Abstract
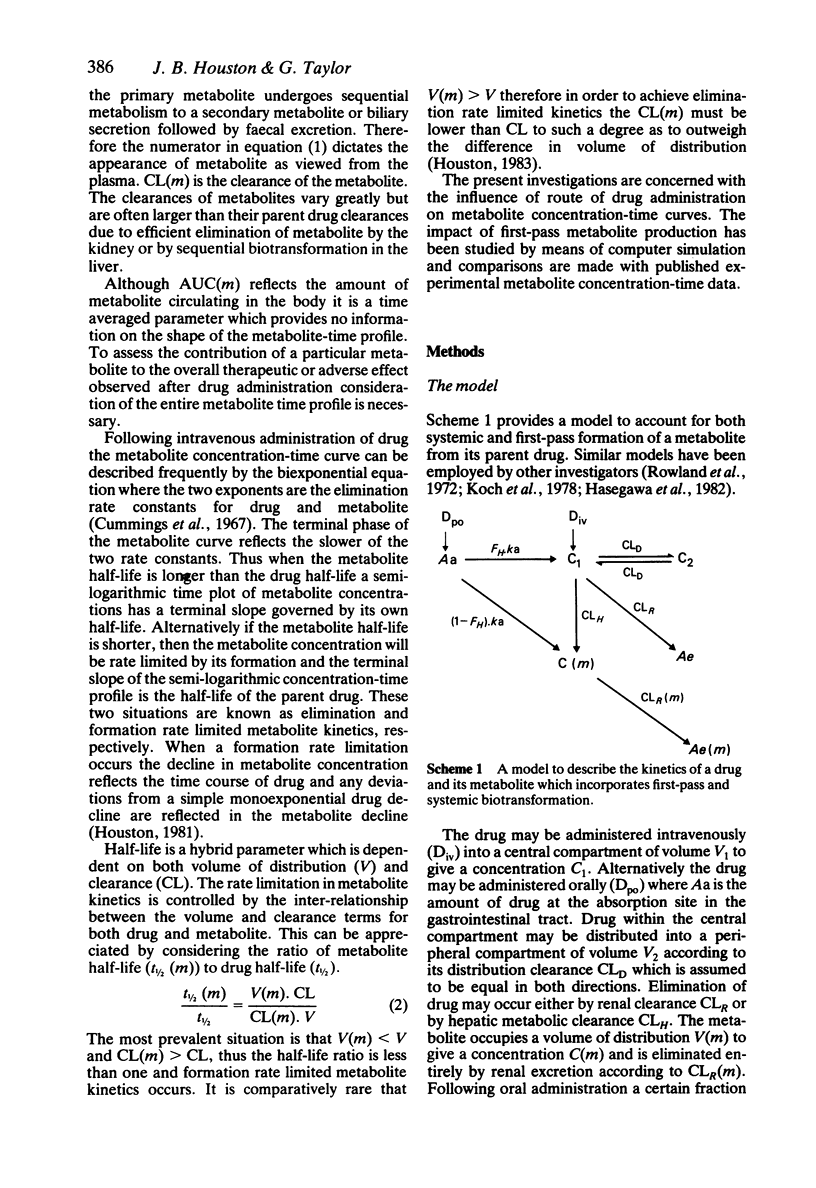
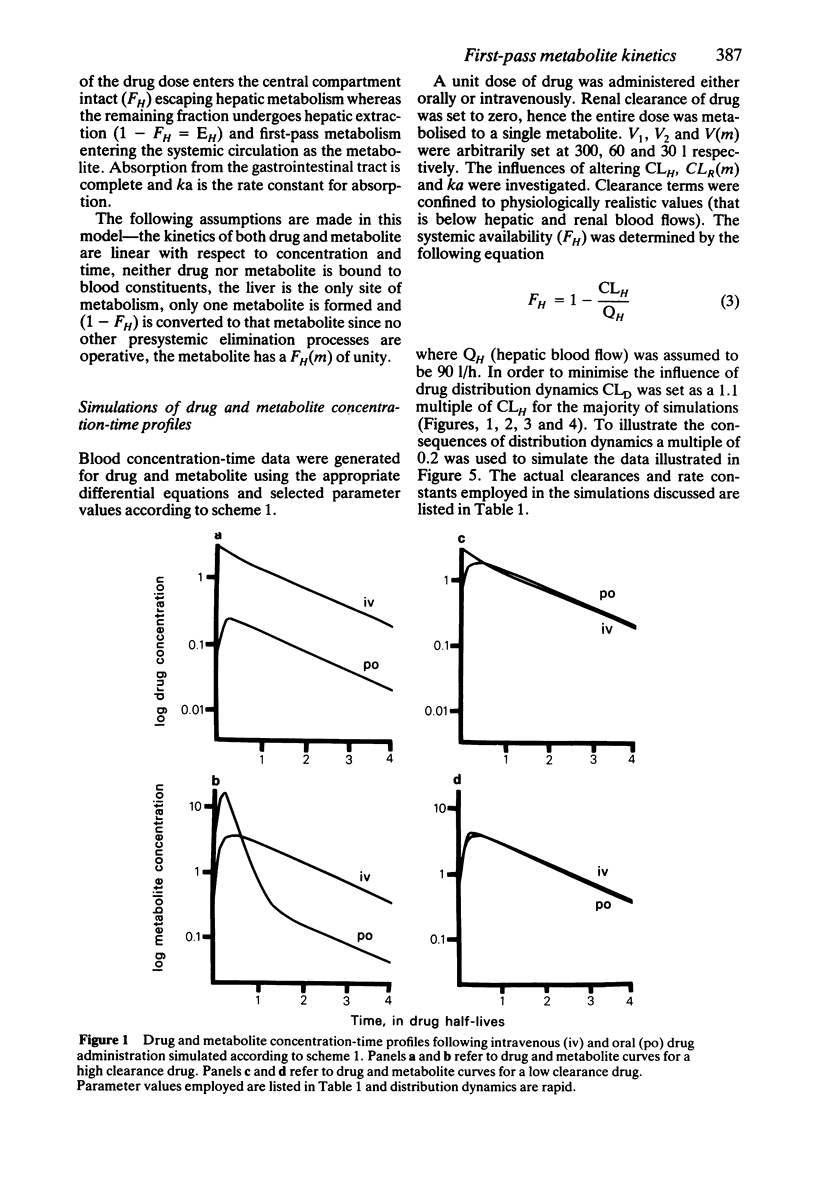
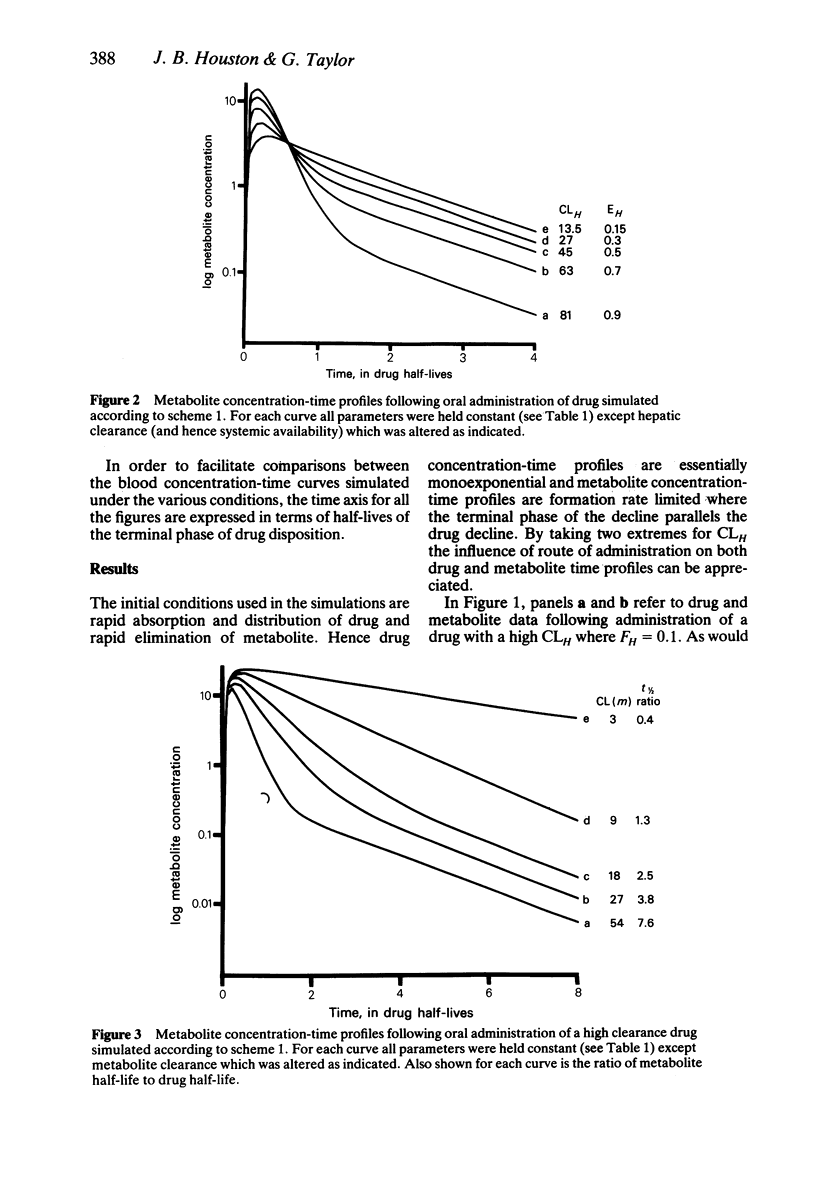
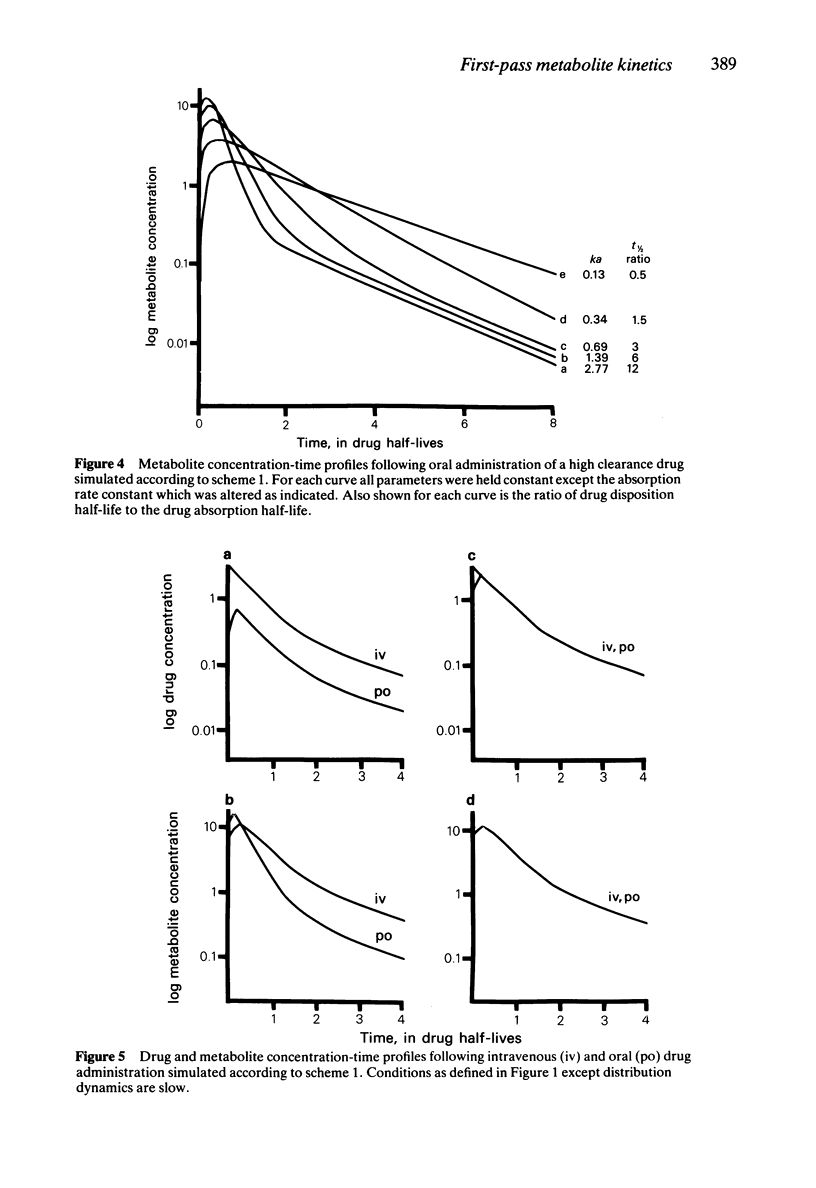
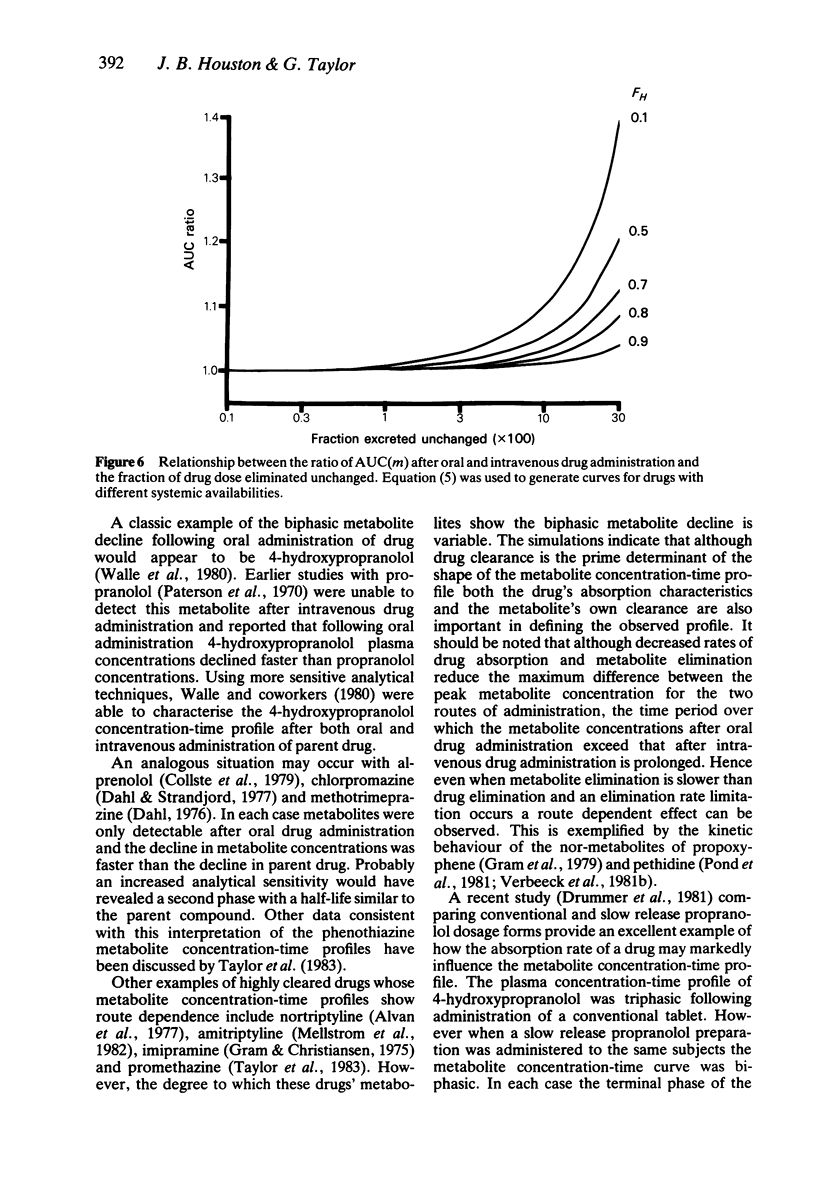
In order to assess the contribution of an active metabolite to the overall pharmacological response following drug administration it is necessary to characterise the metabolite concentration-time profile. The influence of route of drug administration on metabolite kinetics has been investigated by computer simulation. Comparisons between simulated profiles and published concentration-time data have been carried out. A route dependence in metabolite concentration-time curves is readily apparent provided the metabolite kinetics are formation rate limited and the hepatic clearance of drug is greater than 25 l/h (medium to highly cleared). Oral drug administration produces a triphasic metabolite concentration-time profile whereas only two phases are discernable after intravenous drug administration. The magnitude of the difference in maximum metabolite concentration is directly proportional to the hepatic clearance of drug due to first-pass metabolite production. The route dependence in the shape of the metabolite concentration-time curves is most dramatic when the absorption and distribution of drug and the elimination of metabolite is rapid. A reduction in the rate of either of these processes alters the shape of the metabolite concentration-time profile such that the consequence of first-pass metabolite formation may be reduced.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alván G., Borga O., Lind M., Palmér L., Siwers B. First pass hydroxylation of nortriptyline: concentrations of parent drug and major metabolites in plasma. Eur J Clin Pharmacol. 1977 Mar 11;11(3):219–224. doi: 10.1007/BF00606414. [DOI] [PubMed] [Google Scholar]
- Atkinson A. J., Jr, Stec G. P., Lertora J. J., Ruo T. I., Thenot J. P. Impact of active metabolites on monitoring plasma concentrations of therapeutic drugs. Ther Drug Monit. 1980;2(1):19–27. doi: 10.1097/00007691-198001000-00004. [DOI] [PubMed] [Google Scholar]
- Bai S. A., Abramson F. P. Interaction of phenobarbital with propranolol in the dog. 3. Beta blockade. J Pharmacol Exp Ther. 1983 Jan;224(1):62–67. [PubMed] [Google Scholar]
- Collste P., Borg K. O., Aström H., von Bahr C. Contribution of 4-hydroxy-alprenolol to adrenergic beta receptor blockade of alprenolol. Clin Pharmacol Ther. 1979 Apr;25(4):416–422. doi: 10.1002/cpt1979254416. [DOI] [PubMed] [Google Scholar]
- Cummings A. J., Martin B. K., Park G. S. Kinetic considerations relating to the accrual and elimination of drug metabolites. Br J Pharmacol Chemother. 1967 Feb;29(2):136–149. doi: 10.1111/j.1476-5381.1967.tb01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl S. G. Pharmacokinetics of methotrimeprazine after single and multiple doses. Clin Pharmacol Ther. 1976 Apr;19(4):435–442. doi: 10.1002/cpt1976194435. [DOI] [PubMed] [Google Scholar]
- Dahl S. G., Strandjord R. E. Pharmacokinetics of chlorpromazine after single and chronic dosage. Clin Pharmacol Ther. 1977 Apr;21(4):437–448. doi: 10.1002/cpt1977214437. [DOI] [PubMed] [Google Scholar]
- Drayer D. E. Pharmacologically active drug metabolites: therapeutic and toxic activities, plasma and urine data in man, accumulation in renal failure. Clin Pharmacokinet. 1976 Nov-Dec;1(6):426–443. doi: 10.2165/00003088-197601060-00003. [DOI] [PubMed] [Google Scholar]
- Drayer D. E. Pharmacologically active metabolites of drugs and other foreign compounds. Clinical, pharmacological, therapeutic and toxicological considerations. Drugs. 1982 Dec;24(6):519–542. doi: 10.2165/00003495-198224060-00003. [DOI] [PubMed] [Google Scholar]
- Drummer O. H., McNeil J., Pritchard E., Louis W. J. Combined high-performance liquid chromatographic procedure for measuring 4-hydroxypropranolol and propranolol in plasma: pharmacokinetic measurements following conventional and slow-release propranolol administration. J Pharm Sci. 1981 Sep;70(9):1030–1032. doi: 10.1002/jps.2600700916. [DOI] [PubMed] [Google Scholar]
- Gibaldi M., Perrier D. Route of administration and drug disposition. Drug Metab Rev. 1974;3(2):185–199. doi: 10.3109/03602537408993742. [DOI] [PubMed] [Google Scholar]
- Gram L. F., Christiansen J. First-pass metabolism of imipramine in man. Clin Pharmacol Ther. 1975 May;17(5):555–563. doi: 10.1002/cpt1975175555. [DOI] [PubMed] [Google Scholar]
- Gram L. F., Schou J., Way W. L., Heltberg J., Bodin N. O. d-Propoxyphene kinetics after single oral and intravenous doses in man. Clin Pharmacol Ther. 1979 Oct;26(4):473–482. doi: 10.1002/cpt1979264473. [DOI] [PubMed] [Google Scholar]
- Hasegawa J., Lin E. T., Williams R. L., Sörgel F., Benet L. Z. Pharmacokinetics of triamterene and its metabolite in man. J Pharmacokinet Biopharm. 1982 Oct;10(5):507–523. doi: 10.1007/BF01059034. [DOI] [PubMed] [Google Scholar]
- Holford N. H., Coates P. E., Guentert T. W., Riegelman S., Sheiner L. B. The effect of quinidine and its metabolites on the electrocardiogram and systolic time intervals: concentration--effect relationships. Br J Clin Pharmacol. 1981 Feb;11(2):187–195. doi: 10.1111/j.1365-2125.1981.tb01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston J. B. Drug metabolite kinetics. Pharmacol Ther. 1981;15(3):521–552. doi: 10.1016/0163-7258(81)90056-5. [DOI] [PubMed] [Google Scholar]
- Koch P. A., Schultz C. A., Wills R. J., Hallquist S. L., Welling P. G. Influence of food and fluid ingestion on aspirin bioavailability. J Pharm Sci. 1978 Nov;67(11):1533–1535. doi: 10.1002/jps.2600671110. [DOI] [PubMed] [Google Scholar]
- Pang K. S., Gillette J. R. Sequential first-pass elimination of a metabolite derived from a precursor. J Pharmacokinet Biopharm. 1979 Jun;7(3):275–290. doi: 10.1007/BF01060018. [DOI] [PubMed] [Google Scholar]
- Pang K. S., Gillette J. R. Theoretical relationships between area under the curve and route of administration of drugs and their precursors for evaluating sites and pathways of metabolism. J Pharm Sci. 1978 May;67(5):703–704. doi: 10.1002/jps.2600670536. [DOI] [PubMed] [Google Scholar]
- Pang K. S. Metabolite pharmacokinetics: the area under the curve of metabolite and the fractional rate of metabolism of a drug after different routes of administration for renally and hepatically cleared drugs and metabolites. J Pharmacokinet Biopharm. 1981 Aug;9(4):477–487. doi: 10.1007/BF01060890. [DOI] [PubMed] [Google Scholar]
- Pang K. S., Strobl K., Gillette J. R. A method for the estimation of the fraction of a precursor that is converted to a metabolite in rat in vivo with phenacetin and acetaminophen. Drug Metab Dispos. 1979 Nov-Dec;7(6):366–372. [PubMed] [Google Scholar]
- Pond S. M., Tong T., Benowitz N. L., Jacob P., Rigod J. Presystemic metabolism of meperidine to normeperidine in normal and cirrhotic subjects. Clin Pharmacol Ther. 1981 Aug;30(2):183–188. doi: 10.1038/clpt.1981.146. [DOI] [PubMed] [Google Scholar]
- Routledge P. A., Shand D. G. Presystemic drug elimination. Annu Rev Pharmacol Toxicol. 1979;19:447–468. doi: 10.1146/annurev.pa.19.040179.002311. [DOI] [PubMed] [Google Scholar]
- Rowland M., Riegelman S., Harris P. A., Sholkoff S. D. Absorption kinetics of aspirin in man following oral administration of an aqueous solution. J Pharm Sci. 1972 Mar;61(3):379–385. doi: 10.1002/jps.2600610312. [DOI] [PubMed] [Google Scholar]
- Taylor G., Houston J. B., Shaffer J., Mawer G. Pharmacokinetics of promethazine and its sulphoxide metabolite after intravenous and oral administration to man. Br J Clin Pharmacol. 1983 Mar;15(3):287–293. doi: 10.1111/j.1365-2125.1983.tb01501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeeck R. K., Branch R. A., Wilkinson G. R. Drug metabolites in renal failure: pharmacokinetic and clinical implications. Clin Pharmacokinet. 1981 Sep-Oct;6(5):329–345. doi: 10.2165/00003088-198106050-00001. [DOI] [PubMed] [Google Scholar]
- Verbeeck R. K., Branch R. A., Wilkinson G. R. Meperidine disposition in man: influence of urinary pH and route of administration. Clin Pharmacol Ther. 1981 Nov;30(5):619–628. doi: 10.1038/clpt.1981.213. [DOI] [PubMed] [Google Scholar]
- Walle T., Conradi E. C., Walle U. K., Fagan T. C., Gaffney T. E. 4-Hydroxypropranolol and its glucuronide after single and long-term doses of propranolol. Clin Pharmacol Ther. 1980 Jan;27(1):22–31. doi: 10.1038/clpt.1980.4. [DOI] [PubMed] [Google Scholar]
- Walle T., Conradi E. C., Walle U. K., Fagan T. C., Gaffney T. E. Naphthoxylactic acid after single and long-term doses of propranolol. Clin Pharmacol Ther. 1979 Nov;26(5):548–554. doi: 10.1002/cpt1979265548. [DOI] [PubMed] [Google Scholar]


