Abstract
The past few years have seen the application of confocal and especially two-photon microscopy to the dynamic high-resolution imaging of lymphocytes and antigen presenting cells within organs such as lymph nodes and thymus. After summarizing some of the published results obtained to date using these methods, we describe our view of how this technology will develop and be applied in the near future. This includes its extension to a wide variety of non-lymphoid tissues, to the tracking of functional responses in addition to migratory behavior, to the analysis of molecular events previously studied only in vitro, to dissection of the interplay between hematopoietic and stromal elements, to visualization of a wider array of cell types including neutrophils, macrophages, NK cells, NKT cells and others, and to the interaction of the host with infectious agents. Reaching these goals will depend on a combination of new tools for genetic manipulations, novel fluorescent reporters, enhanced instrumentation, and better surgical techniques for the extended imaging of live animals. The end result will be a new level of understanding of how orchestrated cell movement and interaction contribute to the physiological and pathological activities of the immune system.
Keywords: Antigen presenting cell, Dendritic cell, T cell, Chemokine, Lymph node, Microscopy
1. Introduction
In contrast to the cells of most adult tissues, which are typically sessile and do not rapidly change intercellular contacts or body location, the cells of the immune system are highly motile. They move from blood to lymphoid tissues to peripheral sites of infection and inflammation or from the latter sites to lymphoid organs [1]. Within lymphoid tissues, both antigen presenting cells and lymphocytes of multiple lineages interact transiently and then change partners or location within these organs. Lymphoid and myeloid cells also exit the circulation and enter tissues in response to local signals [2]. In all of these sites, hematopoietic cells undergo differentiation, leading to changes in their patterns of gene expression, alterations in their repertoire of surface proteins, and production of effector molecules. They interact not only with each other but also with parenchymal cells throughout the body, as well as with infectious agents.
Until recently, our understanding of when, where, and in what manner lymphocytes and other cells of the immune system engaged in cell–cell interactions, and what the consequences were for variations in the partners involved, the duration of association, or the specific molecular signals exchanged, came from serial static analysis of tissue sections combined with in vitro dynamic studies [3]. Over the past 3 years, new methods that utilize advanced light microscopy techniques for visualizing cell behavior directly in intact lymph nodes and other tissues have provided a new window on the dynamic aspects of cellular immune function. Some initial studies tracked the movements of naïve B lymphocytes or CD4+ and CD8+ T cells after adoptive transfer in the absence of antigenic stimulation, revealing the rapid and extensive trafficking of all these cell types within their respective follicular or paracortical zones of accumulation in non-inflamed lymph nodes [4,5]. Other analyses involved the transfer into a single animal of T cell receptor (TCR) transgenic lymphocytes and antigen-bearing dendritic cells, revealing a sequence of cell interaction behaviors within lymph nodes that included early transient associations, followed by prolonged (>1 h) contacts, and then separation and rapid movement of the activated T cells [5-10]. Very recent work has visualized the antigen-dependent association of T and B lymphocytes at the border of the T and B cell zones [11], the migration of thymocytes undergoing positive selection [12], the movement of NKT cells within liver sinusoids [13], and the behavior of effector cells in the central nervous system of animals with autoimmune encephalomyelitis [14].
In the process of conducting these studies, many previous insights into lymphoid behavior and the relationship between lymphoid tissue architecture and function [15-17] have been rediscovered and some new ones revealed. A renewed appreciation for the connection between the anatomy of the lymph node and efficient onset of adaptive immune responses is emerging [1,18], as is an understanding of the need to develop better tools for visualizing not only one or another lymphoid or DC subset, but simultaneously the tissue context in which these cells conduct their business [18-20]. This latter consideration has pushed the limits of available technology, leading to multiplex analysis with three and even four distinct fluorescent probes. Yet these published methods still lack a capacity to reveal the biological response of the involved cells in terms of intracellular signaling events or to routinely report the redistribution of molecules whose location we believe reflects their function based on in vitro studies [21-23]. Techniques for co-tracking of infectious agents, the production of immune mediators such as effector cytokines, or the visualization of the interaction of activated immune cells with host non-lymphoid tissues are still very limited. Finally, only a subset of all the relevant cell types involved in innate and adaptive immunity has been examined and only a small number of tissues have been imaged, all under a very limited set of conditions.
Here we review past work that established the basic methods for dynamic in situ imaging, the relationship of our early studies on T-DC interactions to those that have followed, and our ongoing attempts to develop new tools and techniques that will allow a more complete and informative analysis of immune cell function in vivo.
2. Modes of T-DC interaction
A key step in the development of an adaptive immune response is the recognition of processed antigen on the surface of dendritic cells (DCs) by CD4+ and CD8+ T cells [24]. Prior to the emergence of live tissue imaging tools, two widely cited in vitro studies explored the relationship between lymphocyte activation and the dynamics of T cell contacts with DCs bearing cognate antigen. While both studies focused on the duration of such associations in relation to the resulting functional state of the T cell, they reached drastically differing conclusions. Using pMHC-coated plastic and soluble anti-CD28 antibody, Iezzi et al. found that naïve T cells required approximately 10 h of co-stimulation and TCR signaling or more than 20 h of TCR signaling alone before they committed to cell division [25]. A subsequent report using a more physiologic model with antigen on live presenting cells suggested that continuous stimulation during this prolonged interval was required for a proliferative response [26]. In contrast, by employing a system involving DCs as antigen presenting cells and a collagen gel matrix to mimic a three-dimensional tissue environment, Gunzer et al. found that T cells engaged in sequential and short (10–15 min) contacts with the same or different DCs, eventually resulting in the activation of these T cells as measured by cell proliferation and changes in surface marker expression [27,28]. Consistent with these data, a recent report found evidence for “biochemical memory” in T cells after separation from TCR ligand [29].
Each of these in vitro systems has limitations, with one lacking living antigen-bearing DCs and a three-dimensional environment and the other exposing T cells to collagen fibers, a situation not normally encountered by the T cells in lymph nodes (LNs) [17]. In addition, the conclusion that sequential short interactions support full cell activation as measured by cell division is made problematic by the wide range of cell–cell interaction times observed in the collagen matrix cultures. While most interactions were short-lived, some lasted more than an hour. As discussed below, it may be the case that only these long-lived interactions result in cytokine (especially IL-2) secretion, which could then recruit into cell cycle the remainder of the cells in the gel whose transient interactions with antigen-bearing DCs have led to CD25 upregulation without cytokine secretion. From this perspective, long-lived T-DC association would be required for a productive response, but the trans-activity of soluble factors could serve as an amplifier that acts on cells engaging in short-lived contacts. Resolution of these apparently conflicting datasets required direct observation of T-DC interactions in situ, accompanied by an assessment of individual T cell activation (cytokine) responses and cell division.
The high-resolution dynamic studies necessary to address these issues are now possible using new techniques for applying conventional laser-scanning confocal microscopy and, most importantly, two-photon imaging to intact lymphoid tissues studied either as explants or in the living animal [30,31]. While standard epifluorescence microscopy yields an image that contains emitted light from both above and below the focal plane within a specimen, both confocal and two-photon imaging limit the collected light to a small axial (z) distance, thus improving the resolution of objects in three-dimensional space. In addition, these methods can generally penetrate deeper within dense tissue and are less prone to image degradation due to light scattering. Based on these properties, confocal and two-photon imaging techniques allow for the simultaneous dynamic tracking of the shape, movement, and even gene activation responses of several cells or cell types within a tissue through the rapid sequential collection of multispectral x–y images at successive z levels in a thick specimen over time. Two-photon methods of data collection offer benefits over confocal methods in terms of deeper tissue penetration and less photodamage of tissue samples and have become the techniques of choice for in situ fluorescence imaging [19,30-32].
Two methods of tissue preparation have been employed for in situ dynamic imaging studies: tissue explant microscopy and intravital microscopy. By using two-photon imaging of explanted LNs bathed in medium perfused with 95% O2, Miller et al. provided the first detailed quantitative description of the movement of adoptively transferred, ex vivo fluorescent dye-labeled naïve B and CD4+ T cells in lymphoid tissue at a depth >100 μm below the LN capsule [4]. They found that both B and CD4+ T cells were extremely mobile, moving at 4 and 11 μm/min, respectively. Remarkably, this is the same migration rate seen for these two lymphocyte types in vitro after their isolation from the thoracic duct of rats [15]. A comparable motility for CD8+ T cells was subsequently reported based on data from a similar explant tissue preparation at similar imaging depths [5]. Although these data were obtained using surgically isolated LNs, which lack blood flow, lymph flow, and innervation, these lymphocyte motility characteristics were subsequently confirmed using intravital microscopy of LNs in anesthetized animals [7,10,33,34]. Although clearly a superior method in terms of physiology, one limitation of intravital imaging is that anatomical constraints can prevent effective data collection due to restrictions on the field of view within the target lymphoid structure. Explant methods, on the other hand, circumvent this problem by allowing multiple imaging angles and the collection of more representative data throughout the sample, while also minimizing motion artifacts that prevent effective dynamic data collection [12,19]. The combination of the two methods provides a means of obtaining more complete and physiologically relevant data sets.
Contemporaneously with the migration studies of Miller et al., other groups first used explant imaging to explore the dynamics of T cell interaction with antigen-bearing DCs. Initial studies were conducted using a method first established for static imaging of cell location during immune responses in which T cells from TCR transgenic mice with a known specificity were adoptively transferred into recipients that also received injections of cultured DCs bearing the corresponding peptide-Major Histocompatibility Complex molecule (pMHC) ligands [35,36]. Employing explants of intact LNs from such recipient animals and dual color, confocal time-lapse volume imaging to visualize both the transferred T cells and DCs, Stoll et al. provided the first clear dynamic evidence in support of a long-lived interaction model. In their system, the bulk of transferred CD4+ T cells present in the subcapsular region of the LN (30–80 μM deep) formed highly stable, antigen-dependent associations with DCs during the first 24 h, with individual unbroken associations tracked for >8 h in some cases [6]. This situation changed between 30–36 h after cell transfer, at the time when cell division of the antigen-activated CD4+ T cells began. Many of the lymphocytes left their initial DC partner and began to move rapidly within the paracortical region of the LN, making only transient secondary contacts with antigen-bearing DCs.
Concurrent with the Stoll et al. report, Cahalan and colleagues also reported stable (>50 min) clusters of DO11.10 CD4+ TCR transgenic cells in draining LNs 24 h after a subcutaneous ovalbumin immunization [4]. Although the antigen-bearing DCs were not directly visualized in this study, previous experiments demonstrated that with the same combination of antigen and transgenic T cells, antigen-specific T cells formed clusters exclusively around DCs at this time after immunization or direct injection of antigen-pulsed DCs [36,37]. This suggested that the T cell aggregates observed by Miller et al. most likely contained antigen-bearing DCs, consistent with a long-lasting T-DC conjugation model [30], a conclusion confirmed in more recent studies from this group [9]. Recently, Shakhar et al. visualized resident DCs that had taken up antigens under tolerogenic and priming conditions by using a chimeric DEC-205 antibody to target antigen to DCs in vivo and again observed prolonged CD4+ T cell-DC interactions for the first 18 h after CD4+ T cells entered the LN [10].
In agreement with the long-lived CD4+ T cell-DC contacts seen by several groups, Bousso et al. first reported prolonged associations between CD8+ T cells and antigen-bearing DCs in explanted LNs [5]. Other investigators refined these results through the use of intravital techniques. Mempel et al. used a monoclonal antibody specific for CD62L two hours after transfer of dye-labeled CD8+ T lymphocytes to interfere with the tethering of circulating lymphocytes to high endothelial venules (HEV) [1,2,38] and to prevent T cells that had not already entered the LN from doing so [7]. Using this in vivo manipulation, it was possible to conduct intravital imaging studies of the behavior of a labeled T cell cohort within the LN that was synchronized in terms of its residence time in the tissue. This analysis led to evidence for three rather than the two sequential phases of T-DC interactions first described by Stoll et al. [6]. For the first few hours after entering into the LN, T cells engaged in brief and multiple contacts with antigen-bearing DCs. This “Phase 1” was followed by a 12-h interval (“Phase 2”) during which antigen-specific T cells established and maintained stable conjugates with DCs. After this period of prolonged interaction, T cells left the DCs and moved rapidly within the tissue, with many undergoing cell division (“Phase 3”), in accord with the behavior also reported by Stoll et al. [6]. Hugues et al. studied CD8+ T cell-DC interactions in a model involving antigen-loading of resident DC and also found that T cells established prolonged interactions with antigen-carrying DC 15–20 h after immunization [8]. Thus, accumulating in situ observations involving both CD4+ and CD8+ T cells now support the notion that long-lasting physical conjugation with well-activated DCs bearing high antigen densities is the predominant mode of lymphocyte interaction in the period preceding initiation of clonal expansion. This conclusion echoes observations made more than 25 years ago involving guinea pig lymphocytes and antigen presenting cells analyzed in vitro, which showed evidence of prolonged antigen-specific interactions that preceded the onset of cell division [39].
Nonetheless, some of these studies clearly indicated that short-lived, repetitive T-DC interactions, as first reported in vitro [27,28] also occurred in vivo, although their physiological significance is currently unclear. Miller et al. observed “swarming” behavior by some activated, antigen-specific T cells in which the lymphocytes confined their migration to a relatively small volume (presumably the neighborhood of an antigen-bearing DC) [4]. However, this behavior seems to involve T cells that had been activated several hours previously, not the naïve lymphocytes whose initial responses are in question. In other cases, transient contacts involving naïve CD4+ T cells were clearly delineated [9]. Analyses at higher imaging resolution raise the question of whether in some instances the T cells appearing to make transient contact are actually touching dendrites [34] or if they are truly dissociated from the DC under these conditions. Most relevant from a functional perspective are the data of Mempel et al. [31]. These investigators reported that T cells engaged in brief and multiple contacts with antigen-bearing DCs during “Phase 1” but did not produce IL-2 or interferon-γ (IFNγ) during this period, despite their multiple contacts with DCs. A similar type of transient contact without full activation that preceded prolonged cell–cell association was also seen in another study of CD8+ T cell in explanted LNs [8].
One explanation offered for this phase of transient contact without full activation is that T cells need to “resensitize” their TCR signaling capacity after they emerge from residence in the blood [31], where these cells are deprived of the self-recognition necessary for optimal foreign antigen reactivity [40]. Until such re-sensitization can occur, the T cells may be unable to upregulate integrin affinity upon initial antigen recognition [41,42] and establish tight binding to antigen-bearing DC. An alternative interpretation is that the change in contact duration is not the result of alterations in T cell physiology, but rather reflects the further differentiation/maturation of the co-transferred DCs over the observation period. This is supported by various studies showing that more mature or activated DCs express increased levels of adhesion molecules [43] that are important in establishing long-lived contacts with T cells [44]. Hugues et al. also reported serial brief contacts between CD8+ T cells and antigen-carrying DCs, but they visualized this behavior at a time (15–20 h) well beyond “Phase 1” as reported by Mempel et al. and only under tolerogenic conditions [8]. Presently it is not clear whether these late-occurring brief interactions between T cells and DCs is a special feature of CD8+ T cells undergoing tolerization or may be generalized to other T cell subsets.
3. Relationship of lymph node structure to development of T cell responses
Another important consideration when evaluating data from in vivo imaging studies of the LN is the question of where within the tissue the images have been collected. Several recent studies emphasize the importance of conducting imaging studies in the context of appropriate anatomic landmarks [18-20]. Sequential static imaging has shown that T cells first accumulate in contact with antigen-bearing DCs just outside the HEV through which the lymphocytes entered the LN [45]. Likewise, T and B lymphocytes, as well as DCs, congregate in a novel stromal structure at the boundary between T and B zones termed the “cortical ridge” that is rich in HEV (S movie 1) and is the preferential location of T-DC interactions after immunization with antigen in adjuvant [46]. In a model involving DO11.10 CD4+ T cells and peptide-pulsed splenic DCs, T-DC clusters could be seen in draining lymph nodes as early as 8 h after subcutaneous DC injection [36]. The size and number of these clusters increased substantially within the next 16 h near the HEV-dense paracortical region that corresponds to this cortical ridge structure [16,17]. Likewise, soluble antigen given subcutaneously first accumulated and was processed by DCs that are positioned along lymph conduits associated with HEV in this same subregion of the LN [47]. Earlier studies had revealed these conduits as the routes by which inflammatory mediators and antigen reached the interior of LNs [15-17]. The peri-HEV resident DCs were the initial activators of naïve T cells, in accord with other findings [45]. A second wave of antigen-presentation involved DC migrating from the inoculation site, and these cells were initially concentrated in the same interfollicular, HEV-rich region before moving into the deeper paracortex.
Such preferential distribution of incoming DCs to HEV regions has also been confirmed by two published live imaging analyses [7,9]. Recently, additional information has been provided by Lindquist et al., who found that both antigen-bearing, migrating DCs from the periphery as well as resident steady-state DCs form an extensive sessile network near HEV to facilitate antigen presentation to, and interaction with, migrating T cells exiting the circulation [20]. The migrating DCs function to present antigens acquired in peripheral sites, while soluble antigens that reach the draining LN are taken up by resident DCs embedded within the fibroblastic reticular cell layer that lines the conduit system [16,17,48].
In aggregate, these various findings argue that T cells arriving from the circulation meet a large network of antigen-bearing DCs immediately after egress from the HEV in what is termed the cortical ridge region. The early phases of T-DC contact and response occur in this more superficial region, or in the peri-HEV areas of what is traditionally thought of as the T cell zone, rather than throughout the latter region. Both the very early transient and subsequent prolonged (“Phase 2”) T-DC interactions that occur in the first 24–30 h of a response appear to take place in these sites, leading to cytokine secretion and the initiation of clonal expansion. The prolonged binding that characterizes this first T-DC interaction makes sense if a series of reciprocal signaling events must take place to achieve useful T cell activation. Partially activated, antigen-bearing DC that have received innate signals either while in an infected site or via soluble material draining in the lymph would first induce partial T cell activation involving upregulation of CD40L and OX40. Reciprocal signaling within the cell pair via T cell CD40L engaging DC-expressed CD40 would then promote full DC activation [49], increasing pMHC display, costimulatory molecule levels, and expression of OX40L. These DC changes then further stimulate the bound T cell and also endow it with the capacity to survive during the proliferative and effector differentiation phases that ensue [50]. All of these events involve transcription, translation, and protein trafficking, processes that require hours to complete, consistent with the duration of T-DC contact in this part of the response.
After this time, the progeny of the dividing, activated T cells would move more rapidly within the LN, now making transient interactions with the antigen-rich DCs that have entered from the infected site [47] and are in the process of moving throughout the deeper paracortex. Such transient contacts would likely function to sustain signaling in these pre-activated T cells, based on evidence that such lymphocytes have a heightened capacity to signal in response to low antigen density and to do so more rapidly than naïve T cells [51-53]. This sampling of many antigen-laden DCs may be crucial to the development of effector function among the expanding T cell population [54].
An intriguing possibility is that this mode of behavior enables the T cells to estimate the amount of antigen present in the host. When antigen levels are high and many DCs display ligand, most contacts in this dynamic polygamous phase of T cell migration would likely involve productive TCR signaling. This condition would be a sign that more effector function is needed to fight the infection and the effects of the many productive contacts would promote just this outcome. In contrast, if only a few DCs bore antigen, then only a few contacts would be able to sustain T cell signaling and fewer effectors would be generated. This state would be consistent with host defense having been effective in clearing the infection, so that fewer effectors producing potentially harmful molecules would be needed. Instead, the clonally expanded cells would begin the contraction phase of the response, with some cells resting down into memory cells and others dying [55]. In sum, this model proposes that the enrichment of presenting cells near entry sites for naïve T cells (and central memory cells [56]) fosters effective activation for clonal expansion during an initial phase of prolonged cell–cell contact, whereas later transient contacts among many cells within the larger volume of the paracortical region regulates the extent of effector cell differentiation in proportion to the antigen load (extent of infection) (S movie 1).
4. Imaging functional responses
The model just proposed incorporates ideas about effector cell differentiation that remain untested in situ. A shared deficiency in all published live imaging studies is the lack of real-time functional read-outs. For example, we are still unable to accurately estimate how long a particular T cell needs to contact an antigen-bearing DC in situ in order to activate a relevant gene, e.g., IL-2. This leaves the field unable to draw firm conclusions about whether multiple transient contacts or only prolonged interactions actually result in a functional response from a given T cell. In vitro studies are more advanced in this regard. For example, green fluorescent protein (GFP) expressed under the control of an IL-2 promoter fragment was used to assess the relationship between the longevity of a T cell-DC conjugate and the capacity of a given T cell to activate the IL-2 reporter in a dispersed cell culture environment [57]. In this study, several hours of stable contact were necessary before a T cell would show reporter GFP activity. Brief encounters allowed CD69 upregulation but never IL-2 gene activity.
Fortunately, production of suitable fluorescent reporter animals is poised to provide the tools needed for in vivo analyses. Two different “knock-in” mouse lines with GFP under the control of the IL-4 gene regulatory apparatus have been described [58,59]. Surprisingly, the behavior of the reporter differs markedly in the two strains, raising questions about how to interpret data obtained with cells from these animals. The IL4/GFP mouse [59] appears to report the onset of IL-4 gene transcription whereas the 4GET mouse [58] appears better at labeling cells that are “competent” to produce IL-4. Another mouse expressing yellow fluorescent protein (YFP) under the control of the interferon γ (IFNγ) locus also has been generated [60]. However, the bicistronic mRNA transcripts encoding IFNγ and YFP are longer-lived than unmanipulated IFNγ mRNAs, leading to a pathological inflammatory state that limits the utility of these animals. They seem best suited for detection of the onset of IFNγ production in cells that are clearly YFP-negative at the start of any imaging session. Other animals make GFP under the control of a large fragment of the IL-2 gene (D. Bruniquel and R. H. Schwartz, unpublished observations). We have recently been able to show that the latter animals produce a fluorescent signal upon T cell activation that can be readily visualized by intravital two-photon methods, whether a bacterial superantigen is used to stimulate polyclonal responses or specific peptide is employed to trigger cells transferred from a TCR transgenic animals crossed to the reporter strain (A.Y.C. Huang, D. Bruniquel, R.H. Schwartz, unpublished observations). In concert with methods for intravital imaging that allow tracking dye-labeled naïve T cells or central memory cells as they first exit the HEV (when they should be negative for the cytokine reporter protein), these various animals should permit an assessment of the functional responses of T cells in the context of the duration of interaction with DCs, their location within the LN, and with selective antigen targeting to DC subsets, the nature of the presenting cell.
Imaging molecular events such as protein redistribution during immune synapse formation remain problematic. Although in situ live imaging of a fluorescent chimeric protein expressed by T cells (CD43-GFP) has been reported [6], the physiological level of expression of most relevant proteins is such that it is difficult to attain a sufficient signal from cells deep in a tissue without gross over-expression of the reporter molecule. Yet such over-expression is highly likely to perturb the system, making the results obtained of questionable physiological relevance. Because the quantum yield cannot be substantially increased per fluorescent protein molecule, the solution to this problem must come from better instrumentation that enables effective imaging at lower fluorescence levels. In the near term, a new generation of photodetectors employing gallium arsenide phosphide (GaAsP) may provide the two to four-fold increase in sensitivity necessary to make such studies possible. The use of fluorochromes that emit a signal well separated from any autofluorescent signal will be essential for utilizing this approach, because one needs to increase the signal relative to the noise, not just enhance all signal capture.
5. Stromal influences on hematopoietic cell behavior
Although many of the movies generated using explant or intravital imaging of LNs show cells that appear to be swimming in a dark void lacking underlying structure, nothing could be further from reality. In addition to a dense packing of non-fluorescent lymphocytes and a complex meshwork of DCs [20], intravital visualization of blood vessels using fluorescent tracers or of stromal elements such as collagen using second harmonic emission [18] reveals some of the classically described 3D micro-architecture of lymphoid tissues [15-17]. It is obvious that at a minimum, migrating cells must respect the physical boundaries of these stromal elements as they move and interact. Furthermore, the intricate patterning of vessels, conduits, and other non-hematopoietic components is consistent with them playing an important role in the behavior and function of the hematopoietic elements [15-18]. There is also a wealth of data on the secreted mediators within lymphoid tissue, especially chemokines, and on the role of at least some of the latter in guiding the localization of lymphoid subsets (e.g., CXCL13 (BLC) in the accumulation of CXCR5-expressing B lymphocytes within primary follicles [61,62]). Indeed, the clear segregation between the T zone and the B cell follicles appears to involve remarkably sharp reversals of lymphocyte migratory direction at the borders of these zones, suggesting that lymphocytes respond very precisely to some combination of physical and chemical cues to arrange themselves spatially within lymphoid tissue. The dissection of such guidance cues and the elucidation of the molecular machinery involved in directional sensing are thus important to understanding the development, homeostasis, and activation of the immune system.
To date, all published dynamic imaging studies examining lymphocyte trafficking within LNs have reached the conclusion that naïve T and B cells show “random walk” behavior [4,5,7,8,11,33,34,44]. By this, the authors mean that the cells do not show any preferred direction of movement, changing course in a non-directed fashion so that over time, their progress resembles that of a gas molecule undergoing diffusion. Some deviation from a pure random-walk has been noted in certain analyses [7], but these findings have not changed the prevailing view that T cells in particular interact with DCs based on random collisions [63]. But is this the necessary conclusion from the data? Studies in chemical physics have shown that the linear distance/time1/2 relationship obtained in these imaging analyses is a necessary but not sufficient condition to prove random walk behavior (A. Chakraborty, personal communication). If the field within which an object moves is studded with many small local attractors (e.g., many DC making chemokines that act locally), the overall movement of the objects in that field will fit the linear distance/time1/2 relationship even though near to the source of the attractor, the migration of the objects is non-random. Such local directional movement can be distinguished from true random walk by a correlation analysis and several groups are now collecting the large datasets involving T cells in LNs containing either unactivated or activated DCs that will be necessary for such analyses (A. Chakraborty, personal communication).
This is a critical issue, because there is a great difference in the biological implications of the conclusion that all DCs are equivalent targets for T cells once they enter a LN versus the view that a subset of DCs (perhaps those having arrived from an infected site or those acquiring inflammatory signals through sampling of the lymph draining along with antigen through conduits [16,17,48]) selectively attract T cells and show an elevated rate of interaction with lymphocytes. Indeed, recent studies in this laboratory have documented such local chemokine-guided attraction of naïve CD8+ T cells to DCs that have been activated by TLR signaling and by interaction with antigen-triggered CD4+ T cells (Castellino et al., submitted). This chemokine activity increases CD8+ T cell contacts with the activated DC by three to five-fold over DCs in the same small region of the LN that have not interacted with CD4+ T cells. Furthermore, interference with this chemokine attraction blocks CD4+-dependent memory CD8+ T cell development. Such findings point to a crucial role of local directional cues in the efficient physiological functioning of the LN, at least during primary adaptive immune responses. It would be surprising if this were not the case for many other cell–cell interactions that involve rare antigen specific cells and antigen-laden presenting cells. On a broader scale, these data fit well together with the evidence that antigen-bearing DCs initially form a dense network just outside of HEV and that emerging T cells first recognize and respond to antigen as they negotiate this gauntlet of cells [20,45,48,64]. With this anatomic organization favoring T-DC interactions in a small concentrated region of the node and with local chemokine signals further enhancing useful associations (for example, CCL19 produced by DCs following stimulation by innate or inflammatory signals [65] attracting CCR7-expressing naïve or central memory cells), one begins to understand how the immune system can function efficiently with only rare antigen-specific cells in the repertoire and an ongoing race with rapidly replicating infectious agents.
While these concepts provide a broad framework for understanding cell–cell interactions, we need to develop greater knowledge of the detailed biochemical and structural basis of lymphocyte mobility and cell localization within complex lymphoid and peripheral tissues. To date, most of our understanding of the molecular events governing leukocyte directional sensing and movement derives from in vitro models of neutrophils and of the single cell eukaryote Dictyostelium discoideum [66]. A powerful tool in these analyses has been chimeric fluorescent protein reporters for observing the distribution of specific molecules in migrating cells in the presence and absence of chemoattractants [67]. Despite the insights gained from these in vitro systems, however, it has been difficult to verify these biochemical models using other cell types in vitro and in any cells in vivo. The most useful in vitro systems should accommodate various levels of physical and chemical complexity while allowing experimental manipulations that may not be feasible in a fully physiological in vivo system. For example, one can move from the 2D substrates used in the neutrophil and D. discoideum models to matrices providing a 3D context in which cells can crawl along fibers, squeeze between structural elements, and even remodel their surroundings, all while being tracked at high optical resolution suitable for determining fluorescent protein reporter distribution. Among available model matrices, networks made of polymerized collagen type III fibers have been the most widely used [27,28,68]. At high magnifications, these macromolecular strands can be imaged using differential interference contrast, confocal reflection microscopy, or multi-photon second harmonic generation. When placed in a 3D network, activated T lymphocytes show movement adapted to the physical structure of the gel matrix (S movie 2).
These in vitro observations emphasize the obvious fact, sometimes ignored by viewers of published intravital images, that a cell's path and speed are markedly influenced by contact with its physical surroundings. One striking example involves T cells emerging from HEV. These cells move slowly at best until they exit into the surrounding parenchyma [19] and if an imaging field is rich in these vessels, as was the case for the confocal work of Stoll et al. [6] because of limited imaging depth, then it will appear that the majority of the T cells are poorly motile for no apparent reason (S movie 3). Without co-visualization of the vessels, this could be taken to suggest poor sample viability [5,9], but in truth, it reflects a physiological behavior whose origin is obscure without the added information provided by vessel illumination.
What are some of the methods that can be used to investigate control of immune cell motility and provide information beyond that available from the present intravital methods? While the collagen matrix model is easy to manipulate and image, in normal lymph nodes lymphocytes are not in direct contact with collagen fibers [15]. Lymph nodes are largely supported by a reticular network established by fibroblasts and their secreted extracellular matrix products, and collagen fibers are encased within a sheath of fibroblast cytoplasm and secreted matrix material [15-17]. Experimental tools other than collagen gels are thus needed to provide a more physiologic 3D environment for lymphocyte migration studies. Recently, fibroblast reticular cell lines have been established that when co-cultured with lymphocytes or exposed to an agonist antibody to the lymphotoxin β receptor and to TNF-α form a reticular network expressing the marker ER-TR7 and closely resembling that seen in frozen sections of lymph nodes [69]. The network formed by these cells represents an attractive in vitro reductionist model for investigating the role of this stromal element of LN structure in lymphocyte migration, complementing more difficult intravital studies aimed at dissecting the physical and chemical cues that regulate lymphocyte mobility.
Leukocyte adhesion was originally studied using cryostat sections that retain at least the cell-associated molecular signals and the stromal structure of the original node [70] and a variant of this technique has been used to study DC migration in vitro [71]. With this experimental setup, biochemical treatment or immunostaining can be used to modify the environment of the cells being studied or to reveal structural elements that would be hidden from view in current intravital imaging protocols, respectively. In a recent variant of the cryosection model, thick vibratome sections of thymus have been employed for analysis of the relationship between Ca2+ signaling and thymocyte migration [72]. We have been able to adapt this method to LNs, observing the same type of movement of adoptively transferred T cells within such sections as seen by intravital imaging of lymph nodes from the same animals (L.Y. Koo, unpublished observations; S movie 4). These sections can be stained with at least some antibodies without affecting this migratory behavior. T cell mobility in these sections is quite dependent on super-perfusion with medium containing high O2 levels, as previously reported for thymic sections (L.Y. Koo, unpublished observations; S movie 5), a result that differs from what is seen with explants in our hands, which often [but not always] show T cell movement indistinguishable from that obtained using intravital methods despite the use of normal medium and room air (A.Y.C. Huang, unpublished observations; S movie 6).
Thus, one can study lymphocyte migration in systems of increasing complexity, ranging from 2D culture plates, to artificial 3D matrices, to complex 3D cell cultures, to thin and then thick tissue sections, and finally directly in organized lymphoid tissues themselves. Each model has its limitations in terms of physiological relevance and ease of experimental manipulation as well as imaging resolution. Used in conjunction with fluorescent reporters that can be employed in multiplex mode to follow several parameters simultaneously, and with modifiers of the cellular and extracellular environment such as siRNA and blocking antibodies, the factors controlling lymphocyte migration and localization can be dissected at various scales.
6. Non-lymphoid tissues
In addition to providing insight into how adaptive immune responses are primed within lymphoid organs and how haematopoietic cell navigate in LNs, in situ and intravital imaging has also been applied to visualizing the immune response in peripheral non-lymphoid tissues. Indeed, much of our understanding of leukocyte recruitment to inflamed tissues and subsequent extravasation out of blood vessels has come from imaging blood flow in tissues of living animals or in explanted, externally perfused organs [31]. In general, these studies have employed video-rate transmitted or epi-fluorescence wide-field imaging systems to investigate leukocyte rolling and tethering behavior in a variety of tissue inflammation models, including the spleen [73], lymph nodes [74], mesentery [75], skin [76], cremaster muscle [77], liver [78] and retina [79]. While wide-field video-rate imaging is well suited for studying leukocyte dynamics in blood vessels, as it can provide data acquisition rates capable of capturing rapid intravascular cell dynamics, it is limited in its ability to visualize cells outside of the blood vessel due to greater light-scattering and light-absorbing properties of the tissue parenchyma. Consequently, our understanding of cell recruitment to an inflamed tissue greatly exceeds our knowledge of how that cell behaves once it leaves the blood vessel lumen and enters the tissue.
Using the recently developed microscopy techniques discussed in the preceding sections, we and others have begun to investigate the extra-vascular behavior of immune cells by both intravital and explanted tissue imaging in a variety of non-lymphoid organs, including the intestines, liver, kidney and skin. In addition, we are working towards developing methods that will permit microscopic observation of cellular dynamics in the lung. While technical obstacles exist for all intravital microscopy applications, including limitation of motion artifacts due to breathing, heart-beat and skeletal muscle contractions, preservation of physiological conditions during periods of prolonged anesthesia and surgery, and identification of cells and structures in a non-invasive manner, each organ has unique challenges related to anatomical location and tissue-specific physiology. The rationale for developing imaging methods and inflammatory models for multiple non-lymphoid tissues, despite the associated technical challenges, is two-fold. First, the behavior of immune cells at an inflammatory site may depend upon structural elements that are unique to a particular tissue. For instance, cellular dynamics within the extravascular space of the intestines may differ from what is observed within the epidermis of the skin. Second, different infectious agents and autoimmune diseases may produce unique pathologies in different tissues. Thus, the relevant study of the interface between the immune system and infection or autoimmunity requires the ability to visualize cell behavior in multiple involved organs.
The digestive tract contains the largest collection of lymphocytes in the body and is a site of constant contact between microbes and the host [80]. How the immune system gathers information about the microbial flora and directs the host response in a productive rather than destructive direction are key questions. In the small bowel, there are intraepithelial lymphocytes, lamina propria dendritic cells, Peyer's patches with associated T and B cells, as well as several DC subpopulations [81]. We have focused our attention on the lamina propria subepithelial DC population and its activities. For many years, bacterial transfer from the intestinal lumen to lymphoid tissue has been attributed to the subset of epithelial-derived cells called M cells [82]. In 2001, a new mechanism was described for immune sensing of lumenal small bowel content, one that involved trans-epithelial DC capture of bacteria [83,84]. These in vitro studies used a monolayer of a human colon-derived cell line (CACO-2) able to mimic both the basolateral and the lumenal sides of the intestinal epithelium. Human monocyte-derived DCs were able to extend a dendrite through the tight junction between epithelial cells in the CACO-2 monolayer without disrupting the seal between these cells. The extended process of the DC could capture bacteria added on the lumenal side of the monolayer. Recently Niess et al. broadened this observation using explants from mice heterozygous (sufficient) or homozygous (deficient) for a targeted mutation that replaced the CX3CR1 gene with GFP [85]. DCs in the terminal ileum express CX3CR1, the receptor for CX3CL (fractalkine). Using high-resolution static confocal analyses, this group demonstrated that the presence of a functional receptor for CX3CL on DCs is associated with the capacity of lamina propria DCs to extend dendrites into the lumen of the terminal ileum and acquire bacteria. Additional evidence for epithelial cell-DC communication comes from two other recent studies. In unperturbed conditions, the epithelial cells are able to condition resident DCs to promote non-inflammatory immune responses, whereas in the presence of bacterial compounds, these cells produce chemokines that attract new immature DCs that can differentiate into inflammation-promoting presenting cells [86,87].
We have now developed an intravital technique for two-photon microcopy that permits imaging the small bowel wall from the lumenal surface. Using this method, we have acquired dynamic images of DCs extending membrane processes through the epithelial monolayer into the intestinal lumen, under both normal conditions and in response to pathogenic bacteria (M. Chieppa, in preparation). This process preserves the barrier of the epithelium to small molecules, consistent with the in vitro data [84], while permitting the movement of bacteria from the lumen of the bowel into the body of DCs in the lamina propria. Strikingly, the predominant shape of the DC extensions is not finger-like, as might be expected, but spherical, giving rise to what we term “balloon bodies” (Fig. 1). The cell biological basis for this structure and its physiological significance remain unclear. Ongoing studies are examining the nature of the signals arising from epithelial cell responses to bacterial stimuli interacting with innate receptors such as TLRs.
Fig. 1.
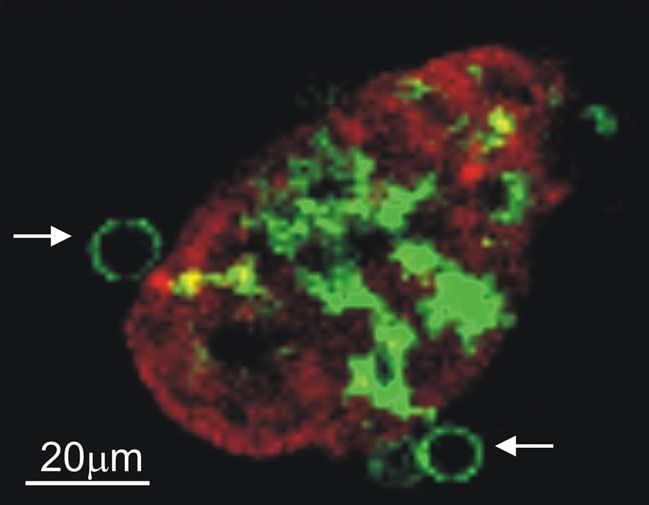
Lamina propria DCs extend trans-epithelial processes with a “balloon-body” appearance. The terminal ileum of a CD11c-EGFP mouse was isolated and the lumen exposed. Villous epithelial cells were stained using the red fluorescent dye SNARF and the tissue imaged by two-photon microscopy. A reconstructed transverse section shows a single z slice from one villus with 2 “balloon bodies” formed by DC trans-epithelial membrane extensions marked by white arrows. The cropped field is 170 μm × 133 μm from an original field of 560 μm × 560 μm.
Recently, several studies have used intravital and explant imaging techniques to investigate lymphoid cell behavior in other non-lymphoid tissues. Employing intravital confocal microscopy and a knock-in animal in which EGFP replaces the gene encoding the chemokine receptor CXCR6, Geissmann et al. [13] demonstrated that NKT cells continually patrol the liver sinusoids and stop upon antigen receptor engagement, presumably providing intravascular immune surveillance of hepatocyte status. Our own non-lymphoid intravital imaging studies have revealed similar patterns of intravascular myeloid cell migration in the liver sinusoids. For instance, after induction of endotoxemia, large numbers of neutrophils are recruited to the hepatic vasculature. These neutrophils show a highly dynamic migration pattern, traveling both with and against blood flow and often making contact with resident cell populations (J.G. Egen, unpublished observations; S movie 7). In addition to visualizing immune cell dynamics in the liver vasculature, we are also utilizing intravital microscopy techniques to investigate cell migration in the blood vessels and parenchyma of the kidney. To this end, we have developed methods that permit imaging of the renal vasculature and tubular network (J.G. Egen, unpublished observations; S movie 8) and are currently applying these new approaches in studies of immune cell dynamics during renal autoimmune responses and after allogeneic transplantation.
In situ live cell imaging techniques have also been applied to the study of effector T cell dynamics at sites of tissue inflammation. Kawakami et al. found that TCR signaling plays a role in influencing effector T cell migration patterns within an explanted rat spinal cord section after the induction of experimental autoimmune encephalomyelitis [14]. These studies demonstrated that the myelin basic protein-specific effector T cells within the spinal cord that mediate disease had reduced motility compared with a population of antigen non-specific T cells that were also recruited to the inflamed tissue. This difference in motility was attributed to antigen-specific interactions made by the T cells with resident MHC class II expressing APCs. These latter antigen-induced stopping data are reminiscent of findings showing that thymocytes undergoing positive selection flux Ca2++ and stop migrating [72] and agree with earlier in vitro studies on the arrest of T cell migration upon contact with an antigen-bearing cell [41].
These types of studies are just the beginning in terms of what we can learn from imaging studies of immune responses in the periphery. Visualization of immune cell dynamics in non-lymphoid organs holds promise for elucidating a number of fundamental biological processes, such as uptake and transport of pathogens/antigens to lymphoid organs by tissue resident presenting cells, inflammatory cell migration and interactions at sites of tissue damage or infection, and local regulation or dysregulation of inflammatory responses by the adaptive arm of the immune system. Combining these studies with more traditional approaches for investigating immune function should yield insight into such diverse fields as infection, autoimmunity, and transplantation.
7. Closing comments
What might be called anatomical immunology is undergoing a renaissance with the introduction and dissemination of methods for live imaging of dynamic cell behavior in situ. As the technology becomes more established and the necessary instrumentation more widely available, such studies will become a mainstream part of immunological investigation. Although certain aspects of immune behavior can be profitably analyzed solely by such methods, in most cases in situ imaging will supplement more conventional experimental tools, providing additional insight into the processes being examined.
Here we have summarized the current state of the field, with an emphasis on the nature of T-DC interactions and lymphocyte mobility that have been the subjects of most published studies. We have tried to point out the underappreciated influence of local tissue organization, especially of non-visualized stromal elements, on lymphocyte migratory behavior, and what new experimental tools might be employed to better probe this relationship. The limitations of current technology in terms of imaging sensitivity, measurement of cell activation status and response, and the localization of specific proteins in single cells have been described and possible solutions to these problems discussed. Additional emphasis has been placed on the study of cell activity outside the lymph node, especially inflamed peripheral tissues. If one can be permitted to employ the types of puns that seem obligatory in this area of research, the future of immune system imaging is “bright” and poised to make an increasingly large contribution to putting decades of deconstructive immunological research back into a holistic perspective.
Supplementary Material
Acknowledgements
The authors wish to thank all the past and present members of the LBS who have contributed to our imaging studies and to discussions that led to the concepts presented in this review, especially Sabine Stoll, Marc Bajenoff, Ina Ifrim, and Parizad Parizi-Torabi. We also thank Owen Schwartz for his help in establishing the confocal protocols used in our early work, Thorsten Mempel and Uli von Andrian for generously helping to teach us their intravital imaging methods, and Mike Dustin for sharing technical details of his imaging procedures. We also are grateful to all those working in this area who have provided critical analyses of our results and have pointed out issues (both methodological and otherwise) that we should consider in interpreting our imaging data. Lastly, we are grateful to Marc Jenkins, whose foray into Flash-based presentations inspired S movie 1. This work was supported in part by the Intramural Research Program of the NIH, NIAID. A.Y.C. Huang and H. Qi are supported by Cancer Research Institute Postdoctoral Fellowships.
Abbreviations
- DC(s)
dendritic cell(s)
- GFP
green fluorescent protein
- HEV
high endothelial venule
- IFNγ
interferon-γ
- LN(s)
lymph node(s)
- TCR
T cell receptor
- YFP
yellow fluorescent protein
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.smim.2005.09.003.
References
- 1.von Andrian UH, Mackay CR. T cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–34. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 2.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–53. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 3.Germain RN, Jenkins MK. In vivo antigen presentation. Curr Opin Immunol. 2004;16:120–5. doi: 10.1016/j.coi.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–73. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 5.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–85. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 6.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–6. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 7.Mempel TR, Henrickson SE, Von Andrian UH. T cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–9. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 8.Hugues S, Fetler L, Bonifaz L, Helft J, Amblard F, Amigorena S. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–42. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- 9.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–56. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, et al. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–14. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witt CM, Raychaudhuri S, Schaefer B, Chakraborty AK, Robey EA. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol. 2005;3:e160. doi: 10.1371/journal.pbio.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawakami N, Nagerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flugel A. Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J Exp Med. 2005;201:1805–14. doi: 10.1084/jem.20050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson AO, Anderson ND, White JD. In: Lymphocyte locomotion, lymphatic tissues and lymphocyte circulation in the rat, in Animal Models of Immunological Processes. Hays JB, editor. Academic Press; London: pp. 26–95. [Google Scholar]
- 16.Gretz JE, Kaldjian EP, Anderson AO, Shaw S. Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. J Immunol. 1996;157:495–9. [PubMed] [Google Scholar]
- 17.Gretz JE, Anderson AO, Shaw S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev. 1997;156:11–24. doi: 10.1111/j.1600-065x.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 18.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–78. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 19.Huang AY, Qi H, Germain RN. Illuminating the landscape of in vivo immunity: insights from dynamic in situ imaging of secondary lymphoid tissues. Immunity. 2004;21:331–9. doi: 10.1016/j.immuni.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, et al. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–50. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 21.Delon J, Germain RN. Information transfer at the immunological synapse. Curr Biol. 2000;10:R923–33. doi: 10.1016/s0960-9822(00)00870-8. [DOI] [PubMed] [Google Scholar]
- 22.Krummel M, Wulfing C, Sumen C, Davis MM. Thirty-six views of T cell recognition. Philos Trans R Soc Lond B Biol Sci. 2000;355:1071–6. doi: 10.1098/rstb.2000.0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol. 2000;1:23–9. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 24.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 25.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 26.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–55. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 27.Gunzer M, Schafer A, Borgmann S, Grabbe S, Zanker KS, Brocker EB, et al. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–32. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 28.Friedl P, Gunzer M. Interaction of T cells with APCs: the serial encounter model. Trends Immunol. 2001;22:187–91. doi: 10.1016/s1471-4906(01)01869-5. [DOI] [PubMed] [Google Scholar]
- 29.Munitic I, Ryan PE, Ashwell JD. T cells in G1 provide a memory-like response to secondary stimulation. J Immunol. 2005;174:4010–8. doi: 10.4049/jimmunol.174.7.4010. [DOI] [PubMed] [Google Scholar]
- 30.Cahalan MD, Parker I, Wei SH, Miller MJ. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat Rev Immunol. 2002;2:872–80. doi: 10.1038/nri935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mempel TR, Scimone ML, Mora JR, von Andrian UH. In vivo imaging of leukocyte trafficking in blood vessels and tissues. Curr Opin Immunol. 2004;16:406–17. doi: 10.1016/j.coi.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Bousso P, Robey EA. Dynamic behavior of T cells and thymocytes in lymphoid organs as revealed by two-photon microscopy. Immunity. 2004;21:349–55. doi: 10.1016/j.immuni.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Miller MJ, Wei SH, Cahalan MD, Parker I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc Natl Acad Sci USA. 2003;100:2604–9. doi: 10.1073/pnas.2628040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc Natl Acad Sci USA. 2004;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–39. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 36.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4+ T cells. J Exp Med. 1997;185:2133–41. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ingulli E, Ulman DR, Lucido MM, Jenkins MK. In situ analysis reveals physical interactions between CD11b+ dendritic cells and antigen-specific CD4 T cells after subcutaneous injection of antigen. J Immunol. 2002:169, 2247–52. doi: 10.4049/jimmunol.169.5.2247. [DOI] [PubMed] [Google Scholar]
- 38.Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med. 1998;187:205–16. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipsky PE, Rosenthal AS. Macrophage-lymphocyte interaction. II. Antigen-mediated physical interactions between immune guinea pig lymph node lymphocytes and syngeneic macrophages. J Exp Med. 1975;141:138–54. doi: 10.1084/jem.141.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–34. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 41.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci USA. 1997;94:3909–13. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dustin ML. Making a little affinity go a long way: a topological view of LFA-1 regulation. Cell Adhes Commun. 1998;6:255–62. doi: 10.3109/15419069809004481. [DOI] [PubMed] [Google Scholar]
- 43.Teunissen MB, Rongen HA, Bos JD. Function of adhesion molecules lymphocyte function-associated antigen-3 and intercellular adhesion molecule-1 on human epidermal Langerhans cells in antigen-specific T cell activation. J Immunol. 1994;152:3400–9. [PubMed] [Google Scholar]
- 44.Benvenuti F, Lagaudriere-Gesbert C, Grandjean I, Jancic C, Hivroz C, Trautmann A, et al. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J Immunol. 2004;172:292–301. doi: 10.4049/jimmunol.172.1.292. [DOI] [PubMed] [Google Scholar]
- 45.Bajenoff M, Granjeaud S, Guerder S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J Exp Med. 2003;198:715–24. doi: 10.1084/jem.20030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katakai T, Hara T, Lee JH, Gonda H, Sugai M, Shimizu A. A novel reticular stromal structure in lymph node cortex: an immuno-platform for interactions among dendritic cells T cells B cells. Int Immunol. 2004;16:1133–42. doi: 10.1093/intimm/dxh113. [DOI] [PubMed] [Google Scholar]
- 47.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 48.Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 49.Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–62. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 50.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–7. [PubMed] [Google Scholar]
- 51.Pihlgren M, Dubois PM, Tomkowiak M, Sjogren T, Marvel J. Resting memory CD8+ T cells are hyperreactive to antigenic challenge in vitro. J Exp Med. 1996;184:2141–251. doi: 10.1084/jem.184.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curtsinger JM, Lins DC, Mescher MF. CD8+ memory T cells (CD44high, Ly-6C+) are more sensitive than naive cells to (CD44low, Ly-6C-) to TCR/CD8 signaling in response to antigen. J Immunol. 1998;160:3236–43. [PubMed] [Google Scholar]
- 53.Slifka MK, Whitton JL. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–7. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 54.Bajenoff M, Wurtz O, Guerder S. Repeated antigen exposure is necessary for the differentiation, but not the initial proliferation, of naive CD4(+) T cells. J Immunol. 2002;168:1723–9. doi: 10.4049/jimmunol.168.4.1723. [DOI] [PubMed] [Google Scholar]
- 55.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–42. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 56.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 57.Hurez V, Saparov A, Tousson A, Fuller MJ, Kubo T, Oliver J, et al. Restricted clonal expression of IL-2 by naive T cells reflects differential dynamic interactions with dendritic cells. J Exp Med. 2003;198:123–32. doi: 10.1084/jem.20022230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–11. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 59.Hu-Li J, Pannetier C, Guo L, Lohning M, Gu H, Watson C, et al. Regulation of expression of IL-4 alleles: analysis using a chimeric GFP/IL-4 gene. Immunity. 2001;14:1–11. doi: 10.1016/s1074-7613(01)00084-x. [DOI] [PubMed] [Google Scholar]
- 60.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–76. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–14. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 62.Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–9. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 63.Catron DM, Itano AA, Pape KA, Mueller DL, Jenkins MK. Visualizing the first 50 h of the primary immune response to a soluble antigen. Immunity. 2004;21:341–7. doi: 10.1016/j.immuni.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med. 2004;200:783–95. doi: 10.1084/jem.20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ngo VN, Tang HL, Cyster JG. Epstein-Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J Exp Med. 1998;188:181–91. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parent CA. Making all the right moves: chemotaxis in neutrophils and Dictyostelium. Curr Opin Cell Biol. 2004;16:4–13. doi: 10.1016/j.ceb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 67.Halet G. Imaging phosphoinositide dynamics using GFP-tagged protein domains. Biol Cell. 2005;97:501–18. doi: 10.1042/BC20040080. [DOI] [PubMed] [Google Scholar]
- 68.Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4(+) T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta 1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Euro J Immunol. 1998;28:2331–43. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 69.Katakai T, Hara T, Sugai M, Gonda H, Shimuzu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med. 2004;200:783–95. doi: 10.1084/jem.20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stamper HB, Jr, Woodruff JJ. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976;144:828–33. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sixt M, Sorokin L, Lutz MB. Migration routes of dendritic cells into lymph nodes. Seventh International Symposium on Dendritic Cells. 2002:36. [Google Scholar]
- 72.Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6:143–51. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt EE, MacDonald IC, Groom AC. Interactions of leukocytes with vessel walls and with other blood cells, studied by high-resolution intravital videomicroscopy of spleen. Microvasc Res. 1990;40:99–117. doi: 10.1016/0026-2862(90)90011-f. [DOI] [PubMed] [Google Scholar]
- 74.Von Andrian UH. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation. 1996;3:287–300. doi: 10.3109/10739689609148303. [DOI] [PubMed] [Google Scholar]
- 75.Atherton A, Born GV. Quantitative investigations of the adhesiveness of circulating polymorphonuclear leucocytes to blood vessel walls. J Physiol. 1972;222:447–74. doi: 10.1113/jphysiol.1972.sp009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Falkvoll KH, Rofstad EK, Brustad T, Marton P. A transparent chamber for the dorsal skin fold of athymic mice. Exp Cell Biol. 1984;52:260–8. doi: 10.1159/000163269. [DOI] [PubMed] [Google Scholar]
- 77.Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc Res. 1973;5:384–94. doi: 10.1016/0026-2862(73)90054-x. [DOI] [PubMed] [Google Scholar]
- 78.Vollmar B, Glasz J, Senkel A, Menger MD, Messmer K. Role of leukocytes in the initial hepatic microvascular response to endotoxemia. Zentralbl Chir. 1993;118:691–6. [PubMed] [Google Scholar]
- 79.Nishiwaki H, Ogura Y, Kimura H, Kiryu J, Miyamoto K, Matsuda N. Visualization and quantitative analysis of leukocyte dynamics in retinal microcirculation of rats. Invest Ophthalmol Vis Sci. 1996;37:1341–7. [PubMed] [Google Scholar]
- 80.Mowat AM, Parker LA, Beacock-Sharp H, Millington OR, Chirdo F. Oral tolerance: overview and historical perspectives. Ann N Y Acad Sci. 2004:1029–8. doi: 10.1196/annals.1309.001. [DOI] [PubMed] [Google Scholar]
- 81.Garside P, Millington O, Smith KM. The anatomy of mucosal immune responses. Ann N Y Acad Sci. 2004;1029:9–15. doi: 10.1196/annals.1309.002. [DOI] [PubMed] [Google Scholar]
- 82.Jang MH, Kweon MN, Iwatani K, Yamamoto M, Terahara K, Sasakawa C, et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci USA. 2004;101:6110–5. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rescigno M, Rotta G, Valzasina B, Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology. 2001;204:572–81. doi: 10.1078/0171-2985-00094. [DOI] [PubMed] [Google Scholar]
- 84.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 85.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 86.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–14. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 87.Rimoldi M, Chieppa M, Larghi P, Vulcano M, Allavena P, Rescigno M. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood. doi: 10.1182/blood-2004-11-4321. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


