Abstract
In addition to their well-known effects on the development of the mushroom body, mud mutants are also female sterile. Here we show that, although the early steps of ovary development are grossly normal, a defect becomes apparent in meiosis II when the two component spindles fail to cohere and align properly. The products of meiosis are consequently mispositioned within the egg and, with or without fertilization, soon undergo asynchronous and spatially disorganized replication. In wild-type eggs, Mud is found associated with the central spindle pole body that lies between the two spindles of meiosis II. The mutant defect thus implies that Mud should be added to the short list of components that are required for the formation and/or stability of this structure. Mud protein is also normally found in association with other structures during egg development: at the spindle poles of meiosis I, at the spindle poles of early cleavage and syncytial embryos, in the rosettes formed from the unfertilized products of meiosis, with the fusomes and spectrosomes that anchor the spindles of dividing cystoblasts, and at the nuclear rim of the developing oocyte. In contrast to its important role at the central spindle pole body, in none of these cases is it clear that Mud plays an essential role. But the commonalities in its location suggest potential roles for the protein in development of other tissues.
MUTATIONS in the X-linked mushroom body defect (mud) gene were originally identified in a screen for altered brain anatomy in adult flies (Heisenberg 1980). Prominent among the alterations seen in mud mutants are greatly enlarged calyces, the input neuropil for the mushroom bodies (MBs). Mud mutants are also characterized by severely shrunken or absent lobes and peduncles, the output tracts of the MBs. The basis for these defects is only partially understood but appears to be complex. On the one hand, the calyx overgrowth phenotype may be related alterations in the timing and extent of neuroblast proliferation in the larval central nervous system (CNS) (Prokop and Technau 1994). On the other hand, reduction of the lobes appears to reflect defects in axon retraction/extension during pupal remodeling of the MB (Technau and Heisenberg 1982). This complexity suggests that mud is a gene with pleiotropic effects on development.
Insight into the function of mud was anticipated (Levine et al. 1995) from its molecular identification, but the gene proved to encode a protein with no obvious functional signature (Guan et al. 2000). Each of the known splice isoforms encodes a protein that is dominated by a long stretch of residues (>1600 aa) that are predicted to form a coiled coil. Flanking this region are smaller segments that are predicted to form globular domains, none of which contain clear-cut sequence motifs. The only recognizable feature outside of the coiled-coil region is a carboxy-terminal transmembrane domain. This is found in all known isoforms and suggests that, despite the absence of a signal sequence, the protein is capable of association with membranes. Publicly available genomic and cDNA information permits the identification in several insect species of probable orthologs of Mud: proteins with long coiled-coil regions flanked by segments with significant, albeit spotty, similarity to the globular domains of the melanogaster protein. The conservation suggests that Mud protein plays an important role in insect development but the imperfect alignment among the orthologs (see Figure 1 below) suggests that this function can tolerate liberal substitutions not only in the coiled coil but also in the predicted globular domains. This may explain why, although proteins with long coiled-coil regions are very common throughout the eukaryotic kingdom, no clear ortholog of mud has yet been identified in vertebrates.
Figure 1.
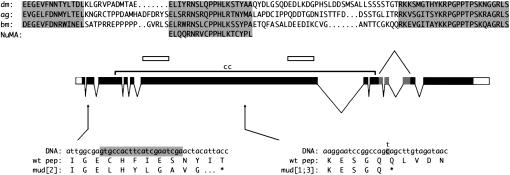
The mud gene and protein. The central line shows the splicing pattern that results in the longest-known cDNA (Guan et al. 2000). The position of the coiled coil (cc) in the encoded ORF is marked by a bracket and the regions used as immunogens are marked by open bars. Below the central line are shown the sequence changes and resulting coding changes that are associated with previously uncharacterized alleles. In mud2, a 20-bp deletion (shaded) near the beginning the second exon leads to a frameshift and a premature stop in the 27th amino acid after the deletion site. The mud1 and mud3 alleles have the identical change (shaded), a C-to-T transition leading to a stop codon in the middle of the coiled-coil region. The mud4 change was shown previously (Guan et al. 2000) to be a nonsense mutation in the first exon. Above the central line is shown the sequence of a region encoded by the shaded exons that is conserved between mud orthologs in distant insect species: Drosophila melanogaster (dm), Anopheles gambiae (ag), and Bombyx mori (bm). Matches to all three conserved regions are also found in available sequences from Apis mellifera and Aedes aegypti (not shown). Below the silkworm sequence is presented a stretch of residues that is found in many vertebrate NuMA proteins; as in Mud, this sequence is located downstream of the NuMA coiled-coil region. Near the amino terminus of Mud a few patches of similarity are also found between the dm and ag orthologs (not shown) but the genomic sequence of other insect species is too fragmentary to permit confident identification of conservation.
Determining the fundamental role for this fascinating protein would be expedited by identification of a system in which the mutant phenotype could be ascribed to a simple defect. In this work we have explored the female sterility of mud mutants and present evidence that this phenotype reflects the contribution of the protein to formation of a unique structure, the meiosis II spindle.
MATERIALS AND METHODS
Fly stocks and DNA sequencing:
When mud mutants (de Belle and Heisenberg 1996) are maintained as balanced lines using the FM7i, JMR3 balancer (FlyBase Consortium 2002), homozygous females are readily obtained with the mud4 allele but rarely with the other alleles. Accordingly, most of the studies in this work used the mud4 strain. To test whether the abnormal replication observed in early eggs (Figure 2) was due to the mud mutation in this strain and not to an adventitious mutation, we compared mud4 homozygotes with flies trans-heterozygous for this mutation and either another point mutant (mud1 and mud3) or a deficiency for the region [Df(1)KA9, Df(1)CO1, and Df(1)CO2]. In all cases, the identical phenotype was seen.
Figure 2.
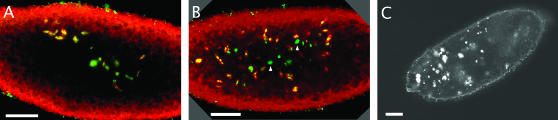
Defective early embryonic development of mutant eggs. Eggs laid by mud4 homozygote mothers stained for DNA (A and B, green; C, gray) and α-tubulin (A and B, red) are shown. (A and B) Embryos from mated mothers that have been aged for 2–4 hr contain numerous nuclei of varying size, many of which are associated with apparently anastral spindles and thus appear to be in the process of asynchronous division. When in moderate numbers, these nuclei are irregularly clustered near the center (A), but, when in larger numbers, the nuclei are distributed more broadly (B). Many of these nuclei are much larger and more intense than normal and do not appear to be associated with microtubules (arrowheads). (C) Unfertilized mutant eggs do not arrest normally after meiosis, but undergo DNA replication in the same manner as fertilized eggs. Bar, 50 μm.
As a source of genomic DNA for PCR amplification we used males bearing the mud1, mud2, and mud3 alleles that were maintained as attached-X stocks. Following amplification of overlapping segments of the 16-kb region that contains the mud gene (Guan et al. 2000), DNA sequence was obtained commercially or via the National Institute of Neurological Disorders and Stroke Sequence Facility, assembled by hand, and compared to the wild-type sequence (Adams et al. 2000; Guan et al. 2000). This revealed many minor and/or silent polymorphisms, all of which were identical in the three mutant lines and thus probably reflect the common origin of these mutations from the wild-type Berlin strain (de Belle and Heisenberg 1996). In addition to these polymorphisms, each mutant line contained a single major change, which is described in the text.
Reagents for histochemistry:
To make anti-Mud antibodies, two segments of the gene (corresponding to amino acids 375–549 and 1510–1693 of the protein annotated in GenBank accession AF174134) were cloned into pET28b(+) vectors (Novagen, Madison, WI) and expressed in Escherichia coli. The resulting His-tagged polypeptides were purified in the presence of 6 m guanidine by chromatography on immobilized nickel as per manufacturer's instructions and, after dialysis to remove denaturant, were used to immunize rabbits (Research Genetics, Huntsville, AL). Specific antibodies were purified from the resulting antisera by chromatography on columns containing covalently linked immunogen (Sterogene BioSeparations, Carlsbad, CA). Most of the experiments in this article used a 1:500 dilution of the affinity-purified antibody raised against amino acids 1510–1693; the distribution patterns of Mud in wild-type oocytes undergoing meiosis II and in early cleavage wild-type embryos were confirmed with the antibody raised against the other polypeptide. Monoclonal mouse antibodies (Sigma, St. Louis) against α-tubulin (clone DM1A) and against nuclear pore complex (clone 414) were used at 1:250 and 1:200, respectively. Monoclonal mouse anti-hts 1B1 (Iowa Developmental Hybridoma Bank) was used at 1:10. DNA was labeled using Oligreen or 7-aminoactinomycin D (7-AAD; both from Molecular Probes, Eugene, OR) at 1:1000. Alexa488 and Alexa546 conjugated secondary antibodies (Molecular Probes) were used at 1:333.
Western blotting:
All samples were homogenized in HE buffer (100 mm KCl, 20 mm HEPES pH 7.5, 5% glycerol, 10 mm EDTA, 0.1% Triton X-100) and loaded onto Tris-Acetate SDS-PAGE gels (Novex, Encinitas, CA), using the manufacturer's sample buffer and reducing agent. After electrophoresis the protein was transferred in the presence of 12% methanol onto nitrocellulose membranes, which were then stained with Ponceau S (Sigma). After destaining in distilled water, the membrane was blocked with a 5% powdered milk solution in TBST (10 mm Tris pH 7.5, 140 mm NaCl, 0.05% Tween-20) and incubated with the affinity purified anti-Mud (1:2000), followed by HRP-conjugated anti-rabbit secondaries (1:1000; Pierce Chemical, Rockford, IL). The West Femto kit (Pierce) was used to detect the protein.
Ovary staining:
To examine fixed material, well-fed Canton-S and mud4 mutant females were dissected in EBR (10 mm HEPES pH 6.9, 130 mm NaCl, 5 mm KCl, 2 mm CaCl2) and treated with 4% paraformaldehyde for 10 min or, for tubulin staining, with cold methanol for 10 min followed by cold acetone for 7 min. To stain stage 14 oocytes, methanol-fixed ovaries were further processed as described (Mathe 2004). Briefly, fixed oocytes were cut across the middle using a no. 15 scalpel blade, largely stripped of their chorion and vitelline membranes with Dumont no. 5 standard tip tweezers (Fine Science Tools, Foster City, CA), and incubated again with cold methanol and acetone for 10 and 7 min, respectively. Following fixation, ovaries or oocytes were washed with phosphate-buffered saline (PBS) containing 0.1% Triton-X (PBT) and blocked in 2.5% fish gelatin (Sigma) in PBT for 30 min. They were then incubated with primary antibody for 3–4 hr or overnight at 4°. After washing for 2 hr with many changes of PBT, they were incubated with secondary antibody (and, when OliGreen stain was to be used, 1 mg/ml of RNAase) for 2–4 hr. To stain for DNA, ovaries and oocytes were further incubated with 7-AAD or OliGreen for 20 min. After multiple washes in PBT and a final wash in PBS, the ovaries and oocytes were mounted for visualization in 90% glycerol or Vectashield (Vector Laboratories, Burlingame, CA). To examine unfixed material, ovaries from wild-type or mud4 flies bearing a tubulin–GFP transgene (Grieder et al. 2000) were dissected in EBR and individual stage 14 oocytes were then separated using tungsten electrode needles. The oocytes were transferred to a slide and imaged directly in EBR.
Egg staining:
Following a 1-hr precollection, well-fed Canton-S or mud4 females were allowed to deposit eggs on microscope slides coated with grape juice agar for 30 min or 1 hr. These were collected either immediately or after being allowed to incubate at room temperature for up to 4 hr. For visualizing meiotic spindles, eggs were collected by squeezing them out from the ovipositors of anesthetized females. Following collection, eggs were promptly dechorionated in 50% bleach and fixed with cold methanol/heptane (Gonzalez and Glover 1993) for 10 min, followed by acetone for 7 min. They were then washed in PBT, blocked, and stained as described above.
Confocal microscopy:
Samples were imaged using a Nikon Eclipse TE2000-S confocal microscope equipped with argon/krypton and helium/neon lasers. Series of images taken from several focal planes were projected onto a single image using ImageJ. Levels and color balances were adjusted using Adobe Photoshop or the GNU Image Manipulation Program (GIMP).
RESULTS
Infertility of mud mutants:
There are four classical alleles of mud and all have been reported to be female sterile (de Belle and Heisenberg 1996). Like the other aspects of the mutant phenotype, the female sterility of mud homozygotes is rescued by a cosmid bearing a functional copy of the gene (Guan et al. 2000). To assess the range of damage to gene function that can lead to infertility, we determined the sequence of mud1, mud2, and mud3. Like the previously sequenced mud4 allele (Guan et al. 2000), these lead to changes in coding potential that are quite severe. The mud2 allele is a small out-of-frame deletion near the beginning of the gene (Figure 1). The mud1 and mud3 alleles (which have the identical change) create a stop codon in the middle of the gene (Figure 1), a region in which no alternative exons are apparent (Guan et al. 2000). All of these mutations thus should result in a protein that is truncated well before the globular domain that follows the coiled coil and is partially conserved among insect species (Figure 1). We infer that female sterility is a consequence of strong reduction-of-function mutations in this gene but we cannot predict whether weak hypomorphs would show this phenotype.
To explore the basis of the female sterility, we examined large numbers of mutant females—>100 mud4 homozygotes and >50 trans-heterozygotes of mud4 with mud1, mud3, or deficiencies that remove the gene. In every case ovaries dissected from these animals appeared grossly normal. Moreover, compared to wild-type females that were examined in parallel, they laid normal numbers of eggs that had no defects in shape, size, or position of appendages. To test for sperm penetration, we mated mud4 virgin females to males carrying a GFP-marked transgene that is expressed in sperm tails (Santel et al. 1997). In more than two-thirds of the eggs examined we detected a fluorescent signal of the expected shape (Fitch and Wakimoto 1998), leading us to conclude that the mutants are readily fertilized. However, even when inseminated by wild-type sperm, mutant eggs never proceed through normal development to produce hatchling larvae. Thus, mud mutants are best described as fully penetrant maternal-effect lethals. In contrast, males that are hemizygous for each of the classical alleles are fully fertile and produce normal numbers of motile sperm (K. F. Osborne and H. A. Nash, unpublished observations).
The maternal-effect lethal phenotype:
In wild type, replication of the female pronucleus requires formation of a zygote and results in the generation of an orderly array of daughter nuclei. In contrast, when collected 1–4 hr after deposition and stained with OliGreen, both fertilized and unfertilized mutant eggs show the gradual proliferation of disorganized chromatin masses. Representative examples of the >300 cases examined are shown in Figure 2. A similar pattern of irregularly distributed DNA is seen when eggs are stained with 7-AAD or with the classical Feulgen reagent (not shown). When costained with anti-tubulin, the chromatin masses are frequently seen to be associated with anastral spindle structures. In some cases, the DNA clumps appear to have migrated toward the center of the egg, superficially resembling a cycle 6 or 7 wild-type embryo. However, these clumps are not regularly distributed, and in no case did any mutant egg develop to a recognizable syncytial blastoderm. Some, but not all, of the chromosome masses are enclosed in a lamin sheath (J. X. Yu, unpublished observation). Mutant eggs that are ∼4 hr old typically contain a large number of chromatin masses of varying ploidy spread throughout the egg. In older mutant eggs, most of these chromatin masses lose coherence and are not associated with a microtubule structure, possibly suggesting DNA breakdown. In addition, some mutant eggs contain clear areas within their cytoplasm, possibly due to necrosis (not shown). All these defects are completely recessive: mud4 heterozygotes with an X chromosome bearing a wild-type gene are not only fertile, but disorganized chromatin masses are undetectably rare in eggs laid by these females. In contrast, eggs laid by heterozygotes with one chromosome bearing the mud4 mutation and the other bearing a different point mutation or a deletion of the gene are not only sterile but also accumulate disorganized chromatin. We conclude that loss of mud function is associated with highly aberrant DNA replication that leads to a spectacular collapse of early embryonic development.
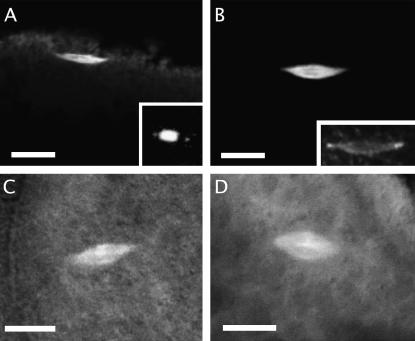
To pinpoint the origin of this defect, we examined the internal anatomy of developing oocytes from mud4 homozygotes and compared it with that described for wild type (see Megraw and Kaufman 2000 for an overview). Up to stage 14 no defect is apparent: in >100 cases examined, the oocyte is well positioned in the cyst, its germinal vesicle has undergone proper migration and condensation, and its cytoplasm appears to have received a full contribution from nurse cells. Stage 14 is characterized by an arrest of meiosis in metaphase I; two methods were used to examine the corresponding spindle structure in mud mutants. First, we fixed, transected, and immunostained unactivated mutant oocytes (Mathe 2004). In the few cases where anatomical integrity was preserved, the meiosis I spindles of the mutant and wild-type oocytes were indistinguishable (see Figure 3, A and B, for examples). Second, to permit more facile study, we constructed a mud4 mutant line containing a transgene that marks the spindle with GFP (Grieder et al. 2000). In each of the 15 cases examined, the spindle of unfixed stage 14 mutant oocytes resembled wild type in having a bipolar shape with well-focused poles (see Figure 3, C and D, for examples). Although we cannot rule out a subtle defect at these early stages, our data imply that maternal lethality is due to a defect in subsequent stages of egg development.
Figure 3.
Meiosis I in stage 14 oocytes. (A and B) Fixed oocytes stained for α-tubulin. Both mud4 (A) and wild-type (B) oocytes display a bipolar anastral spindle with focused poles that is oriented parallel to the oocyte surface. Staining of a mutant ooctye for DNA (inset in A; not the same oocyte as in A) reveals that chromosome positioning is normal, with the nonexchange fourth chromosomes visible away from the center. Staining of a wild-type oocyte for Mud (inset in B; the same oocyte as in B) shows that the protein is localized at the poles. (C and D) Unfixed oocytes from lines bearing an α-tubulin–GFP transgene examined by confocal microsopy. The spindle of both mud4 (C) and wild-type (D) oocytes is of normal shape and position. Bars, 10 μm.
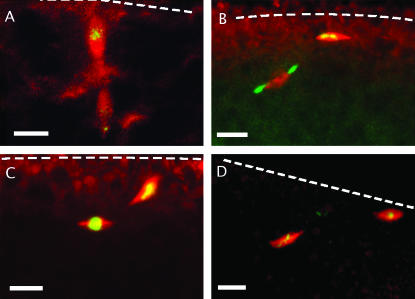
In wild-type flies, activation of oocytes by passage through the uterus results in completion of meiosis I and the separation of two chromatin masses, each associated with an anastral spindle (Page and Orr-Weaver 1997 and references cited therein). In contrast, although pairs of spindles are evident when freshly laid eggs from mud4 homozygotes are immunostained, their organization is aberrant. Specifically, while the two spindles of a wild-type meiosis II (Figure 4A) are in close apposition and are aligned perpendicular to the oocyte cortex (Riparbelli and Callaini 1996; Endow and Komma 1998), the mutant spindles are disconnected and poorly aligned, with respect both to each other and to the oocyte cortex (Figure 4, B–D). Another abnormality concerns the meiosis II central spindle pole body. This is normally a large and complex structure that features a central ring and aster of α-tubulin (Riparbelli and Callaini 1996, 1998; Endow and Komma 1998). Under our conditions, the ring is seen only in eggs with a favorable orientation (an example is shown in Figure 6A) but a diffuse aster is consistently observed (Figures 4A and 6B). In contrast, in none of the 10 mud mutant eggs examined immediately after either natural or manually assisted oviposition could tubulin staining be detected in the region between two spindles. In the absence of Mud this structure fails to form at all, forms but is so short lived as to escape detection, or forms in a way that fails to protect microtubules from dissolution during fixation.
Figure 4.
Meiosis in activated eggs. Meiotic spindles of fixed wild-type (A) and mud4 (B–D) eggs stained for α-tublin (red) and DNA (green) are shown. In each of the three mutant cases shown, the axes of the spindles are not colinear nor are they oriented perpendicular to the egg cortex (whose position is indicated by a dotted line) but make a rather shallow angle with it. In some eggs the two spindles are at least 40 μm apart. In contrast to the wild-type case, the region between the mutant spindles is devoid of tubulin, indicating the absence of a central pole body and its associated microtubule aster. Bars, 10 μm.
Figure 6.
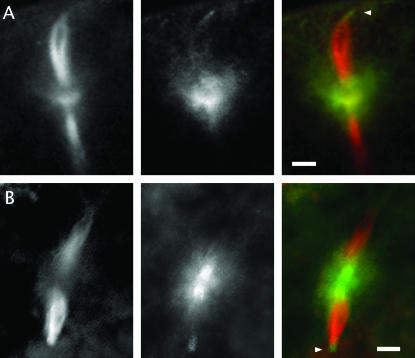
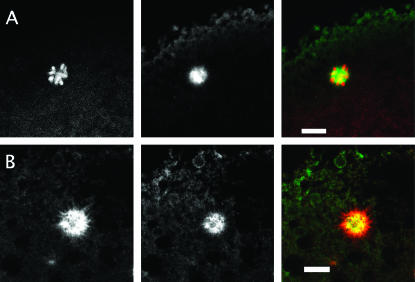
The distribution of Mud protein in wild-type eggs undergoing meiosis. Freshly laid eggs (whose cortex is oriented toward the top of the page) stained for α-tubulin (left column; red in composite) and Mud (middle column; green in composite) are shown. In these respresentative examples of eggs in meiosis II, Mud protein is found in a broad belt that includes and surrounds the central pole body. It is also expressed at the anastral poles (marked by arrowheads when in focus) of the meiosis II apparatus. (A) An example with the same anti-Mud antibody as used in Figure 5. (B) An example with an anti-Mud antibody directed against a more amino-terminal portion of the protein (see Figure 1). Bars, 10 μm.
Mud protein is associated with meiotic and mitotic spindle poles:
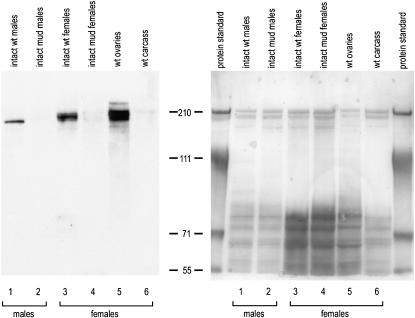
The long coiled coil predicted for the ORF encoded by the mud gene is commonly found in proteins that serve a structural role (Rose and Meier 2004). To see if Mud is associated with structures of the oocyte, we performed immunocytochemistry with an affinity-purified antibody that had been raised to a segment of the protein. The specificity of this antibody was first demonstrated by Western blotting. Samples extracted from wild-type strains show a tight cluster of bands migrating in the size range expected from the known cDNAs (Guan et al. 2000). Comparison of extracts from intact vs. dissected wild-type flies reveals that almost all of the signal in adult females comes from the gonads; the same is true for adult males (K. F. Osborne, unpublished observations). Critically, samples extracted from mud4 animals yield no detectable signal (Figure 5). Antibody specificity was confirmed by staining of ovaries and eggs from mud4 animals. In contrast to the specific patterns seen with wild-type material (see below), only a diffuse and often imperceptible background was seen when affinity-purified antibody was used to stain 2- to 4-hr-old mutant eggs (not shown), stage 1 mutant egg chambers (Figure 8B), or stage 8 mutant egg chambers (Figure 10D).
Figure 5.
Detection of Mud protein by Western blotting. Extracts were made from intact wild-type or mud4 males (lanes 1 and 2), intact wild-type or mud4 females (lanes 3 and 4), ovaries dissected from wild-type females (lane 5), and carcasses of wild-type females from which the ovaries had been removed (lane 6). An amount of each extract corresponding to 0.75 male or 1.0 well-fed female was loaded on the indicated lane of a polyacrylamide gel. After electrophoresis and blotting, the nitrocellulose membrane was stained with PonceauS to confirm the evenness of the transfer (right side). After destaining, Mud in these extracts was detected (left side) with an affinity-purified antibody that was raised against a segment of the protein that spanned amino acids 1510–1693 (see Figure 1). The position and size of marker proteins are shown flanking the experimental lanes. The slightly smaller apparent size of Mud extracted from males is consistent with the size of a male-specific splice isoform (Guan et al. 2000).
Figure 8.
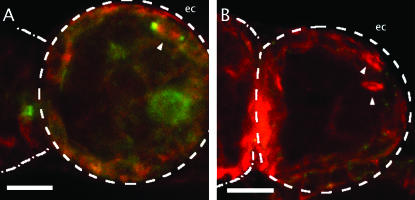
Mud in dividing follicle cells. Stage 1 egg chambers (ec) from wild type (A) and the mud4 mutant (B), visualized for tubulin (red) and Mud (green) at a focal plane that intersects a layer of follicle cells, are shown. Mitotic spindles can be detected both wild-type (wt) and mud4 ovaries (arrowheads). Mud localizes to the poles of these spindles in wt (A), whereas no signal can be detected on mutant spindles (B), which nevertheless have grossly normal shape and bipolarity. Although out of focus, Mud staining around the rim of the germinal vesicle can also be seen in A. Bars, 10 μm.
Figure 10.
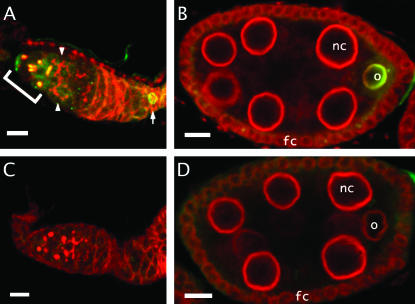
Mud in internal structures of the ovariole. (A and C) Wild-type and mutant germaria stained for Mud (green) and Hts (red) at an internal focal plane. In wild type (A), note that mud protein is found on spectrosomes and fusomes in region 1 (bracketed), but not on the fusomes (arrowheads) in region 2. Mud can also be seen on the germinal vesicle of a stage 1 oocyte (arrow). In germaria from the mud4 mutant (C), fusomes appear normal despite the absence of Mud. (B and D) Stage 8 egg chambers stained for Mud (green) and a nuclear rim component (red). Chambers from wild-type mothers (B) show that Mud persists in the oocyte (o), where it prominently outlines the nuclear rim. Note the absence of Mud in the nuclear rim of follicle cells (fc) and nurse cells (nc). Chambers from mud4 mothers (D) show that, despite the undetectable levels of Mud staining, the oocyte and its nuclear rim appear normal. Bars, 10 μm.
Our survey of wild-type ovaries and eggs stained with this antibody revealed that Mud is a common component of spindles and closely related structures. Most critically, in freshly laid eggs from wild-type mothers, Mud is found in the region of the meiosis II central spindle pole body, where it forms a cloud that encompasses the microtubule aster of this structure (Figure 6A). Both features of this localization—positioning of Mud between the two component spindles of meiosis II and its distribution in a broad belt surrounding the tubulin aster—are seen when eggs undergoing meiosis II are stained with an antibody raised to a different segment of the protein (Figure 6B). Thus, Mud is associated with the central spindle pole body and, since this structure cannot be found in the mutants (Figure 4), it is simplest to conclude that protein plays a direct and important role in its construction and/or stability.
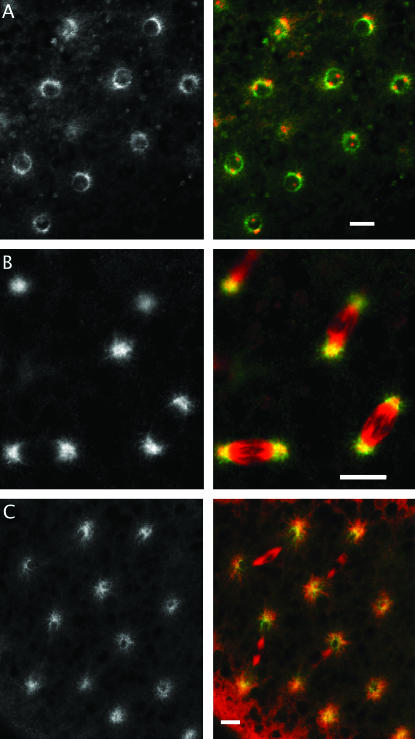
The protein is also found associated with other spindles but in these cases there is little or no evidence that it is essential. For example, in wild-type oocytes Mud can be seen at the poles of meiosis I spindles, just beyond the tip of the α-tubulin signal (Figure 3B), and it persists there to be seen at the distal poles of meiosis II spindles (Figure 6). In the mutants, these poles not only form (Figure 3, A and C) but remain focused (Figure 4). Mud is also found in association with the spindle poles of cleavage and syncytial-stage embryos (Figure 7, B and C). Here, double immunofluorescence experiments reveal that Mud strongly overlaps and extends beyond the core of γ-tubulin (data not shown). Of course, because eggs that lack maternally supplied Mud fail to reach these stages properly, we cannot judge whether the protein plays an essential or redundant role at their spindles. However, Mud is also associated with spindles at a time when the zygotic contribution should completely outweigh the maternal contribution. For example, in egg chambers from mature wild-type females the protein is found on spindles of dividing follicle cells (Figure 8A). And, in the testis of mature wild-type males, it is found on spindles of spermatocytes undergoing meiosis (K. F. Osborne and H. A. Nash, unpublished observations). In both these cases, Mud staining is not observed when mutant individuals are raised from heterozygous mothers but normal-appearing spindles nevertheless form (Figure 8B and J. X. Yu, unpublished observations).
Figure 7.
The distribution of Mud during the mitotic cycle of wild-type syncytial embryos. Immunochemical staining is for Mud (left side, green in composite) and α-tubulin (red in composite). (A) During interphase, Mud is found around the nuclear envelope, distinct from the α-tubulin that is concentrated on the two centrosomes. (B) During metaphase and anaphase, Mud can be detected in the pericentrosomal regions, extending into the astral microtubules. (C) Near the end of telophase, Mud reforms around the nascent nuclear envelope. Bars, 10 μm.
Association of Mud with other structures:
In addition to its association with spindles, within oocytes and eggs Mud is also prominently found at three other subcellular locations, all of which have at least some connection with microtubules. First, Mud is present within the polar bodies that are formed at the end of meiotic anaphase II by the condensation of microtubules with the dead-end haploid products of meiosis (Figure 9). The importance of Mud for the function of these structures is unclear since it is hard to distinguish polar bodies from the disorganized chromatin masses that accumulate in the mutants. In addition, in germaria Mud is found strikingly colocalized with spectrosomes and the fusomes of region 1 (Figure 10A). These structures are not only rich in cytoskeletal proteins like Hts (Lin et al. 1994) but also rich in ER-derived membranes (McKearin 1997; Snapp et al. 2004). Thus, their association with Mud could depend on its carboxy-terminal transmembrane-spanning domain. Whatever its cause, this association must be conditional since Mud is not found at fusomes in region 2 (Figure 10A). Moreover, the protein does not appear to be essential for these structures. Ovarioles from the mutant line, despite the absence of detectable staining for Mud, consistently have normal-appearing fusomes (Figure 10C) and cysts with an oocyte and normal numbers of nurse cells (J. X. Yu, unpublished observations). Finally, Mud is frequently found in association with the nuclear rim. This can be seen in early embryos, where Mud resides on the nuclear envelope from telophase through prophase (Figure 7, A and C). The importance of Mud for this structure cannot be ascertained because, as described above, mutant eggs are so disrupted at this stage. However, dispensability of Mud for the nuclear envelope is suggested from observations on developing egg chambers. Here, an intense Mud signal surrounds the oocyte nucleus from the time of its specification to the time of nuclear envelope breakdown (Figures 8A and 10, A and B). As with spectrosomes/early fusomes, no Mud signal is found in mud mutants (Figures 8B and 10, C and D). Despite this, no structural defect in the envelope of the germinal vesicle is apparent and the mutants suffer no obvious change in egg development that can be ascribed to envelope malfunction.
Figure 9.
The distribution of Mud in polar bodies. Wild-type eggs, collected shortly after deposition, are stained for Mud (middle column and green in the composites) and DNA (left side and red in A) or α-tubulin (left side and red in B). Mud can be seen throughout the center of the rosette formed by meiotic DNA products. This rosette structure contains at its center a microtubule aster that almost perfectly coincides with the distribution of Mud. Bars, 10 μm.
DISCUSSION
In this work we have described a fully penetrant phenotype that is associated with strong mutations in the mud gene. Specifically, mud mutants have a striking maternal-effect lethal phenotype: although eggs produced by mutant females look normal and accept sperm, they fail to undergo normal cleavage divisions. Instead of forming a well-ordered syncytial blastoderm, these eggs accumulate disordered arrays of replicated material and then necrose. A number of mutants in other genes have been reported to have abnormal early embryonic mitoses. One class of these, comprising strong alleles of the genes png, plu, and gnu, reflects defects in a complex with protein kinase activity (Lee et al. 2003). It seems unlikely that mud is a member of this class. While png, plu, and gnu mutants typically form one to five giant nuclei, strong alleles of mud characteristically generate dozens of smaller chromatin masses that are scattered throughout the egg. Moreover, in contrast to mutations in genes of the giant nuclei class (Tadros et al. 2003), mud mutations do not interfere with degradation of maternal mRNA (W. Tadros and H. Lipshitz, personal communication). A closer resemblance to the mud phenotype is shown by mutants that have defects in spindle-associated proteins (Matthews et al. 1993; Endow and Komma 1997; Tavosanis et al. 1997). In such mutants, as in mud, the meiotic products undergo inappropriate and disorganized mitoses that produce eggs with scattered chromatin masses. We considered the possibility that Mud and the products of one or more of these genes (ncd, αTub67C, or γTub37C) serve mutually interdependent roles. If so, double heterozygotes might display a maternal-effect phenotype lacking in any of the single heterozygotes. However, when either the mud3 or the mud4 mutation was combined with the ncdD, αTub67C1, or γTub37C3 mutation, no decrease in fertility was seen over the robust levels seen in the single heterozygotes (J. X. Yu, unpublished observations). Although this outcome is not decisive, it argues against possibilities such as Mud being involved in the localization of these spindle components. In any case, it should be emphasized that our observation that unfertilized mutant eggs undergo the same rounds of disorganized mitoses as do early embryos strongly implies that the mud maternal lethal phenotype is due to a defect in meiosis and not in a subsequent stage of development.
What is the basis for the meiotic defect? A central spindle pole body that incorporates several centrosomal proteins together with a diffuse aster of α-tubulin is normally found between the two spindles that make up the meiosis II apparatus (Riparbelli and Callaini 1996; Endow and Komma 1998). As judged by α-tubulin staining, this structure is defective or absent in mud mutant eggs. In wild-type eggs, Mud is found associated with the spindle pole body, implying that the protein is needed for the formation or stability of this structure. Because Mud staining appears to surround the α-tubulin framework, an attractive possibility is that the former is needed for the latter to be recruited to or be maintained at the central spindle pole body. In any case, just as with weak alleles of polo, which also show defects in the formation of the central spindle pole body, disjoined meiosis II spindles, and disorganized early mitoses (Riparbelli et al. 2000), our observations provide a plausible scenario for the female sterility of mud mutants. To wit, without a functional spindle pole body the two meiosis II spindles are not properly held together and the products of meiosis do not get correctly positioned with respect to the egg cortex. As has been suggested before (Riparbelli et al. 2000), we presume that positioning is important for condensation of the meiotic products into inactive polar bodies. These structures may normally serve to shield the dead-end meiotic products from the replication machinery, which according to this scenario is competent to operate in unfertilized Drosophila eggs. If so, when proper condensation fails, inappropriate replication ensues. Regardless of the correctness of this model, the similar phenotypes of mud and polo mutations suggest that corresponding proteins might serve interdependent roles in activated eggs. But no decrease in fertility was observed in double heterozygotes of polo1 and either mud3 or mud4 (J. X. Yu, unpublished observations). It remains to be seen whether Polo and Mud are similarly distributed in activated oocytes.
In addition to its association with the central spindle pole body, Mud protein is readily detected at other spindle poles. However, in those cases where it can be tested, it appears that Mud is not essential for the formation or function of these structures. In this regard, Mud is reminiscent of the fly orthologs of CP190 and pericentrin, which, despite being consistently found at the centrosome, are dispensable for mitosis (Butcher et al. 2004; Martinez-Campos et al. 2004). It may be that the structures built at the spindle poles are designed to withstand the undersupply of a few ingredients. If so, it might be useful to look for synthetic phenotypes when mud mutations are combined with those of other genes whose products are concentrated at spindle poles. Of course, because eggs that lack a maternal contribution of functional Mud develop so anomalously, we cannot decide whether it plays an essential role at the spindle poles of embryonic mitoses or in polar bodies. Insight into these cases will have to await the availability of a conditional mutant that can be shifted to nonpermissive conditions after meiosis II is completed. Despite the doubts about its role at places other than the central spindle pole body, the frequent associations of Mud with microtubular structures in the oocyte and egg suggest that the protein might be regarded as a MAP; direct tests of this hypothesis are underway (T. Raabe, personal communication). Another attractive, albeit speculative, idea is that Mud is functionally related to the vertebrate NuMA protein. Like Mud, NuMA is a large coiled-coil protein that is found at spindle poles (Kisurina-Evgenieva et al. 2004 and references cited therein). The two proteins are apparently not orthologous in that they cannot be globally aligned outside of their coiled-coil regions; they also differ in subcellular localization during interphase. However, the carboxy terminus of one particular isoform of Mud shares a short region of similarity with NuMA (Figure 1), hinting at a conserved interaction.
Two other sites of Mud localization that suggest a connection with microtubules are fusomes and spectrosomes. These are membrane-rich structures that form during the earliest stages of ovary development and are surrounded by microtubules (Grieder et al. 2000). Mud is present within these structures at a time when they serve to anchor the mitotic spindles of the dividing cystoblasts but not later, when they serve to focus the microtubule network in postmitotic cells (Deng and Lin 1997). Although this distribution hints at a role for Mud in spindle anchoring, inactivation of the gene causes no obvious defect in fusomes or spectrosomes. These structures not only form normally in mud mutants but the processes they govern during early oogenesis (Deng and Lin 1997; Roper and Brown 2004 and references cited therein) proceed without defect. We conclude that, if Mud plays a role in building fusomes and spectrosomes or connecting them to spindles, it is a role for which there is adequate redundancy.
In cells that are not in metaphase or anaphase, Mud can also be found at the nuclear envelope. It is not clear how Mud gets from the nuclear rim of the oocyte and the early embryo, respectively, to the meiotic and mitotic spindle apparatus. On the one hand, Mud might lose contact with the nuclear envelope, where it could have been held by its transmembrane domain, and be directed to the spindle by a distinct targeting signal. On the other hand, since the spindles of the Drosophila germ line and pre-syncytial egg are typically enclosed by membranous structures (Wolf 1995; Kramer and Hawley 2003), the movement of Mud might be part of a concerted redistribution of elements of the nuclear envelope. Although the mechanism is thus unclear, it should be pointed out that shuffling between the nuclear envelope and the spindle is not unique to Mud but has been reported for several other proteins (Theodoropoulos et al. 1999; Joseph et al. 2002). Another open question is whether Mud plays a microtubule-related role at the nuclear rim. However, even though the oocyte nucleus is surrounded by a cage of microtubules and the dynactin complex is concentrated at its rim (Januschke et al. 2002), the structure and positioning of the oocyte nucleus are not grossly affected by loss of Mud function. Accordingly, we favor the idea that Mud is simply stored at the nuclear rim to ensure a local supply for subsequent delivery to the spindle apparatus but we cannot rule out the possibility that a parallel system renders obscure a more active function for Mud at the nuclear envelope.
To what extent do our observations on the female sterility shed light on the other phenotypes of mud mutants, particularly the role of Mud in the development of the adult nervous system? On this subject we must be cautious. The brain anatomy phenotype of mud mutants is not only complex but seems to reflect the alteration of processes that are distinct from those ongoing in meiosis II and early cleavage mitoses. Nevertheless, it is easy to imagine that a protein that can associate with the nuclear envelope might be part of the mechanism that prepares the way for exit from the cell cycle (Barbie et al. 2004). And a protein that is commonly found around spindle poles might be involved in the regulation of spindle orientation that governs the transition from symmetric to asymmetric division of neuroblasts (Kaltschmidt and Brand 2002) and thus the switch from neuroblast proliferation to stem cell behavior (Prokop and Technau 1994). Similarly, a protein that associates with microtubules might play a role in the precision of growth cone movement that is needed for proper axon pathfinding. Thus, although speculative, concrete suggestions for places to look for Mud action in the nervous system can be gleaned from the insights gained from our description of this protein in the oocyte and early embryo.
Acknowledgments
We acknowledge Ward Odenwald for advice and instruction about egg collection and immunostaining, Robert Scott, Debasmita Alone, and Kianna Fowlkes for assistance with these procedures, and Carolyn L. Smith for instruction and assistance with confocal microscopy. Special thanks are due to Kate Osborne for her guidance of J. X. Yu during his introduction to this project and for her permission to cite her unpublished observations. We also are grateful to Sharyn Endow for her comments on an early version of this manuscript.
Sequence data from this article have been deposited with the EMBL/GenBank Data Libraries under accession no. DQ465527.
References
- Adams, M. D., S. E. Celniker, R. A. Holt, C. A. Evans, J. D. Gocayne et al., 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195. [DOI] [PubMed] [Google Scholar]
- Barbie, D. A., B. A. Kudlow, R. Frock, J. Zhao, B. R. Johnson et al., 2004. Nuclear reorganization of mammalian DNA synthesis prior to cell cycle exit. Mol. Cell. Biol. 24: 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher, R. D., S. Chodagam, R. Basto, J. G. Wakefield, D. S. Henderson et al., 2004. The Drosophila centrosome-associated protein CP190 is essential for viability but not for cell division. J. Cell Sci. 117: 1191–1199. [DOI] [PubMed] [Google Scholar]
- de Belle, J. S., and M. Heisenberg, 1996. Expression of Drosophila mushroom body mutations in alternative genetic backgrounds: a case study of the mushroom body miniature gene (mbm). Proc. Natl. Acad. Sci. USA 93: 9875–9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, W., and H. Lin, 1997. Spectrosomes and fusomes anchor mitotic spindles during asymmetric germ cell divisions and facilitate the formation of a polarized microtubule array for oocyte specification in Drosophila. Dev. Biol. 189: 79–94. [DOI] [PubMed] [Google Scholar]
- Endow, S. A., and D. J. Komma, 1997. Spindle dynamics during meiosis in Drosophila oocytes. J. Cell Biol. 137: 1321–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow, S. A., and D. J. Komma, 1998. Assembly and dynamics of an anastral:astral spindle: the meiosis II spindle of Drosophila oocytes. J. Cell Sci. 111(17): 2487–2495. [DOI] [PubMed] [Google Scholar]
- Fitch, K. R., and B. T. Wakimoto, 1998. The paternal effect gene ms(3)sneaky is required for sperm activation and the initiation of embryogenesis in Drosophila melanogaster. Dev. Biol. 197: 270–282. [DOI] [PubMed] [Google Scholar]
- FlyBase Consortium, 2002. The FlyBase database of the Drosophila genome projects and community literature. Nucleic Acids Res. 30: 106–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, C., and D. M. Glover, 1993. Techniques for studying mitosis in Drosophila, pp. 163–168 in The Cell Cycle: A Practical Approach, edited by P. Fantes and R. Brook. IRL Press at Oxford University Press, Oxford.
- Grieder, N. C., M. de Cuevas and A. C. Spradling, 2000. The fusome organizes the microtubule network during oocyte differentiation in Drosophila. Development 127: 4253–4264. [DOI] [PubMed] [Google Scholar]
- Guan, Z., A. Prado, J. Melzig, M. Heisenberg, H. A. Nash et al., 2000. Mushroom body defect, a gene involved in the control of neuroblast proliferation in Drosophila, encodes a coiled-coil protein. Proc. Natl. Acad. Sci. USA 97: 8122–8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg, M., 1980. Mutants of brain structure and function: what is the significance of the mushroom bodies for behavior, pp. 373–390 in Development and Neurobiology of Drosophila, edited by O. Siddiqi, P. Babu, L. M. Hall and J. C. Hall. Plenum Press, New York. [DOI] [PubMed]
- Januschke, J., L. Gervais, S. Dass, J. A. Kaltschmidt, H. Lopez-Schier et al., 2002. Polar transport in the Drosophila oocyte requires Dynein and Kinesin I cooperation. Curr. Biol. 12: 1971–1981. [DOI] [PubMed] [Google Scholar]
- Joseph, J., S. H. Tan, T. S. Karpova, J. G. McNally and M. Dasso, 2002. SUMO-1 targets RanGAP1 to kinetochores and mitotic spindles. J. Cell Biol. 156: 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt, J. A., and A. H. Brand, 2002. Asymmetric cell division: microtubule dynamics and spindle asymmetry. J. Cell Sci. 115: 2257–2264. [DOI] [PubMed] [Google Scholar]
- Kisurina-Evgenieva, O., G. Mack, Q. Du, I. Macara, A. Khodjakov et al., 2004. Multiple mechanisms regulate NuMA dynamics at spindle poles. J. Cell Sci. 117: 6391–6400. [DOI] [PubMed] [Google Scholar]
- Kramer, J., and R. S. Hawley, 2003. The spindle-associated transmembrane protein Axs identifies a membranous structure ensheathing the meiotic spindle. Nat. Cell Biol. 5: 261–263. [DOI] [PubMed] [Google Scholar]
- Lee, L. A., D. Van Hoewyk and T. L. Orr-Weaver, 2003. The Drosophila cell cycle kinase PAN GU forms an active complex with PLUTONIUM and GNU to regulate embryonic divisions. Genes Dev. 17: 2979–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, R. B., D. B. Morton and L. L. Restifo, 1995. Remodeling of the insect nervous system. Curr. Opin. Neurobiol. 5: 28–35. [DOI] [PubMed] [Google Scholar]
- Lin, H., L. Yue and A. C. Spradling, 1994. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development 120: 947–956. [DOI] [PubMed] [Google Scholar]
- Martinez-Campos, M., R. Basto, J. Baker, M. Kernan and J. W. Raff, 2004. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J. Cell Biol. 165: 673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathe, E., 2004. Immunocytological analysis of oogenesis. Methods Mol. Biol. 247: 89–127. [DOI] [PubMed] [Google Scholar]
- Matthews, K. A., D. Rees and T. C. Kaufman, 1993. A functionally specialized alpha-tubulin is required for oocyte meiosis and cleavage mitoses in Drosophila. Development 117: 977–991. [DOI] [PubMed] [Google Scholar]
- McKearin, D., 1997. The Drosophila fusome, organelle biogenesis and germ cell differentiation: if you build it. BioEssays 19: 147–152. [DOI] [PubMed] [Google Scholar]
- Megraw, T. L., and T. C. Kaufman, 2000. The centrosome in Drosophila oocyte development. Curr. Top. Dev. Biol. 49: 385–407. [DOI] [PubMed] [Google Scholar]
- Page, A. W., and T. L. Orr-Weaver, 1997. Activation of the meiotic divisions in Drosophila oocytes. Dev. Biol. 183: 195–207. [DOI] [PubMed] [Google Scholar]
- Prokop, A., and G. M. Technau, 1994. Normal function of the mushroom body defect gene of Drosophila is required for the regulation of the number and proliferation of neuroblasts. Dev. Biol. 161: 321–337. [DOI] [PubMed] [Google Scholar]
- Riparbelli, M. G., and G. Callaini, 1996. Meiotic spindle organization in fertilized Drosophila oocyte: presence of centrosomal components in the meiotic apparatus. J. Cell Sci. 109(5): 911–918. [DOI] [PubMed] [Google Scholar]
- Riparbelli, M. G., and G. Callaini, 1998. gamma-tubulin is transiently associated with the Drosophila oocyte meiotic apparatus. Eur. J. Cell Biol. 75: 21–28. [DOI] [PubMed] [Google Scholar]
- Riparbelli, M. G., G. Callaini and D. M. Glover, 2000. Failure of pronuclear migration and repeated divisions of polar body nuclei associated with MTOC defects in polo eggs of Drosophila. J. Cell Sci. 113(18): 3341–3350. [DOI] [PubMed] [Google Scholar]
- Roper, K., and N. H. Brown, 2004. A spectraplakin is enriched on the fusome and organizes microtubules during oocyte specification in Drosophila. Curr. Biol. 14: 99–110. [PubMed] [Google Scholar]
- Rose, A., and I. Meier, 2004. Scaffolds, levers, rods and springs: diverse cellular functions of long coiled-coil proteins. Cell. Mol. Life Sci. 61: 1996–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel, A., T. Winhauer, N. Blumer and R. Renkawitz-Pohl, 1997. The Drosophila don juan (dj) gene encodes a novel sperm specific protein component characterized by an unusual domain of a repetitive amino acid motif. Mech. Dev. 64: 19–30. [DOI] [PubMed] [Google Scholar]
- Snapp, E. L., T. Iida, D. Frescas, J. Lippincott-Schwartz and M. A. Lilly, 2004. The fusome mediates intercellular endoplasmic reticulum connectivity in Drosophila ovarian cysts. Mol. Biol. Cell 15: 4512–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros, W., S. A. Houston, A. Bashirullah, R. L. Cooperstock, J. L. Semotok et al., 2003. Regulation of maternal transcript destabilization during egg activation in Drosophila. Genetics 164: 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavosanis, G., S. Llamazares, G. Goulielmos and C. Gonzalez, 1997. Essential role for gamma-tubulin in the acentriolar female meiotic spindle of Drosophila. EMBO J. 16: 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Technau, G., and M. Heisenberg, 1982. Neural reorganization during metamorphosis of the corpora pedunculata in Drosophila melanogaster. Nature 295: 405–407. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos, P. A., H. Polioudaki, M. Koulentaki, E. Kouroumalis and S. D. Georgatos, 1999. PBC68: a nuclear pore complex protein that associates reversibly with the mitotic spindle. J. Cell Sci. 112(18): 3049–3059. [DOI] [PubMed] [Google Scholar]
- Wolf, K. W., 1995. Spindle membranes and spindle architecture in invertebrates. Micron 26: 69–98. [Google Scholar]