mutant originating in Asia and subsequently invading Africa [6]. The evolution of this mutant after its local emergence and its spread across Africa has not been studied.
Specific sets of mutations in pfcrt have been associated with chloroquine resistance. All but one in vitro CQ-sensitive (CQS) isolates carry a CVMNK haplotype at residues 72–76, whereas in vitro CQR isolates and isolates collected from CQ treatment failures harbour a different 72–76 haplotype. Strikingly, in spite of numerous studies done in Africa, only one resistant haplotype (CVIET) has been ever found so far in this continent. This haplotype carries in addition to the set of mutations in codons 72–76 within exon 2, a A to S mutation at codon 220 located within exon 4, resulting in a CVIET/S haplotype [7]. Nine bp downstream from this mutation starts intron 4, which contains a polymorphic (TAAA)n(TA)m microsatellite (Figure 1). Because microsatellites evolve much faster than coding sequences, intron-associated microsatellite polymorphism can be used to ear-mark individual pfcrt alleles and to pin-point the spread of specific alleles. In order to test the 'one event invasion theory', a characterization of CVMNK/A and CVIET/S alleles from Asia and Africa was carried out.
Figure 1.
pfcrt genomic sequence available in EMBL. In bold, the mutation leading to the amino acid change in 220 position, in italic the beginning of the 4th intron of the pfcrt gene showing the (TAAA)m(TA)n microsatellite (underscore).
Methods
Sampling
Eleven clinical isolates of P. falciparum from Cambodia, 112 from West and Central Africa (Senegal, Ivory Coast, Bukina Faso, Mali, Guinea, Togo, Benin, Niger, Congo) and 126 from East and South Africa (Madagascar, Comoros Islands, Tanzania, Kenya, Mozambique, Cameroun, Gabon) were collected during active case detection surveys carried out between 1996 and 2001. Briefly, 5 mL blood samples were collected in EDTA vacutainers from patients diagnosed as having uncomplicated P. falciparum malaria. Immediately after blood collection, the patients were treated according to national recommendations. Blood samples were kept at 4°C for in vitro drug sensitivity testing. An aliquot of each isolate was frozen at -20°C for molecular analysis. Informed consent was obtained from the patients. Ethical clearance was obtained from the respective National Ethical Commitees.
DNA extraction, PCR analysis and sequencing
Parasite DNA was extracted from frozen blood aliquots by the phenol-chloroform method as described previously [8]. The pfcrt haplotype was determined by sequencing PCR fragments, amplified from single clone infections (as determined using msp2 amplification profiles see [8] for detail) using two sets of primers designed to amplify a 194 bp exon 2 fragment (CRT76-sense 5'-GGTGGAGGTTCTTGTCTTGG-3 and CRT76-antisense 5'-ATAAAGTTGTGAGTTTCGGATG-3') and 309 bp fragment from exon 4 to exon 5 (CRT220-sense 5'-TTATACAATTATCTCGGAGCAG-3' and CRT220-antisense 5'-CATGTTTGAAAAGCATACAGGC-3'). The primers were selected on the basis of the sequence of the pfcrt gene of the Dd2 clone (accession number AF030694). PCR was performed in a PTC-100 thermal cycler (MJ Research, Merck Eurolab) under the following conditions: 50 μL final volume containing 2 μL DNA, 200 μM each dNTP, 0.5 μM each primer, 2 mM MgCl2, and 2 U TaqI DNA polymerase (Promega) in the buffer supplied by the manufacturer.
The amplification conditions were: 1 min at 95°C, 1 min 30 at 59°C, and 1 min at 72°C for 1 cycle; 10 s at 95°C, 1 min 30 at 59°C, and 2 min at 72°C for 35 cycles, and a final extension step at 72°C for 10 min. The resulting PCR products contained codons 76 and 220 as well as intron 4, respectively. All fragments were subjected to gel electrophoresis on agarose gels containing ethidium bromide and were sequenced using the fluorescent dye terminator method as described in [9]. The multiple infections still found on the basis of sequencing of the microsatelittes were discarded from the analysis (two Cambodian and six African isolates). Each sequence was analysed using Chromas® v 1.45 software.
Results
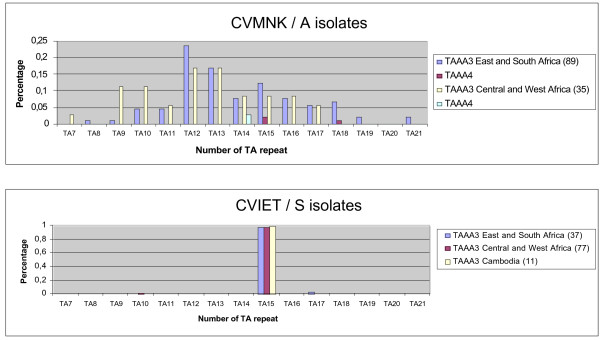
Briefly, 124 isolates with a sensitive (CVMNK/A) haplotype and 125 isolates with a resistant CVIET/S haplotype were typed for the intron 4 microsatellite. Polymorphism of this locus was large, with total of 17 distinct microsatellite alleles identified, namely forteen (TAAA)3 (TA)7-21 and three (TAAA)4 (TA)11-18 (Figure 2). All 17 types were detected in the isolates carrying the CVMNK/A haplotype. Thus, numerous distinct alleles carry the "wild type" CQS haplotype. The individual distribution of the various microsatellite types was consistent with a random generation of polymorphism, as expected for microsatellites and there were no indication of clustering. In contrast, all 125 isolates with a CVIET/S haplotype but two had a single microsatellite type, namely (TAAA)3(TA)15, whatever the location or time of collection. The same microsatellite type was found in the CQR Dd2 clone of the IndochinaIII strain used for the original characterization of the resistant CVIET/S allele (Figure 1). On the other hand, the (TAAA)3(TA)11 type of the CQS 3D7 reference clone was observed in only six of 124 field isolates with the CVMNK/A haplotype, consistent with the extensive Pfcrt intron 4 polymorphism in CQS isolates.
Figure 2.
pfcrt intron 4 microsatellite allelic frequencies in different populations. The relative frequency of the various allelic forms within each haplotype subgroup is indicated. Number of CVMNK/A isolates investigated was 89 from East and Austral Africa and 35 from West and Central Africa. Number of CVIET/S isolates investigated was 37 from East and Austral Africa, 77 from West and Central Africa and 11 from Cambodia. (TAAA)m(TA)n alleles are classified according to the number (n) of TA repeats that follow (TAAA)3 (plain bars) or (TAAA)4 (hatched bars). The colour codes for the various geographical regions are blue for East and Austral Africa, red for West and Central Africa and yellow for Cambodia.
The strikingly different microsatellite distribution in isolates carrying a resistant or a sensitive haplotype was highly significant (chi-square test P < 0.00001). Intron 4 microsatellite field polymorphism is such that the observation of a single allelic form in all but two isolates carrying a resistant haplotype cannot be explained by chance. This indicates that the resistant CVIET/S haplotype is indeed ear-marked by a specific intragenic microsatellite, and points to the existence of a single progenitor allelic form for this locus in African areas investigated here. This represents a strong population genetic evidence for an absence of endogenous emergence of a resistant allele in Africa. It demonstrates the migration of one single CVIET/S pfcrt allele from South East Asia into Africa and its subsequent spreading throughout the whole African continent.
Discussion
Interestingly, a similar analysis on 15 CQR isolates from French Guiana showed that the typical South American (SVMNT/S) resistant haplotype was also associated with a single (TAAA)3(TA)14 microsatellite type, that differed from the (TAAA)3(TA)15 observed in African and Asian CQR isolates. This indicates that the microsatellite sequence itself has limited phenotypic impact and is reminiscent with the association of resistant pfcrt haplotypes with specific intron 4 microsatellites. This shows that resistant haplotypes that have emerged independently in Asia and in South America more than 30 years ago have limited local diversity nowadays.
Why did resistant parasites emerge in Asia/America and not in Africa, despite the larger size of the overall African P. falciparum population?
One of the explanations could be that there is higher P. falciparum mutation rate in Asia/America than in Africa [10]. But, if so, after two decades without CQ pressure in Asia/America, falciparum pfcrt would have "reversed" to the wild type allele, since the appearance of resistant strains occurred at the very beginning of the introduction of chloroquine on a large scale. The latest studies, that were performed in Asia/America, show no reappearance of wild type pfcrt strains, invalidate this hypothesis [4].
Most likely, this emergence can be attributed to differences in transmission conditions and population structure. The endemic areas in South East Asia and South America can be considered as "localized permanent epidemics" [11], with a metapopulation structure for the P. falciparum populations, in which forces acting on differentiation are more effective than in Africa [12]. Indeed population isolation is thought to lead to an increased parasite diversity or local sub-speciation. In a subpopulation of a small number of parasites, genetic drift will act as a major force. This will increase polymorphism on coding sequence without any link with the possible fitness of the selected allele (this also as been shown for cytochrome b sequence in Asia/America compared to Africa [13]). When CQ pressure increased, some pfcrt alleles associated with low fitness in CQ free environment will become associated with a high fitness in this CQ environment and could thus invade the African parasite population. If CQ pressure stops, and if the ''wild type allele" is still present in the subpopulation, it will replace the mutant allele (studies in Malawi show that the relative fitness of the mutant pfcrt allele in the absence of CQ pressure is 0.76 or 0.86 [15,16]).
Conclusion
Field isolates collected from clinical cases confirm that the current continent-wide CQR in Africa is due to the invasion of a single mutant allele. Therefore, monitoring the gene flow regionally can provide valuable information in assessing selection of candidate resistance gene by selective drug pressure for other antimalarials or vaccine escape.
Moreover, despite the overall PfCRT protein polymorphisms described so far in Asia [4], only one major allele has invaded Africa. This can probably be explain by the importance of this protein in the fitness of the parasite and thus to the difficulty for a pfcrt mutant to be selected in CQ-free area of high transmission where fierce competition occurs between strains. This strongly suggest that pfcrt is still one of the major potential target for antimalarial drugs and reinforces the need for a better understanding of its role in Plasmodium metabolism [17].
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
FA, TF, RD, MR, RJ, EL, JBD, VR, JLB and OMP paritcipate to the sampling, the molecular typing of isolates, the data analysis and contributed for the elaboration of manuscript. MTE, CB and SC performed the molecular analysis of the majority of the samples. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was funded by the French Academy of Sciences and the FSP-RAI project from the French government. We are indebted to the field workers (physicians, nurses and technicians) for participation in collecting parasitized blood samples.
Contributor Information
Frédéric Ariey, Email: fariey@pasteur-kh.org.
Thierry Fandeur, Email: thierry.fandeur@univ-tours.fr.
Remy Durand, Email: remy.durand@avc.ap-hop-paris.fr.
Milijaona Randrianarivelojosia, Email: milijaon@pasteur.mg.
Ronan Jambou, Email: rjambou@pasteur.sn.
Eric Legrand, Email: elegrand@pasteur-cayenne.fr.
Marie Thérèse Ekala, Email: mtekala@pasteur.fr.
Christiane Bouchier, Email: bouchier@pasteur.fr.
Sandrine Cojean, Email: sandrine.cojean@wanadoo.fr.
Jean Bernard Duchemin, Email: duchemin@cermes.org.
Vincent Robert, Email: v.robert@mnhn.fr.
Jacques Le Bras, Email: jacques.lebras@bch.ap-hop-paris.fr.
Odile Mercereau-Puijalon, Email: omp@pasteur.fr.
References
- May J, Meyer CG. Chemoresistance in falciparum malaria. Trends Parasitol. 2003;19:432–435. doi: 10.1016/j.pt.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LM, Sidhu AB, Naude B, Deitsch KW, Su XZ, Wootton JC, Roepe PD, Wellems TE. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6:861–871. doi: 10.1016/S1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton JC, Feng X, Ferdig MT, Cooper RA, Mu J, Baruch DI, Magill AJ, Su XZ. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- Lim P, Chy S, Ariey F, Incardona S, Chim P, Sem R, Denis MB, Hewitt S, Hoyer S, Socheat D, Merecreau-Puijalon O, Fandeur T. pfcrt polymorphism and chloroquine resistance in Plasmodium falciparum strains isolated in Cambodia. Antimicrob Agents Chemother. 2003;47:87–94. doi: 10.1128/AAC.47.1.87-94.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhleman AC, Yuthavong Y, Fidock DA. Mechanisms of antimalarial drug action and resistance. In: Sherman IW, editor. Molecular approaches to malaria. Washington DC. ASM Press; 2005. pp. 429–461. [Google Scholar]
- Mehlotra RK, Fujioka H, Roepe PD, Janneh O, Ursos LM, Jacobs-Lorena V, McNamara DT, Bockarie MJ, Kazura JW, Kyle DE, Fidock DA, Zimmerman PA. Evolution of a unique Plasmodium falciparum chloroquine-resistance phenotype in association with pfcrt polymorphism in Papua New Guinea and South America. Proc Natl Acad Sci U S A. 2001;98:12689–12694. doi: 10.1073/pnas.221440898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller KarenaL, Lee Sylvia, Fidock DavidA. Molecular and Cellular Biology of Chloroquine Resistance in Plasmodium falciparum. In: Waters AP, CJ Janse Leiden, editor. Genomes and Molecular Biology. Chapter 16 2004. [Google Scholar]
- Contamin H, Fandeur T, Bonnefoy S, Skouri F, Ntoumi F, Mercereau-Puijalon O. PCR typing of field isolates of Plasmodium falciparum. J Clin Microbiol. 1995;33:944–951. doi: 10.1128/jcm.33.4.944-951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariey F, Randrianarivelojosia M, Duchemin JB, Rakotondramarina D, Ouledi A, Robert V, Jambou R, Jahevitra M, Andrianantenaina H, Raharimalala L, Mauclere P. Mapping of a Plasmodium falciparum pfcrtK76T mutation: a useful strategy for controlling chloroquineresistance in Madagascar. J Infect Dis. 2002;185:710–712. doi: 10.1086/339000. [DOI] [PubMed] [Google Scholar]
- Rathod PK, McErlean T, Lee PC. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc Natl Acad Sci U S A. 1997;94:9389–9393. doi: 10.1073/pnas.94.17.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdrager J. Localized permanent epidemics: the genesis of chloroquine resistance in Plasmodium falciparum. Southeast Asian J Trop Med Public Health. 1995;26:23–28. [PubMed] [Google Scholar]
- Ariey F, Duchemin JB, Robert V. Metapopulation concepts applied to falciparum malaria and their impacts on the emergence and spread of chloroquine resistance. Infect Genet Evol. 2003;2:185–192. doi: 10.1016/S1567-1348(02)00099-0. [DOI] [PubMed] [Google Scholar]
- Ekala M, Legrand E, Kim N, Randrianarivelojosia M, Jambou R, Fandeur T, Ariey F, Bouchier C, Mercereau Puijalon O. Sequencevariations of Plasmodium falciparum cytochrome b in field isolates from a multicentric study. Abstract MIM-ME-142462 Yaounde Cameroun. 2005.
- Mita T, Kaneko A, Lum JK, Zungu IL, Tsukahara T, Eto H, Kobayakawa T, Bjorkman A, Tanabe K. Expansion of wild type allele rather than back mutation in pfcrt explains the recent recovery of chloroquine sensitivity of Plasmodium falciparum in Malawi. Mol Biochem Parasitol. 2004;135:159–163. doi: 10.1016/j.molbiopara.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Kublin JG, Cortese JF, Njunju EM, Mukadam RA, Wirima JJ, Kazembe PN, Djimde AA, Kouriba B, Taylor TE, Plowe CV. Reemergence ofchloroquine-sensitive Plasmodium falciparum malaria aftercessation of chloroquine use in Malawi. J Infect Dis. 2003;187:1870–1875. doi: 10.1086/375419. [DOI] [PubMed] [Google Scholar]
- Cortese JF, Caraballo A, Contreras CE, Plowe CV. Origin and dissemination of Plasmodium falciparum drug-resistance mutations in South America. J Infect Dis. 2002;186:999–1006. doi: 10.1086/342946. [DOI] [PubMed] [Google Scholar]
- Cooper RA, Hartwig CL, Ferdig MT. pfcrt is morethan the Plasmodium falciparum chloroquine resistance gene: a functional and evolutionary perspective. Acta Trop. 2005;94:170–180. doi: 10.1016/j.actatropica.2005.04.004. [DOI] [PubMed] [Google Scholar]