Abstract
Stimulated by recent 13C and 31P NMR studies of exercising muscle, we propose a model of the energetics of contraction. Previous studies of energetics have followed energy consumption. However, the rapidity of contraction, in 10–40 msec, requires that energy be delivered rapidly, so that the muscle has power requirements of rapid energy expenditure that are ultimately met by the slower averaged consumption of carbon and oxygen from blood. We propose that energy is supplied in milliseconds by glycogenolysis and that between contractions, glycogenesis refills the pools. The energy for glycogenesis is supplied by oxidative phosphorylation. This mechanism utilizes the rapid conversion of glycogen phosphorylase, the “fight-or-flight” enzyme, to its active form. Lactate is necessarily generated by this pathway to serve as a time buffer between fast and slow energy needs, which resolves the paradoxical generation of lactate in well oxygenated tissue. Consequences of the glycogen shunt are compatible with numerous biochemical and physiological experiments. The model provides a possible mechanism for muscle fatigue, suggesting that at low but nonzero glycogen concentrations, there is not enough glycogen to supply millisecond energy needs.
Despite intensive biochemical study, the path of energy production in the muscle from glucose is incompletely understood. An example is the generation of lactate during exercise, which is attributed in textbooks to insufficient oxygenation (1). Yet, as has been shown often, there are exceptions to this generalization. Mitochondrial oxidation states are unchanged by exercise in which lactate levels rise (2), and several other experiments show adequate oxygenation during lactate generation (3, 4). Brooks and colleagues, facing this paradox, proposed the lactate shuttle hypothesis, in which lactate distributes carbohydrate energy sources after a meal and during sustained physical exercise (5). However, although this shuttle can describe the flows of lactate, still the reasons suggested for lactate generation have been less convincing.
There are also uncertainties about the metabolic roles of muscle glycogen in exercise. High glycogen levels improve endurance (6), whereas depletion of glycogen is often associated with fatigue (7). However, the specific need for glycogen, especially in the presence of sufficient blood glucose, has not been explained, whereas no specific connection of glycogen levels with fatigue has been possible (8). Nor have the steady-state measurements of glycogen concentrations been related to rapid energy bursts during the millisecond contractions of muscle fibers. Holloszy and Kohrt (9), in summarizing fundamental questions that remain about muscle energetics, led off with “Why is muscle glycogen necessary for exercise of moderate and high intensities?”
In this paper, we propose a physiological function for muscle glycogen metabolism that provides a mechanistic explanation for lactate generation during aerobic exercise and for the requirement of glycogen for exercise.
The Glycogen Shunt Model
Recent 13C and 31P NMR measurements of muscle energetics form the basis of our proposed function of glycogen, which is called the “glycogen shunt,” and which has been proposed to function in mammalian brain metabolism.§ Our discussion centers on moderate exercise, such as long distance running or swimming at ≈20% of maximal voluntary contraction, where measurements of energy consumption and usage are made over periods of minutes or hours. In these exercises, glycogen is initially used as a net fuel. However, after partial depletion, net usage ceases, and the concentration stays constant. 13C NMR studies, reviewed below, indicate continued breakdown of glycogen accompanied by resynthesis during the period of constant glycogen. A diagram of the biochemical pathways in the model is shown in Fig. 1. The physiological function of the glycogen shunt is to provide the rapid release of energy needed to support muscle contractions that last on the order of 20–100 msec. Glycogen phosphorylase, the so-called “fight-or-flight” enzyme that controls the rate of glycogenolysis, is well suited for this purpose because its rapid activation is triggered by calcium and a phosphorylation cascade. The subsequent conversion of glucose 6 phosphate to lactate produces the ATP needed to fuel the contraction. Between contractions, glycogen is resynthesized by glycogen synthase from plasma glucose to replete the pool. Energy to resynthesize glycogen and any net PCr that is broken down for short-term requirements is supplied by oxidation of the lactate generated. The stoichiometry of a complete cycle of the glycogen shunt for one glucosyl unit of glycogen is given in Table 1. The first step occurs during the first ≈15 msec of the contraction and involves PCr being broken down to resynthesize ATP used during the contraction. In the remainder of the contractile phase (≈15–100 msec), the PCr is resynthesized from glycogen breakdown and glycolysis. Finally in the third step, during the intercontraction relaxation period, glycogen lost during the second step is resynthesized. For explanatory purposes, the cycle of contraction and restoration is broken down into three discrete steps. In actual muscle fibers, there can be temporal overlap between these events.
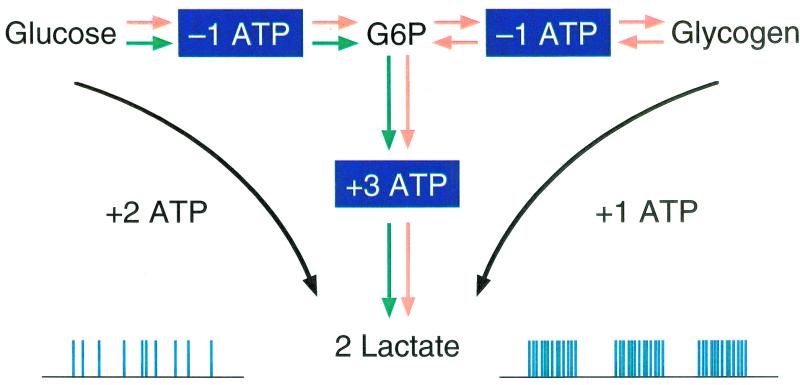
Figure 1.
Schematic of two alternative pathways from glucose to the lactate pyruvate pool. The direct glycolytic pathway produces two lactates and two ATP, whereas the “glycogen shunt” produces only one ATP. We propose that during contractions, glycogenolysis makes a major contribution to the energy needs and that glycogen is replenished between contractions. Because the lactate production is determined by the power requirements during the millisecond contractions, it does not match the average energy requirements that are supplied efficiently by oxidation of lactate/pyruvate. Excess lactate is generated to buffer short- and long-term energy requirements.
Table 1.
Temporal energetics of the glycogen shunt
| Step 1. (0–15 msec) Breakdown of PCR to restore ATP used to fuel contraction |
| 3ATP → 3ADP + 3 Pi |
| 3PCR + 3ADP → 3ATP + 3Cr |
| Net: 3PCR → 3Cr + 3 Pi |
| Step 2. (15–100 msec) ATP from glycogen breakdown and glycolysis restores PCr |
| Glycogen (n + 1) + Pi → glycogen (n) + G6P |
| G6P + 3ADP + 2Pi → 2 lactate + 3ATP |
| 3 Cr + 3ATP → 3ADP + 3PCR |
| Net: 3Cr + 3Pi + Glycogen (n + 1) → Glycogen (n) + 3 PCr + 2lactate |
| Step 3. Resynthesis of glycogen from ATP produced by lactate oxidation |
| glycogen (n) + glucose + 2ATP → Glycogen (n + 1) + 2ADP + 2 Pi |
| 2lactate + 0.6 O2 + 2ADP + 2Pi → 0.6 CO2 + 2ATP + 0.6 H2O + 1.8 lactate |
| Sum of 3 steps: glucose + 0.6 O2 → 0.6 CO2 + 0.6 H2O + 1.8 lactate (note that 1.8 lactates per glycosyl unit transported out of muscle cell) |
The inefficient production of ATP by the continuous synthesis and degradation of glycogen results in the production of more lactate than is needed for oxidation to restore glycogen and PCr levels. The excess lactate is transported to other sites of oxidation, presumably as outlined by the lactate shuttle model (5). The “glycogen shunt” couples the rapid needs for power during contraction to the long-term oxidation of glucose and the excess lactate production is a consequence.
Experimental Evidence for the Glycogen Shunt
We first present experimental data obtained by in vivo 13C NMR studies of glycogen in the exercising human gastrocnemus muscle (10, 11) and 31P NMR (12) measurements of ATP and PCr during rat muscle contraction. These results are combined to suggest that glycogenolysis provides a large fraction of the energy needed for contractions. These 13C and 31P NMR results have been the basis of the “glycogen shunt” hypothesis, which is subsequently tested against a variety of biochemical and physiological data. Additional evidence from a variety of studies is presented and is seen to be consistent with this model.
Evidence from 13C NMR of Human Muscle for Glycogen Turnover During Exercise
Accurate noninvasive measurements of human muscle glycogen concentrations may be obtained from the natural abundance 13C NMR signals (13, 14). Values of glycogen concentrations obtained in this way from muscle agree with simultaneous biopsy measurement (13) and chemical analysis (14) and, because they are noninvasive, have allowed precise repetitive determinations. The infusion of isotopically enriched [1-13C] glucose allows the determination of the separate rates of glycogen synthase and glycogen phosphorylase (11).
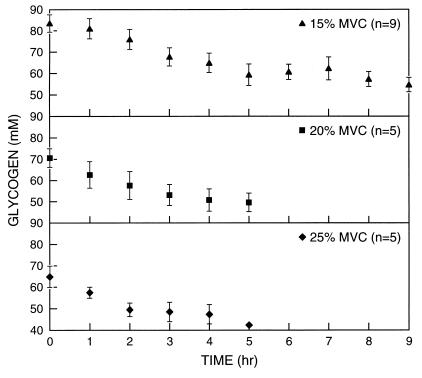
The concentration of gastrocnemius glycogen was measured by using naturally abundant 13C NMR during prolonged low-to-moderate intensity exercise for several hours. The NMR study clearly separated two phases of glycogen metabolism during muscular activity (10). In the first phase, glycogen is consumed at a rate that provides a significant net contribution to the energy requirements. In Fig. 2, this phase is seen during the first 2–2.5 h of exercise. In the second phase, glycogen concentrations become constant, indicating negligible net contributions of glycogen consumption to energy production. These measurements are in general agreement with previous studies (15, 16). However, they provide more detailed information on the initial rate of glycogen utilization and show more quantitatively the constant glycogen levels during the next several hours of continued exercise. This accurate quantitation of constant glycogen concentration for several hours by 13C NMR, showing that it supplies no net energy, highlights Holloszy and Kohrt's (9) question as to the function of glycogen under these conditions.
Figure 2.
Gastrocnemius glycogen concentration vs. exercise time for low-intensity exercise from rest at 15, 20, and 25% of maximal voluntary contraction (MVC). Values are means ± SE [reproduced with permission from ref. 11 (Copyright 1991, The American Physiological Society)].
To address the question of whether the cessation of net glycogen utilization was caused by deactivation of glycogen phosphorylase or, alternatively, by dynamic synthesis and degradation, we performed a study using 13C NMR in conjunction with an infusion of enriched 1-13C glucose during the late period of constant glycogen concentration (11). During this period, 13C label was incorporated at a substantial rate, showing high activity of glycogen synthesis. The absence of net synthesis during label incorporation therefore resulted from simultaneous and equal activity of synthesis and degradation. The rate of this glycogen turnover was calculated to fall between 25 and 100% of the initial rate of net glycogen breakdown. Because the total rate of muscle glycolysis (glucose uptake plus net glycogenolysis) does not change significantly during the early and late periods of this kind of exercise (6, 7), this result suggests that a large fraction of the glucose being phosphorylated by the muscle passes through the glycogen pool (Table 1).
The Relative Role of PCr and Glycogen During Millisecond Contractions
The conventional view of short-term energetics in the muscle is that PCr supplies all the energy needed for a sustained burst of contractions for ≈10 seconds, after which it is replaced by glycogenolysis. Although this view is presented in textbooks, it is not supported by experiments. In a recent paper (17), Greenhaff and Timmons report, “It is now accepted, however, that PCr hydrolysis and lactate production do not occur in isolation, and that both are initiated rather rapidly at the onset of contraction.”
In moderate-intensity exercise, the contractile period is much shorter, and sequential contractions are separated by durations several times longer than the contractile time. Just how rapidly energy is obtained from PCr during single isolated contractions was shown directly by recent brilliant experiments by Chung et al. (12). Reasoning that even the fastest freeze-clamp measurements, with a time resolution of ≈100 msec, did not follow high-energy phosphates during contraction and relaxation, they developed a gated 31P NMR technique with ≈1-msec time resolution. ATP remained constant at all times during contractions, whereas PCr immediately fell by 3 μM ⋅ g tissue−1 ⋅ twitch−1 with a half time of 8 msec. PCr then returned to baseline with a half time of 14 msec so that the whole PCr response to a twitch was finished in ≈40 msec, too rapidly to have been observed by even the fastest extraction methods. Chung et al. showed that this consumption of PCr/twitch is ≈40 times greater than the values reported by dividing the drop in PCr after several seconds of contraction by the number of twitches. This 31P NMR study showed that the traditional method for calculating ΔPCr/twitch underestimates the high energy consumption during the millisecond contraction cycle.
The rapid resynthesis of PCr over the short contractile period appears to be consistent only with ATP generation from glycogenolysis. The energy cannot come from oxidation, because the rate of oxygen consumption is ≈103 times slower than the rate of PCr repletion. Nor could the ATP required during relaxation come from glycolytic intermediates. Except for intense anaerobic exercise, the combined concentration of intramuscular glucose and glucose 6 phosphate is below 0.3 mM, which is insufficient to provide enough flux even if completely depleted (18, 19). Glucose transport flux, which is effectively unidirectional because of the low intracellular concentration and therefore equal to the average rate measured during exercise, is at least 100 times too low to support the rapid flux (20, 21). Therefore, a large fraction of ATP resynthesis must come from glycogenolysis. During recovery, ≈3 mM of PCr is resynthesized, providing a lower limit of energy per contraction, because rat data suggest that additional ATP has probably been synthesized before PCr repletion starts. Therefore, more than 1.5 mmol of glycogen subunits is consumed during each contraction. Because the well fed resting rat muscle has ≈70 mM glucosyl units, glycogen must be resynthesized between twitches. Without resynthesis, glycogen would be depleted after several dozen contractions, which is not observed.
Additional evidence for the ability of glycogenolysis to support the high power requirements during the contractile period comes from studies in which the activity of creatine kinase was reduced in rats with lowered concentrations of PCr (22). 31P NMR spectra of rats on a diet of β-guanidino propionic acid for 6–12 weeks showed that the steady-state PCr level was reduced by ≈90%. Gastrocnemius–plantaris muscles were subjected to tetanic contractions for periods of 1 sec in PCr-depleted rats and in controls. 31P NMR spectra were gated to the onset of tetanic contraction and NMR spectra obtained at 0.2, 0.4, 0.7, and 0.95 s of both depleted rats and controls. The surprising result was that all of the PCr was retained for 0.2 s in both depleted and control rats. Subsequently, the PCr signal decreased more slowly in the controls. Shoubridge and Radda (22) estimated that 3 mM ATP was required to sustain the contraction over this period, which would require 1.5 mM of glucose to be consumed by glycolysis, again well above the level of glucose 6 phosphate and glucose in the muscle during sustained moderate exercise, which leaves glycogen as the likely source.
Watchko et al. (23) created mutant mouse strains missing either or both forms of creatine kinase and compared functional diaphragm muscle performance with controls during isotonic activation. Both singly mutated forms showed no difference from controls in force velocity, power, time of sustained shortening, and work output, whereas in the doubly mutated animal CK [−/−], these were somewhat reduced. In CK [−/−] animals, the velocity of shortening decreased by 20%; maximum power decreased by 16%; work decreased by 30%, and the time to sustain shortening during repetitive isotonic activation decreased by 40%. In contrast to these moderate functional decreases, the creatine kinase activity in CK [−/−] mutants had decreased to less than 1% of control values.
Muscle Fibers and the Lactate Shuttle
If, as proposed in our model, there is an undiminished need for nonoxidative ATP during exercise, then the rate of glucose uptake during the phase of constant glycogen concentration should equal the initial net rate of glycogen breakdown. If other energy sources such as fatty acid oxidation replace the need for glycogen utilization, there should be a substantially lower requirement for glucose consumption during the level period. The rate of muscle glucose consumption and the concentration of muscle glycogen during sustained exercise at various intensities have been measured by Saltin and Gollnick (7), who found that the initial rate of glycogen breakdown at the earliest time is in good agreement with the rate of glucose consumption measured later when glycogen concentration remains constant.
The cessation of net glycogen breakdown may occur because of a change in the muscle fiber recruitment pattern, in which case synthesis and depletion would reflect glycogen synthesis in the longer active fibers that are balanced by glycogen depletion in newly recruited fibers. However, Gollnick and colleagues reported that at 3 h of exercise at 15% of maximal voluntary contraction, all slow-twitch fibers and 40% of all fast-twitch fibers were at least partially glycogen depleted (24). Furthermore, 90% of the slow-twitch fibers were significantly depleted. These findings are not consistent with a steady depletion model but are consistent with the steady state postulated in the glycogen shunt, in which there are simultaneous glycogen synthesis and depletion. However, when considering fiber types, our results do not distinguish between turnover occurring within a fiber from the possibility that lactate is generated in white fibers and transported to red fibers, where it is oxidized.
Evidence That Glucose Cannot Replace Glycogen's Role for Rapidly Generating Glycolytic ATP During Contraction
The proposal that glycogenolysis cannot be replaced by glucose as an energy source for exercise is supported by studies of patients with McArdle's disease. In this disease, glycogen phosphorylase is completely inactive. Patients suffer from exercise intolerance and muscle cramping. 31P NMR studies (25) of the resting muscle in these patients showed normal PCr, ATP, and Pi levels but a decidedly higher pH (≈7.2) than in controls (7.02 ± 0.01). On either aerobic or anaerobic exercise, PCr decreased much more rapidly than in controls, whereas the pH remained high, and ATP levels remained constant. In control subjects exercising at higher intensity, an equivalent drop in PCr was shown to be accompanied by a drop in pH, which is indicative of lactate production. The inferred minimal lactate production in the McArdle's patients relative to control subjects is evidence that the muscle cannot use glucose sufficiently rapidly to satisfy its contractile requirements for glycolytic ATP. Furthermore, as discussed above, the normal levels of PCr present in these patients did not supply enough energy to support mild exercise in the absence of a glycogenolytic flux that, in our models, replenishes PCr levels.
Biochemical Evidence for the Model
The proposed model of the glycogen shunt is consistent with biochemical data on the activities of glycogen phosphorylase, synthase, and glucose transport. First, numerous reports find glycogen phosphorylase in its active form under conditions where glycogen concentrations are constant (26). In studies of the control of the enzyme, Ren and Hultman conclude, “The paradox of a high Pi content and extensive transformation of phosphorylase to the a form but low glycogenolytic activity points to additional factors in the regulation of glycogen breakdown” (27). On the basis of these findings, and in view of the rapid turnover reported here, the allosteric explanation of phosphorylase control in vivo should be reevaluated. Second, a large number of in vitro and in vivo studies have shown that the rate of glycogen synthesis increases as glycogen concentration decreases (28, 29). The lower concentration of glycogen maintained during more intense exercise is consistent with higher rates of synthesis expected only if there is an increased degradation of glycogen during the intense exercise. In this respect, the lower concentrations are explained by the glycogen shunt model.
Finally, glucose transporter activity increases with exercise, consistent with the finding of increased glucose consumption and slower glycogen net consumption. Glucose uptake is controlled by the recruitment of glucose transporters to the plasma membrane and transporter number determines glucose consumption (20, 21). Under conditions of moderate exercise, glucose transport is acting as a unidirectional enzyme with a rate equal to that of the average glucose consumption rate, much slower than the rate of glycolysis required during the contractile period (18, 19). Glucose transporter recruitment can respond to the average needs for glycolysis during exercise by refilling the glycogen pools, but transporter recruitment is slower and cannot respond in the millisecond time frame required to support individual contractions (30).
Proposed Role for Glycogen During Fatigue
Present results provide a possible explanation of the importance of glycogen in muscular fatigue, which has not been explained satisfactorily despite intense study. The traditional explanation for the role of glycogen in fatigue is either as a source of lactate and the associated acidification of the muscle or because of the inability of depleted glycogen to provide net energy. However, these correlations are incomplete. In fatigued McArdle patients, phosphorylase deficiency results in higher pH than in resting controls, which is one of the many results questioning lactate acidosis as the exclusive cause of fatigue.
Normal subjects performing at moderate/heavy workloads fatigue on the depletion of glycogen, even though at the time of depletion the net usage of glycogen for energy is insignificant. In one finding, glycogen levels were measured during exercise with and without continuous ingestion of glucose (8). Glycogen decreased at about the same rate in both cohorts, but because the feeding subjects started at higher concentrations, they took 1 h longer to fatigue. In both cases, fatigue occurred at a particular nonzero concentration of complete glycogen, even though at the time of fatigue, glucose consumption had almost completely replaced net glycogen consumption as an energy source. Previously, this kind of finding was considered paradoxical in that the net energy supplied by glycogen at the fatigue point was small relative to total energy production. In the glycogen shunt model, fatigue on depletion of glycogen could be allowed by energy failure during the contraction. During contraction at very low glycogen concentrations, glycogen levels might be insufficient to provide the rapid burst of glycogenolysis required for the contraction. Fatigue would be the result of an inability to provide the very high initial power requirements of the contraction through glycogenolysis rather than because of the loss of glycogen as a net energy source.
Summary of the Glycogen Shunt
(i) Support of muscle contraction on the millisecond time scale requires rapid ATP production from glycolysis.
(ii) Because of the limited supply of glycolytic intermediates (including intramuscular glucose) and the rapid switching available from glycogen phosphorylase (the fight-or-flight enzyme), glycogenolysis makes the major contribution to the rapid energy requirements during millisecond contractions.
(iii) With increasing activity, lactate production increases, because more glucosyl residues of glycogen are consumed to support rapid contractions than are needed for oxidative energy production to restore glycogen and PCr levels during the intervals between contractions. The inefficiency of ATP production during the obligatory requirement for glycogenolysis explains the generation of lactate during aerobic exercise.
(iv) Low but finite glycogen concentrations at high levels of exercise would not be capable of providing the millisecond bursts of glycogenolytic flux. This could resolve the paradox that, whereas fatigue is associated with low glycogen, still it occurs before complete glycogen depletion.
Acknowledgments
This work was supported by National Institutes of Health Grant NIH R01 DK27121.
Footnotes
Sibson, N. R., Rothman, D. L., Behar, K. L., Wall, J. & Shulman, R. G. (1999) Proc. Intl. Soc. Magn. Reson. Med. 7, 730 (abstr.).
References
- 1.Stryer L. Biochemistry. New York: Freeman; 1988. p. 444. [Google Scholar]
- 2.Jobsis F F, Stainsby W N. Resp Physiol. 1968;4:292–300. doi: 10.1016/0034-5687(68)90035-2. [DOI] [PubMed] [Google Scholar]
- 3.Connett R J, Gayeski T E J, Honig C R. Am J Physiol. 1984;246:H120–H128. doi: 10.1152/ajpheart.1984.246.1.H120. [DOI] [PubMed] [Google Scholar]
- 4.Paul R J. Handbook of Physiology: The Cardiovascular System Vascular Smooth Muscle. II. Bethesda, MD: Am. Physiol. Soc.; 1980. pp. 201–235. [Google Scholar]
- 5.Brooks G A. Comp Biochem Physiol. 1998;120:89–107. doi: 10.1016/s0305-0491(98)00025-x. [DOI] [PubMed] [Google Scholar]
- 6.Coyle E F, Coggan A R, Hammert M K, Ivy J L. J Appl Physiol. 1986;61:165–172. doi: 10.1152/jappl.1986.61.1.165. [DOI] [PubMed] [Google Scholar]
- 7.Saltin B, Gollnick P D. In: Exercise, Nutrition and Energy Metabolism. Horton E S, Tarjung R L, editors. New York: Macmillan; 1988. pp. 45–71. [Google Scholar]
- 8.Hargreaves M. Exer Sport Sci Rev. 1997;25:21–39. [PubMed] [Google Scholar]
- 9.Holloszy J O, Kohrt W M. Annu Rev Nutr. 1996;16:121–138. doi: 10.1146/annurev.nu.16.070196.001005. [DOI] [PubMed] [Google Scholar]
- 10.Price T B, Rothman D L, Shulman R G. J Appl Physiol. 1991;70:1836–1844. doi: 10.1152/jappl.1991.70.4.1836. [DOI] [PubMed] [Google Scholar]
- 11.Price T B, Taylor R, Mason G F, Rothman D L, Shulman G I, Shulman R G. Med Sci Sports Exer. 1994;26:983–991. [PubMed] [Google Scholar]
- 12.Chung Y, Sharman R, Carlsen R, Unger S W, Larson D, Jue T. Am J Physiol. 1998;274:C846–C852. doi: 10.1152/ajpcell.1998.274.3.C846. [DOI] [PubMed] [Google Scholar]
- 13.Taylor R, Price T B, Rothman D L, Shulman R G, Shulman G I. Magn Reson Med. 1992;27:13–20. doi: 10.1002/mrm.1910270103. [DOI] [PubMed] [Google Scholar]
- 14.Gruetter R, Prolla T A, Shulman R G. Magn Reson Med. 1991;20:327–332. doi: 10.1002/mrm.1910200216. [DOI] [PubMed] [Google Scholar]
- 15.Hultman E, Greenhaff P L, Ren J M, Soderlund K. Biochem Soc Trans. 1991;19:347–353. doi: 10.1042/bst0190347. [DOI] [PubMed] [Google Scholar]
- 16.Costill D L, Gollnick P D, Jannson E D, Saltin B, Stein E M. Acta Physiol Scand. 1973;89:374–383. doi: 10.1111/j.1748-1716.1973.tb05532.x. [DOI] [PubMed] [Google Scholar]
- 17.Greenhaff P L, Timmons J A. Exer Sport Sci Rev. 1998;26:1–30. [PubMed] [Google Scholar]
- 18.Shulman R G, Bloch G, Rothman D L. Proc Natl Acad Sci USA. 1995;92:8535–8542. doi: 10.1073/pnas.92.19.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roussel R, Carlier P G, Robert J-J, Velho G, Bloch G. Proc Natl Acad Sci USA. 1998;95:1313–1318. doi: 10.1073/pnas.95.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klip A, Paquet M R. Diabetes Care. 1990;13:228–243. doi: 10.2337/diacare.13.3.228. [DOI] [PubMed] [Google Scholar]
- 21.Goodyear L J, Hirshman M F, King P A, Horton E D, Thompson C M, Horton E S. J Appl Physiol. 1990;68:193–198. doi: 10.1152/jappl.1990.68.1.193. [DOI] [PubMed] [Google Scholar]
- 22.Shoubridge E A, Radda G K. Am J Physiol. 1987;252:C532–C542. doi: 10.1152/ajpcell.1987.252.5.C532. [DOI] [PubMed] [Google Scholar]
- 23.Watchko J F, Daood M J, Sieck G C, LaBella J J, Ameredes B T, Koretsky A P, Wieringa B. J Appl Physiol. 1997;82:1416–1423. doi: 10.1152/jappl.1997.82.5.1416. [DOI] [PubMed] [Google Scholar]
- 24.Gollnick P D, Karlsson J, Piehl K, Saltin B. J Physiol. 1974;241:59–67. doi: 10.1113/jphysiol.1974.sp010640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross B D, Radda G K, Gadian D G, Rocker G, Esiri M, Falconer-Smith J. N Engl J Med. 1981;304:1338–1342. doi: 10.1056/NEJM198105283042206. [DOI] [PubMed] [Google Scholar]
- 26.Ren J-M, Hultman E. J Appl Physiol. 1990;69:919–923. doi: 10.1152/jappl.1990.69.3.919. [DOI] [PubMed] [Google Scholar]
- 27.Ren J-M, Hultman E. J Appl Physiol. 1989;67:2243–2248. doi: 10.1152/jappl.1989.67.6.2243. [DOI] [PubMed] [Google Scholar]
- 28.Danforth W H. J Biol Chem. 1965;240:588–593. [PubMed] [Google Scholar]
- 29.Maehlum S, Hermansen L. Scand J Clin Lab Invest. 1978;38:557–560. doi: 10.1080/00365517809108819. [DOI] [PubMed] [Google Scholar]
- 30.Holloszy J O, Constable S H, Young D A. Diabetes Metab Rev. 1986;1:409–423. doi: 10.1002/dmr.5610010405. [DOI] [PubMed] [Google Scholar]