Abstract
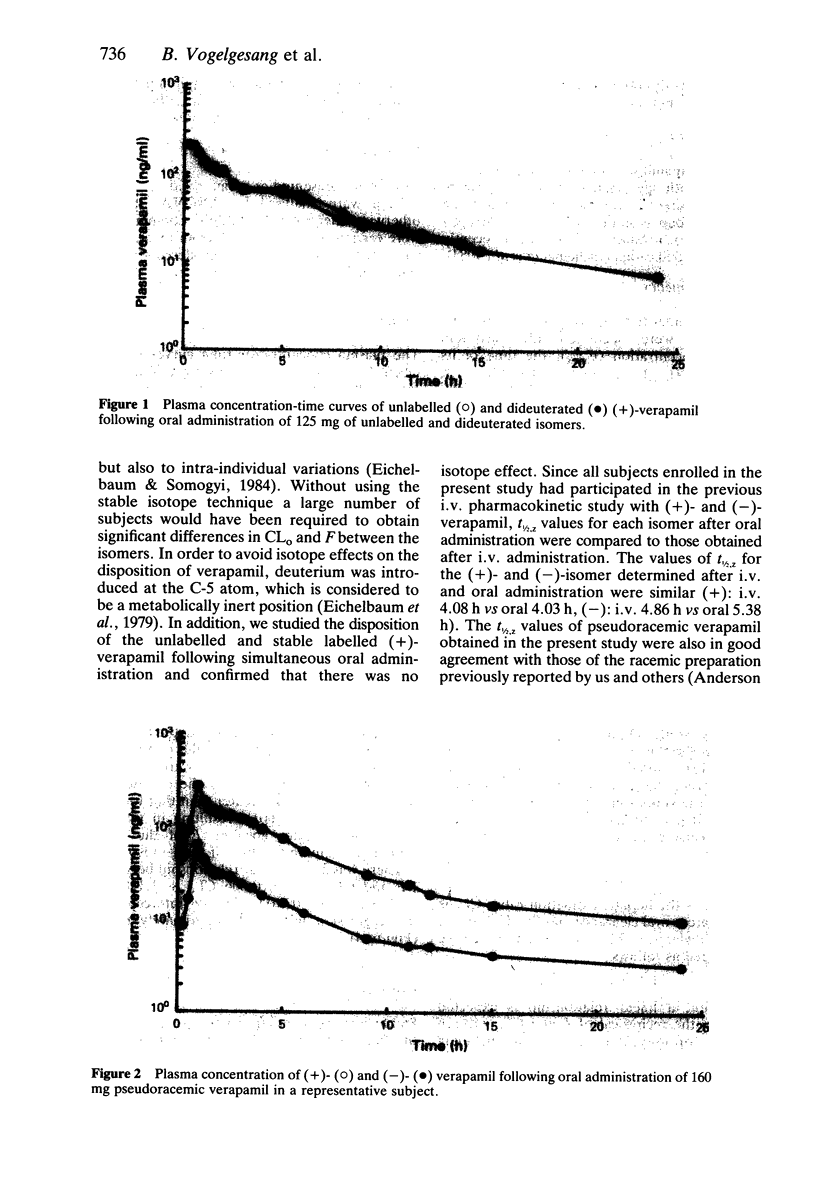
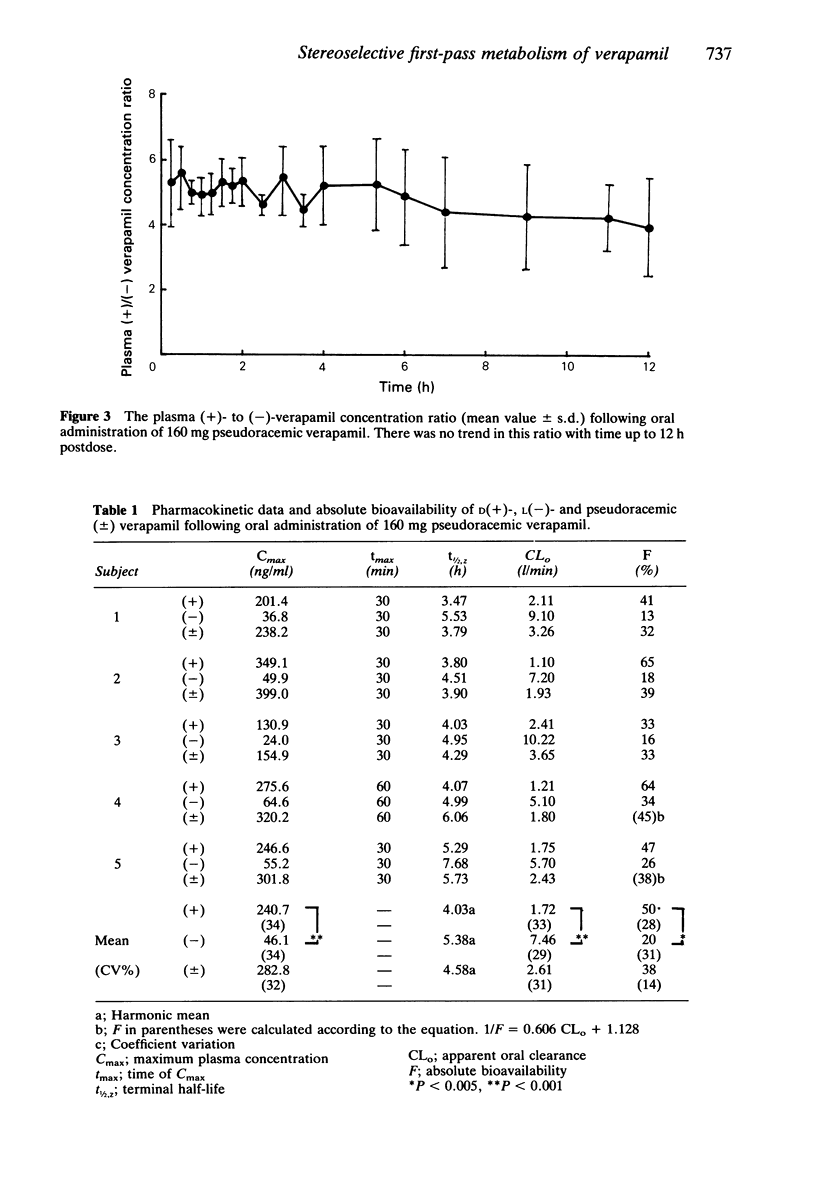
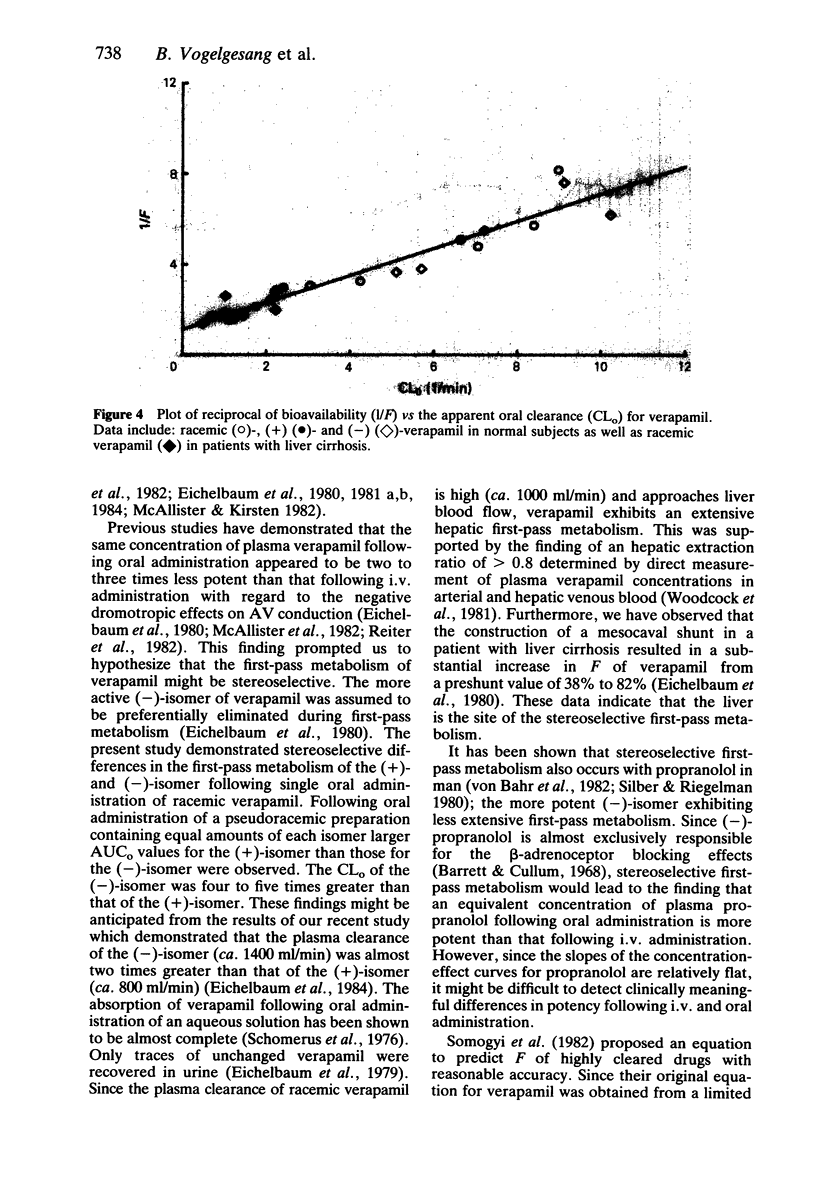
The pharmacokinetics of dextro(+)- and levo(-)-verapamil were studied in five healthy volunteers following oral administration of pseudoracemic verapamil containing equal amounts of unlabelled (-)- and dideuterated (+)-isomer. (+)-verapamil exhibited approximately five times greater Cmax (+): 240 +/- 81.1 ng/ml, (-): 46.1 +/- 15.7 ng/ml, P less than 0.0001) and AUC than (-)-verapamil. The apparent oral clearance (CLo) for (+)-verapamil was significantly smaller than that for (-)-verapamil (+): 1.72 +/- 0.57 l/min, (-): 7.46 +/- 2.16 l/min, P less than 0.001). The bioavailability of (+)-verapamil (50%) was 2.5 times greater than that of (-)-verapamil (20%), P less than 0.005). Thus following oral administration verapamil exhibited a stereoselective first-pass metabolism. Neither tmax nor the elimination t1/2,z were different between the isomers. The elimination of t1/2,z for each verapamil isomer obtained following oral administration (+): 4.03 h, (-): 5.38 h) were similar to those previously obtained following intravenous administration (+): 4.15 h, (-): 5.38 h, respectively. Whereas the (+)- to (-)-verapamil plasma concentration ratio following oral administration was 4.92 +/- 0.48, the ratio following i.v. administration was approximately 2. (-)-verapamil has been demonstrated to possess 8 to 10 times more potent negative dromotropic effect on AV conduction than (+)-verapamil. Therefore, following oral administration the same concentration of plasma verapamil consisting of a two to three times smaller proportion of the more potent (-)-isomer appeared to be less potent than that following i.v. administration with regard to the negative dromotropic effects on the AV conduction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., BondessoN U., Sylvén C. Clinical pharmacokinetics of verapamil in patients with atrial fibrillation. Eur J Clin Pharmacol. 1982;23(1):49–57. doi: 10.1007/BF01061377. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Birkel P., Grube E., Gütgemann U., Somogyi A. Effects of verapamil on P-R-intervals in relation to verapamil plasma levels following single I.V. and oral administration and during chronic treatment. Klin Wochenschr. 1980 Sep 15;58(18):919–925. doi: 10.1007/BF01477049. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Dengler H. J., Somogyi A., von Unruh G. E. Superiority of stable isotope techniques in the assessment of the bioavailability of drugs undergoing extensive first pass elimination. Studies of the relative bioavailability of verapamil tablets. Eur J Clin Pharmacol. 1981 Jan;19(2):127–131. doi: 10.1007/BF00568399. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Ende M., Remberg G., Schomerus M., Dengler H. J. The metabolism of DL-[14C]verapamil in man. Drug Metab Dispos. 1979 May-Jun;7(3):145–148. [PubMed] [Google Scholar]
- Eichelbaum M., Mikus G., Vogelgesang B. Pharmacokinetics of (+)-, (-)- and (+/-)-verapamil after intravenous administration. Br J Clin Pharmacol. 1984 Apr;17(4):453–458. doi: 10.1111/j.1365-2125.1984.tb02371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelbaum M., Somogyi A. Inter- and intra-subject variation in the first-pass elimination of highly cleared drugs during chronic dosing. Studies with deuterated verapamil. Eur J Clin Pharmacol. 1984;26(1):47–53. doi: 10.1007/BF00546708. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Somogyi A., von Unruh G. E., Dengler H. J. Simultaneous determination of the intravenous and oral pharmacokinetic parameters of D,L-verapamil using stable isotope-labelled verapamil. Eur J Clin Pharmacol. 1981 Jan;19(2):133–137. doi: 10.1007/BF00568400. [DOI] [PubMed] [Google Scholar]
- Freedman S. B., Richmond D. R., Ashley J. J., Kelly D. T. Verapamil kinetics in normal subjects and patients with coronary artery spasm. Clin Pharmacol Ther. 1981 Nov;30(5):644–652. doi: 10.1038/clpt.1981.216. [DOI] [PubMed] [Google Scholar]
- Kates R. E., Keefe D. L., Schwartz J., Harapat S., Kirsten E. B., Harrison D. C. Verapamil disposition kinetics in chronic atrial fibrillation. Clin Pharmacol Ther. 1981 Jul;30(1):44–51. doi: 10.1038/clpt.1981.125. [DOI] [PubMed] [Google Scholar]
- Lucas C. L., Wilcox B. R., Coulter N. A. Contrasting pulmonary blood flow profiles in children with atrial and ventricular septal defects. Cardiovasc Res. 1976 Jan;10(1):1–12. doi: 10.1093/cvr/10.1.1. [DOI] [PubMed] [Google Scholar]
- McAllister R. G., Jr, Kirsten E. B. The pharmacology of verapamil. IV. Kinetic and dynamic effects after single intravenous and oral doses. Clin Pharmacol Ther. 1982 Apr;31(4):418–426. doi: 10.1038/clpt.1982.54. [DOI] [PubMed] [Google Scholar]
- Raschack M. Relationship of antiarrhythmic to inotropic activity and antiarrhythmic qualities of the optical isomers of verapamil. Naunyn Schmiedebergs Arch Pharmacol. 1976 Sep;294(3):285–291. doi: 10.1007/BF00508397. [DOI] [PubMed] [Google Scholar]
- Rowland M. Influence of route of administration on drug availability. J Pharm Sci. 1972 Jan;61(1):70–74. doi: 10.1002/jps.2600610111. [DOI] [PubMed] [Google Scholar]
- Satoh K., Yanagisawa T., Taira N. Effects on atrioventricular conduction and blood flow of enantiomers of verapamil and of tetrodotoxin injected into the posterior and the anterior septal artery of the atrioventricular node preparation of the dog. Naunyn Schmiedebergs Arch Pharmacol. 1979 Aug;308(2):89–98. doi: 10.1007/BF00499049. [DOI] [PubMed] [Google Scholar]
- Silber B., Riegelman S. Stereospecific assay for (-)- and (+)-propranolol in human and dog plasma. J Pharmacol Exp Ther. 1980 Dec;215(3):643–648. [PubMed] [Google Scholar]
- Singh B. N., Nademanee K., Baky S. H. Calcium antagonists. Clinical use in the treatment of arrhythmias. Drugs. 1983 Feb;25(2):125–153. doi: 10.2165/00003495-198325020-00003. [DOI] [PubMed] [Google Scholar]
- Somogyi A., Albrecht M., Kliems G., Schäfer K., Eichelbaum M. Pharmacokinetics, bioavailability and ECG response of verapamil in patients with liver cirrhosis. Br J Clin Pharmacol. 1981 Jul;12(1):51–60. doi: 10.1111/j.1365-2125.1981.tb01854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi A., Eichelbaum M., Gugler R. Prediction of bioavailability for drugs with a high first-pass effect using oral clearance data. Eur J Clin Pharmacol. 1982;22(1):85–90. doi: 10.1007/BF00606430. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder B., Eichelbaum M. Determination of verapamil in human plasma by mass fragmentography using stable isotope-labelled verapamil as internal standard. Arzneimittelforschung. 1977;27(1):94–97. [PubMed] [Google Scholar]
- Von Bahr C., Hermansson J., Tawara K. Plasma levels of (+) and (-)-propranolol and 4-hydroxypropranolol after administration of racemic (+/-)-propranolol in man. Br J Clin Pharmacol. 1982 Jul;14(1):79–82. doi: 10.1111/j.1365-2125.1982.tb04937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock B. G., Schulz W., Kober G., Rietbrock N. Direct determination of hepatic extraction of verapamil in cardiac patients. Clin Pharmacol Ther. 1981 Jul;30(1):52–56. doi: 10.1038/clpt.1981.126. [DOI] [PubMed] [Google Scholar]


