Abstract
Propelled by the identification of a small family of NADPH oxidase (Nox) enzyme homologs that produce superoxide in response to cellular stimulation with various growth factors, renewed interest has been generated in characterizing the signaling effects of reactive oxygen species (ROS) in relation to insulin action. Two key observations made >30 years ago—that oxidants can facilitate or mimic insulin action and that H2O2 is generated in response to insulin stimulation of its target cells— have led to the hypothesis that ROS may serve as second messengers in the insulin action cascade. Specific molecular targets of insulin-induced ROS include enzymes whose signaling activity is modified via oxidative biochemical reactions, leading to enhanced insulin signal transduction. These positive responses to cellular ROS may seem “paradoxical” because chronic exposure to relatively high levels of ROS have also been associated with functional β-cell impairment and the chronic complications of diabetes. The best-characterized molecular targets of ROS are the protein-tyrosine phosphatases (PTPs) because these important signaling enzymes require a reduced form of a critical cysteine residue for catalytic activity. PTPs normally serve as negative regulators of insulin action via the dephosphorylation of the insulin receptor and its tyrosine-phosphorylated cellular substrates. However, ROS can rapidly oxidize the catalytic cysteine of target PTPs, effectively blocking their enzyme activity and reversing their inhibitory effect on insulin signaling. Among the cloned Nox homologs, we have recently provided evidence that Nox4 may mediate the insulin-stimulated generation of cellular ROS and is coupled to insulin action via the oxidative inhibition of PTP1B, a PTP known to be a major regulator of the insulin signaling cascade. Further characterization of the molecular components of this novel signaling cascade, including the mechanism of ROS generated by insulin and the identification of various oxidation-sensitive signaling targets in insulin-sensitive cells, may provide a novel means of facilitating insulin action in states of insulin resistance.
Cellular reactive oxygen species (ROS; superoxide and H2O2), especially when chronically raised to high levels and associated with hyperglycemia, have been widely recognized to have an important pathophysiological role in the vascular complications of diabetes as well as in the development of the disease itself (1-3). In contrast, transient bursts of small amounts of ROS are triggered in response to stimulation with a variety of growth factors, cytokines, and hormones, including insulin, making it seem “paradoxical” that localized effects of ROS can enhance the cellular responses to these ligands. The involvement of an oxidation step in the action of insulin has been suggested for decades, but only recently have potential molecular mechanisms been identified that enable researchers to test the hypotheses that insulin-induced ROS serve a second messenger function and play an important role in facilitating the insulin action cascade. Among the signaling enzymes potentially susceptible to inhibition by biochemical oxidation are those that contain reduced cysteine thiol side chains essential for their catalytic activity. These effects have been best demonstrated for the protein-tyrosine phosphatases (PTPs), but other important enzymatic signal regulators may be susceptible to oxidative inhibition, as discussed below. Furthermore, the recent cloning of a novel family of NADPH oxidase (Nox) enzymes has provided evidence that the homolog Nox4 may be a source of the rapid generation of ROS in insulin-stimulated cells. A full understanding of this signaling network may provide a novel means of facilitating insulin action in states of insulin resistance and, potentially, of differentially regulating some of the pleiotropic cellular actions of insulin.
Reversible tyrosine phosphorylation in insulin signaling. Insulin is a critical regulator of pleiotropic metabolic and mitogenic cellular responses (4). The earliest defect in type 2 diabetes is insulin resistance in peripheral tissues, which is also a common pathophysiological feature of related conditions including obesity and the “metabolic” syndrome, which are associated with increased cardiovascular risk (5). Although advances are continually being made in our understanding of insulin action, we still do not have a complete picture of how reversible tyrosine phosphorylation in the insulin signaling pathway is regulated (4,6,7).
Insulin action is initiated by binding to a specific plasma membrane receptor whose tyrosine kinase activity is essential for insulin’s growth-promoting and metabolic effects (4,6). Substrate proteins of the insulin receptor kinase, especially adapters of the insulin receptor substrate (IRS) family and Shc, are efficiently tyrosine phosphorylated (6,7). These sites act as docking scaffolds for the binding and activation of a variety of signaling and adaptor proteins, including the p85 subunit of phosphatidylinositol (PI) 3′-kinase, that are linked to the activation of downstream insulin responses, including glucose transport, p70 S6 kinase, nuclear DNA synthesis, and gene expression.
Role of PTPs in the regulation of insulin signaling. The cellular mechanisms that regulate the steady-state balance of reversible protein-tyrosine phosphorylation in the insulin signaling cascade involve the reciprocal effects of the insulin receptor kinase and the action of PTPs (6,8). In addition to serving as steady-state regulators, PTPs appear to be required for receptor deactivation because purified insulin receptors retain their autophosphorylation state after insulin is removed from the ligand binding site in vitro (9). In vivo, dissociation of insulin from the receptor is followed by its rapid dephosphorylation and by deactivation of its kinase activity (10,11). Specific PTPs, in particular PTP1B, have become important targets for therapeutic intervention in disease states associated with clinical insulin resistance (12-15).
PTPs comprise an extensive group of homologous proteins involved in a variety of signal transduction pathways (16). Receptor and nonreceptor forms of the classical tyrosine-specific PTPs have in common a ∼230 amino acid phosphatase domain that contains the tightly conserved signature catalytic motif VHCSxGxGR[T/S]G (17). The nonclassical, dual-specificity (phosphotyrosine and phosphoserine/phosphothreonine) phosphatases, e.g., mitogen-activated protein (MAP) kinase phosphatase and others (18) and PTEN (phosphatase and tensin homolog deleted on chromosome 10), which dephosphorylates the 3′-phosphate of inositol phospholipids generated by PI 3′-kinase and exhibits dual phosphatase activity in vitro (19,20), are structurally related, sharing a less tightly conserved catalytic motif that retains the essential C(x)5 R core structure (21). This sequence contains a reduced cysteine residue required for catalysis that is involved in the formation of a cysteinyl-phosphate intermediate (22,23). Modification of this catalytic cysteine by oxidation or disulfide conjugation has recently been recognized as a critically important mode of PTP regulation in vivo, including in the insulin action cascade, as discussed in detail below (24-27).
Among several candidate enzymes expressed in insulin-sensitive cells, compelling experimental evidence has best implicated the intracellular single-domain enzyme PTP1B as having a physiological role in the negative regulation of the insulin action cascade (15,28,29). PTP1B is active in vitro against the autophosphorylated insulin receptor (30,31), and it also has a relatively high specific activity toward IRS-1 compared with other candidate PTPs (32,33). Several studies have characterized the unique molecular interactions underlying the close interaction between the insulin receptor and PTP1B, which are facilitated by the presence of a second phosphotyrosine binding site in the PTP1B catalytic region that interacts with the multiple phosphotyrosine residues of the receptor kinase (34-36).
Numerous studies have demonstrated that PTP1B modulates tyrosine phosphorylation and activation of the insulin receptor itself and that it plays a key role in the regulation of early and late steps in postreceptor insulin signaling (rev. in 12,15). The most compelling data supporting a key role for PTP1B in insulin signaling come from studies in independently derived lines of PTP1B knockout mice, which exhibit no obvious disease phenotype but have enhanced glucose tolerance and heightened insulin sensitivity (37,38). Studies of insulin signaling in the knockout mice revealed enhanced insulin-stimulated phosphorylation of the insulin receptor and IRS-1 in skeletal muscle and liver but, interestingly, not in adipose tissue. The knockout mice exhibit decreased adiposity and a striking resistance to weight gain when fed a high-fat diet, with an increased basal metabolic rate (37,38), which has been attributed to enhanced leptin signaling (39,40). Although enhanced epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) receptor activation can be demonstrated in PTP1B-/- fibroblasts isolated from the knockout mice, downstream signaling is not significantly affected, suggesting that compensatory regulatory mechanisms for other growth factor pathways exist (41). PTP1B can also regulate growth hormone signaling by diminishing JAK2 phosphorylation, which may influence the regulation of adiposity under various metabolic conditions (42).
ROS as second messengers for cellular tyrosine kinase signaling. Superoxide and H2O2, collectively called “reactive oxygen species” (ROS), are now well recognized to play an integral role in several growth factor and cytokine signal transduction pathways (43-51). Superoxide [O2·-], hydroxyl [·OH] ions, and H2 O2 generated by cellular redox reactions have a complex physiology (46,52) and are ultimately converted to thioredoxin, H2O + O2 cellular catalase, glutathione by peroxidase, and/or peroxiredoxins (53-56). Relatively low levels of H2O2, generated in response to growth factor stimulation, occur in a concerted fashion with specific signaling targets in the cell, suggesting that it serves as a second messenger (44,51,57). H2O2 activates tyrosine phosphorylation cascades in cultured cells in a manner that mimics ligand-mediated signaling by PDGF and EGF (44,45,58). However, an actual second messenger function of cellular H2O2 generated transiently during stimulation of cells with growth factors has been convincingly demonstrated in pivotal experiments by Sundaresan et al. (44) and Bae et al. (57), showing that autophosphorylation of PDGF and EGF receptors, respectively, and their distal signaling effects are dependent on intracellular H2 O2 production. Numerous studies have also recently emerged supporting the hypothesis that cellular oxidant signaling is mediated by discrete localized redox circuitry, which is distinct from the notion of a generalized “oxidative stress” effect (51). For example, overexpression of the Nox homolog Nox1 increases cellular ROS and specifically activates an oxidant-sensitive reporter gene via activation of c-Jun NH2-terminal kinase (JNK) and extracellular signal-related kinase 1/2 without affecting the overall redox state of glutathione and thioredoxin, the major thiol antioxidant substances in the cell (59).
A novel regulatory paradigm: PTPs are thiol-dependent enzymes regulated by cellular ROS. In parallel with developments in the cellular physiology of H2 O2 generation, studies have also characterized the biochemical inhibition of PTPs by progressive oxidation of their catalytic cysteine thiol moiety by cellular ROS to more inert forms in vivo (Fig. 1) (24,25,27,60,61). The activity of PTP1B is dependent on the oxidation state of its cys-215 residue, which is required for catalytic activity via the formation of a phospho-enzyme intermediate (23,24,62, 63). The catalytic cysteine residue in the PTP active site is particularly sensitive to oxidation because of hydrogen bonding of neighboring side chains, which lowers the thiol pKa to ∼5.5, >3 units below that of a typical sulfhydryl (-SH) group, rendering it in an ionized state at physiological pH. Compared with other typical protein sulfhydryl side chains, the catalytic PTP thiol can be readily oxidized by locally generated H2O2, even in the presence of high cytosolic concentrations (millimolar) of the cysteine-containing tripeptide glutathione (51).
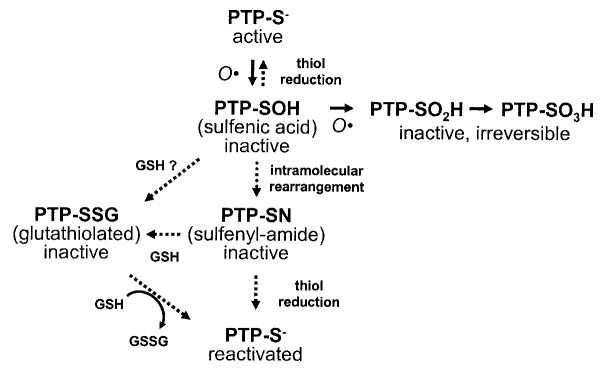
FIG. 1.
Regulation of PTP catalytic activity by oxidation, reduction, and conjugation. The catalytic cysteine residue of PTPs is especially reactive because the low pKa of the sulfhydryl favors a relatively ionized state of the cysteinyl hydrogen (51). When subjected to ROS, including those elicited by cellular insulin stimulation, the cysteine side-chain undergoes stepwise oxidation to increasingly inert forms (27,60,157). The inactive sulfenic derivative may be reduced to regenerate the active thiol form of the protein. Alternatively, it may be directly conjugated with glutathione (GSH) in the cell, producing a catalytically inert PTP derivative that can be reactivated by biochemical reduction or through the action of glutathione reductases (63). Recently, the sulfenic derivative of PTP1B has been shown to undergo an intramolecular rearrangement, forming a novel sulfenyl-amide derivative that also sequesters the PTP in an inactive state (68,69). The sulfenyl-amide form may actually be an obligate intermediate in this reaction scheme because its altered protein conformation opens a groove adjacent to the catalytic center that may render it particularly susceptible to reduction with cytosolic glutathione compared with the sulfenic derivative. GSSH, oxidized glutathione.
The catalytic cysteine thiol is initially oxidized to the sulfenic (-SOH) form, which can be reversed by cellular enzymatic mechanisms or with reducing agents in vitro (Fig. 1) (62,64). Sequential steps of progressive oxidation, to the sulfinic (-SO2H) and sulfonic (-SO H) forms can lead to irreversible PTP inactivation (65-67). 3However, the partially oxidized sulfenic acid intermediate of PTP1B can also be rapidly converted to other forms that may stabilize the molecule and protect it from further irreversible oxidation. One potentially stabilizing modification is conjugation with glutathione, which may be enzymatically reactivated in the cell by glutaredoxin (63). The catalytic cysteine of PTP1B has recently been shown to be reversibly converted to a previously unknown intramolecular sulfenyl-amide species, in which it becomes linked to the main chain nitrogen of an adjacent residue, rendering the enzyme inactive and inducing large conformational changes that inhibit substrate binding (68,69). This novel protein modification not only protects the enzyme catalytic site from irreversible oxidation to sulfonic acid but also permits redox regulation of the enzyme by promoting its reversible reduction by thiols. The conformation of the catalytic cleft assumes a more open structure in the sulfenyl-amide derivative of PTP1B and renders it particularly amenable to reduction by glutathione (68,69). This suggests that this unique protein derivative may, in fact, be an obligatory intermediate in the generation of the glutathionylated form of the oxidized enzyme.
Generation of H2O2 by cellular insulin stimulation. The potential involvement of oxidant species in insulin signaling was initially explored >30 years ago, with the observation by Czech and colleagues (70,71) and May (72) that certain metal cations interacting with albumin could transfer electrons to a cellular target and enhance glucose utilization by adipocytes. Livingston et al. (73) also showed that polyamines and related insulin mimickers acted via the generation of H2O2, and H2O2 itself stimulates lipid synthesis in adipocytes (74). Stimulation of glucose uptake in adipose cells by insulin was also found to be accompanied by sulfhydryl oxidation (75,76), and sulfhydryl blocking agents enhance insulin receptor function (77). Studies have also shown that oxidized vanadate derivatives can irreversibly inactivate PTPs and potently enhance insulin action (78 - 80).
In the late 1970s, insulin itself was shown to elicit the generation of H2O2 in adipocytes (81). An early characterization of the enzymology of this process revealed that insulin activated a plasma membrane enzyme system with the properties of an Nox, resulting in the downstream production of H2O2 (82,83). Further biochemical studies of this activity accounted for insulin-stimulated ROS production in rat adipocyte plasma membranes (84,85) also present in 3T3-L1 adipocytes (86). Nox catalyzes the reduction of oxygen to a superoxide radical: . Although a superoxide anion itself can react with thiols, it is rapidly converted spontaneously or by superoxide dismutase in the cell to generate H2O2 (51).
Closing the loop: insulin stimulation generates H2O2, which negatively regulates PTP1B. Related findings from several research approaches noted above have now been synthesized into a novel regulatory mechanism for insulin action (87). This process involves a plasma membrane oxidase activity stimulated by insulin that generates cellular ROS which, in turn, facilitate the insulin signaling cascade via the oxidative inhibition of cellular PTP activity. As discussed below, these inhibitory oxidative modifications may also affect additional cellular targets that normally serve as negative regulators of insulin action. Consistent with the previous work cited above, we reported that differentiated 3T3-L1 adipocytes that were loaded with a redox indicator dye based on dichlorodihydrofluorescein generated cellular H2O2 within 1 min of stimulation by 0.1-10 nmol/l insulin (25,26). The oxidant signal peaked at 5 min and began to dissipate by 10 min. Blocking the generation of cellular H2O2 with catalase or diphenyleneiodonium (DPI), an inhibitor of cellular Nox activity, reduced the insulin-stimulated autophosphorylation of the insulin receptor and the IRS proteins by up to 48%, suggesting that the oxidant signal inhibited cellular PTPs that serve as negative regulators of the insulin signaling cascade.
We also showed that the enhancement of insulin signaling by the oxidant signal was associated with PTP inhibition, using a novel approach that includes sample handling and analysis under anaerobic conditions to preserve the endogenous activity of PTPs isolated from cultured cells and avoiding the cysteine oxidation that occurs on exposure to air (88). In HepG2 hepatoma cells, stimulation with 100 nmol/l insulin for 5 min resulted in up to a 52% reduction in overall PTP activity in the cell homogenate, the cytosol, and the solubilized particulate fraction (25). Biochemical reduction of the enzyme samples with dithiothreitol (DTT) before PTP assay fully restored the reduced PTP activity of the insulin-treated samples, indicating that they had been reversibly oxidized and inactivated by insulin exposure. Similarly, in 3T3-L1 adipocytes, insulin caused a 62% drop in PTP activity in the cell lysate, which was restored to within control levels by treatment of samples with DTT before enzyme assay. Catalase also blocked the reduction of PTP activity in the 3T3-L1 adipocyte cell lysate induced by insulin to a level that was not significantly different from the control samples, indicating that H2O2 mediated the oxidative inhibition of cellular PTP activity (25). Insulin-stimulated generation of H2 O2 also affected the specific activity of endogenous 22PTP1B isolated from intact cells. In 3T3-L1 adipocytes, insulin treatment also potently reduced the activity of immunoprecipitated PTP1B to 12% of control, which was reversible to 72% of control by preincubation with DTT before PTP assay (25). In the continued presence of insulin, this effect was sustained for at least 10 min. Catalase pretreatment of the cells abolished the insulin-induced inhibition of PTP1B. Oxidative inactivation of PTP1B is thus associated with enhanced insulin signal transduction via the insulin-stimulated H2 O2 signal.
Blocking the insulin-stimulated cellular production of H2O2 with DPI also completely inhibited the activation of PI 3′-kinase activity by insulin and reduced the insulin-induced activation of the serine kinase Akt by up to 49% (26). The H2O2-induced activation of Akt was mediated by upstream stimulation of PI 3′-kinase activity because treatment of 3T3-L1 adipocytes with the PI 3′-kinase inhibitors wortmannin or LY294002 completely blocked the subsequent activation of Akt by exogenous H2O2. Interestingly, the effects of H2O2 on downstream insulin signaling we observed in the adipocyte model were similar to prior observations in other cell types, including fibroblasts, embryonic kidney cells, vascular smooth muscle cells, and astrocytes, where activation of Akt by H2O2 is also abrogated by inhibition of PI 3′-kinase (89-91). These important findings suggest that cellular PI 3′-kinase is a critical upstream mediator of Akt activation by oxidative molecules in a variety of signaling pathways, including insulin action.
Identification of the Nox homolog Nox4 as a potential mediator of insulin-stimulated ROS. In phagocytic cells, the prototypic Nox complex that generates copious for bacterial killing has a multisubunit structure complex and is highly regulated (92). Among its six subunits are two plasma membrane-associated proteins, the catalytic gp91phox protein (now designated as Nox2, which contains the FAD [flavin-adenine dinucleotide] and NADPH binding domains) and p22phox, which together form the flavocytochrome b588, and four regulatory cytosolic factors, p47phox, p67phox, p40phox, and rac, which form the active holoenzyme at the plasma membrane. Using the Nox2 sequence, a small family of five homologous Nox catalytic subunits has recently been cloned (rev. in 93), each encoding a predicted transmembrane protein of ∼65 kDa with functional homology domains similar to Nox2. The Nox enzymes exhibit unique patterns of cellular expression by Northern analysis: Nox1 is predominantly expressed in colon and vascular smooth muscle cells, Nox2 in phagocytes, Nox3 in fetal tissues and the inner ear, and Nox5 in spleen, sperm, mammary glands, and cerebrum.
Nox4 is prominently expressed in kidney (94-96) but was also shown by RT-PCR analysis to be widely expressed among other cell types, including liver, skeletal muscle, and osteoclasts (95,97). Using RT-PCR as well as Northern and Western blot analyses, we found that Nox4 is present in mature adipocytes and differentiated 3T3-L1 cells (98). Evidence for an integral role of Nox4 in the insulin-induced oxidant signal was obtained by adenovirus-mediated expression of Nox4 deletion constructs lacking either the NADPH binding domain or the combined FAD/NADPH domains, which acted in a dominant-negative fashion and attenuated insulin-stimulated generation of H2O2. Functionally, expression of the deletion constructs led to an inhibition of insulin receptor and IRS-1 tyrosine phosphorylation, activation of downstream serine kinases, and glucose uptake.
In parallel studies, transfection of specific small interfering RNA oligonucleotides reduced Nox4 protein abundance by up to 50% in the 3T3-L1 adipocytes and reduced insulin receptor autophosphorylation up to 64% compared with control cells transfected with scrambled small interfering RNA constructs (98). Reduced Nox4 mass was also associated with decreased insulin-stimulated Akt phosphorylation by up to 48%. Together with the wild-type and mutant Nox4 transduction studies noted above, these results suggest that Nox4 overexpression potentiates, and reduced Nox4 mass diminishes, insulin signal transduction in the 3T3-L1 cells. In related work using other cell systems, reduction of Nox4 expression with antisense DNA oligonucleotides markedly decreased the cellular generation of ROS in osteoclasts and mesangial cells (99,100). Lowering Nox4 mass also abolished the activation of Akt by angiotensin II, implying that the oxidant signal is integral to downstream signaling in the mesangial cells (100).
ROS generation by Nox4 in the adipocytes was also associated with oxidative inhibition of cellular PTP1B activity (98). Overexpression of recombinant PTP1B inhibited insulin-stimulated tyrosine phosphorylation of the insulin receptor, which was significantly reversed by co-overexpression of active Nox4. The effect of overexpression of Nox4 on receptor autophosphorylation was closely associated with inhibition of PTP1B catalytic activity, as measured in enzyme immunoprecipitates. Interestingly, PTP1B inhibition was relatively specific because it was evident with no measurable reduction in the overall PTP activity in cell lysate fractions (M.K., X.W., B.J.G., unpublished observations). These findings suggest that Nox4 provides a novel link between the insulin receptor and ROS generation that enhance insulin signal transduction, at least in part via the oxidative inhibition of cellular PTPases, including PTP1B.
Nox2 itself does not appear to be a major mediator of insulin-induced ROS production. Nox2 knockout mice exhibit a severe deficiency of H2O2 production in phagocytes and aortic fibroblasts, but not in vascular smooth muscle cells (101). We recently found that lymphoblasts from Nox2-deficient human subjects (Coriell Institute) had reduced overall H2O2 generation, but they retained a 44% increase in ROS production after insulin treatment, suggesting that Nox2 is not involved in insulin-stimulated ROS generation (X.W., B.J.G., unpublished observations).
Regulation of Nox4 signaling in the insulin action cascade. Although structurally related, the Nox homologs vary markedly in their signaling properties and modes of regulation. Nox1 has mitogenic activity and promotes growth (102), whereas Nox4 has been implicated in cellular senescence (94). In vascular smooth muscle cells, Nox1 and Nox4 show opposite regulation by angiotensinII, and Nox1 colocalizes with caveolin, whereas Nox4 colocalizes with vinculin in focal adhesions (103). Nox1, Nox2, and Nox4 are physically associated with p22phox, an essential component of the catalytic flavocytochrome complex, suggesting a potential commonality in their subunit structure (104). Several groups have very recently reported the cloning of novel Nox1-interacting proteins in nonphagocytic cells (especially colonic epithelia) that dramatically enhance superoxide production by Nox1, termed NOXO1 (Nox organizer 1, or p41) and NOXA1 (Nox activator 1, or p51), which are closely homologous to the well-characterized Nox2 regulatory proteins p47phox and p67phox (105-108). Superoxide production by Nox3 is also enhanced by interactions with regulatory subunits (109,110). Nox5 is regulated by intracellular calcium levels (111). Less is known about the mechanisms underlying the regulation of Nox4 activity. NIH 3T3 fibroblasts transfected with Nox4 exhibit constitutively increased superoxide generation (94), suggesting that Nox4 may not to depend on activation by regulatory subunits as is evident with several other homologs in the Nox family.
H2O2 generated by PDGF and EGF stimulation in various cell models via a Nox1-mediated pathway appears to require PI 3′-kinase activation (112,113). Similarly, in skin fibroblasts, Ceolotto et al. (114) found that insulin-stimulated ROS production was also dependent on PI 3′-kinase activity. In contrast, we reported that insulin-stimulated ROS generation in 3T3-L1 adipocytes, presumably via a Nox4-mediated pathway, was not sensitive to zPI 3′-kinase inhibition (26). These data suggest that Nox catalytic and regulatory components may differ in various cell types, including those responsive to insulin. For example, fibroblast cells are likely to express several Nox homologs as well as some of the regulatory components of the classical Nox2 (gp91phox) system, including p47phox, which apparently undergoes an apocynin-sensitive translocation from the cytosol to the cell membrane in response to insulin stimulation (114). A better understanding of the expression of regulatory components for the Nox system in the classical insulin target tissues and their responsiveness to insulin is clearly required.
Potential role of G-proteins in insulin-stimulated H2O2 and PTP regulation
Rac. This protein is a key component of the Nox complex in a variety of cell types. Rac2 is expressed predominantly in phagocytic cells, and Rac1 is expressed in other cell types (115). The Rac effector domain has been mapped, and a chimeric Rac1-p67phox protein increases Nox2 activity (116,117). Expression of a dominant-negative Rac (T17N) inhibits the rise in ROS seen after stimulation by growth factors or cytokines, and a constitutively active Rac (G12V) stimulates ROS formation in NIH 3T3 cells and in renal mesangial cells (100,118). Because superoxide generation in the renal cell system is likely to involve Nox4, these data also implicate Rac as a potential regulator of this Nox homolog (100).
Is Rac an effector of Nox4 in insulin-sensitive cells? Rac is expressed in adipocytes and skeletal muscle cells and is activated by insulin, albeit in a manner at least partly dependent on PI 3′-kinase activity (119). Although Rac does not couple distal insulin signaling to glucose transport (120), its strong association with Nox activity in other systems warrants its testing as a candidate regulatory protein for Nox4 in insulin target cells (Fig. 2).
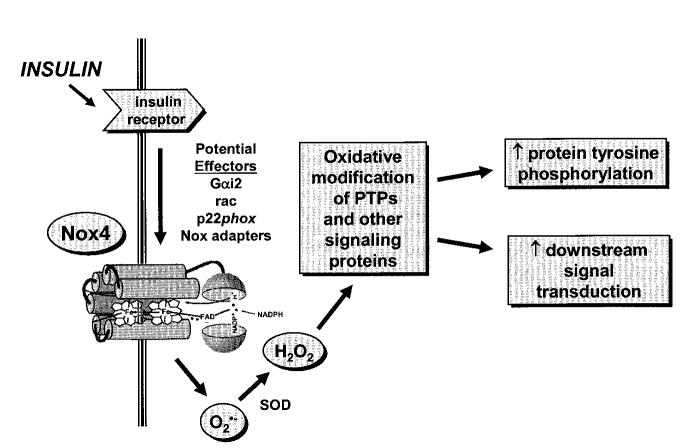
FIG. 2.
Postulated effectors of insulin-induced ROS via Nox4 and influences on downstream events in the insulin action cascade. Insulin stimulation of its target cells, especially adipocytes, elicits a burst of superoxide, with rapid generation of H2O2 catalyzed by cellular superoxide dismutase (SOD). As discussed in the text, the Nox homolog Nox4 may mediate a major part of the insulin-induced ROS generation in adipocytes (98). However, the mechanism(s) coupling Nox4 with the insulin receptor is not known. Potential effectors of the insulin activation of Nox activity may include the G-proteins rac and Gαi2, the flavocytochrome b558 subunit p22phox, or interactions with novel Nox adaptor proteins (perhaps homologous to NOXO1 [Nox organizer 1] or NOXA1 [Nox activator 1]), among other possibilities. Insulin-stimulated ROS are believed to interact with a limited set of cellular proteins that contain catalytic thiol side-chains known to be particularly susceptible to biochemical oxidation (see Table 1). Inhibition of these signaling proteins, e.g., PTPs, leads to enhanced tyrosine autophosphorylation of the receptor and its substrate proteins as well as alterations in downstream signaling in the insulin action cascade. See text for further discussion and references.
Protein complex formation has a major regulatory influence on Nox function, increasing the catalytic activity of some of the Nox homologs, playing a key role in Nox subcellular localization and possible access to substrates. In this regard, it is of interest that the guanine nucleotide exchange factor Trio, which can activate the phagocyte Nox in the absence of GDP-to-GTP exchange on Rac (121), is a widely expressed multidomain protein that also forms a complex with leukocyte antigen-related PTP (LAR) (122). LAR is a receptor-type transmembrane PTP localized to focal adhesions that has been implicated in the regulation of insulin receptor dephosphorylation and insulin resistance (29,123,124). Trio has two functional guanine nucleotide exchange factor domains, one with specificity for Rac and the other for Rho, as well as a protein serine kinase domain. Although highly speculative, it is possible that Trio, with or without the involvement of Rac, interacts with one or more Nox homologs at the plasma membrane and directs the oxidative inhibition of the catalytic PTP domain of LAR. The localization of Nox4 to focal adhesions, demonstrated at least in vascular smooth muscle cells (103), raises the possibility that these regulatory protein interactions may involve this Nox homolog. Studies of the subcellular localization of Nox4 in insulin-sensitive cell types is thus a high priority for further work in this area.
Gαi2. Interesting data from diverse sources has also linked the small G-protein Gαi2 with insulin action and potentially with insulin-stimulated Nox activity (Fig. 2). There has been a steady flow of data over the years linking insulin action particularly with a pertussis toxin-sensitive G-protein (rev. in 125,126). Krieger-Brauer et al. (127) first reported that the insulin-stimulated plasma membrane Nox was coupled to Gαi2. In an insightful recent study using human adipocyte plasma membranes and recombinant protein components in an incubation buffer lacking added MnCl2, this group demonstrated that insulin stimulation led to protein association between Gαi2 and the insulin receptor (128). Furthermore, insulin receptor autophosphorylation was stimulated by activated Gαi2 and blocked by pretreatment with pertussis toxin, consistent with an earlier study in Fao hepatoma cells (129). A recent report also linked the attenuation of platelet activation by insulin with both tyrosine phosphorylation of Gαi2 and complex formation between IRS-1 and Gαi2, but not other Gα subunits (130).
In transgenic mice, Malbon and colleagues (131-133) showed that deficiency of Gαi2 in adipose tissue and liver caused hyperinsulinemia, impaired glucose tolerance, and insulin resistance, although conditional expression of a constitutively active Gαi2 mutant in insulin-sensitive tissues mimicked insulin action, with increased GLUT4 translocation in adipose tissue and skeletal muscle and enhanced overall glucose tolerance supporting a permissive role of Gαi2 in insulin signaling in vivo. In fact, transgenic expression of the Q205L constitutively activated mutant of Gαi2 ameliorates streptozotocin-induced insulin-deficient diabetes (134). Interestingly, the mice lacking Gαi2 also exhibited increased tissue PTP activity (131), implicating a potential loss of insulin-stimulated Nox activity in the Gαi2-deficient animals, with reduced oxidative inhibition of PTPs that regulate the insulin action pathway; however, this has not been directly tested in this model system. Overall, these studies are consistent with the hypothesis that the regulation of tyrosine phosphorylation in the insulin signal cascade is propagated by a wave of H2O2, possibly generated by a link between the insulin receptor and Gαi2, coupled to cellular Nox activity, which transiently inhibits PTP activities.
Novel targets of insulin-stimulated ROS that may potentially influence insulin action. In addition to PTPs that have been implicated in the regulation of insulin signaling (e.g., PTP1B and LAR, as discussed above) (29), a number of additional cellular enzymes are potential targets of oxidative inhibition by insulin-induced ROS (Table 1). A serine-threonine phosphatase, protein phosphatase 2A, implicated in the negative regulation of Akt by dephosphorylation of ser-473, has a redox-sensitive cysteine residue that is potentially susceptible to inhibition by H2O2 (135). The dual-specificity phosphatase MAP kinase phosphatase-1, which attenuates insulin-stimulated MAP kinase activity (136), is also dependent on a reduced thiol for activity. The lipid phosphatase PTEN can modulate downstream insulin signaling (137) and is also inactivated by oxidation of essential cysteine residues in its active site, which can be reactivated by thioredoxin in the cell (138,139). Further research is needed to determine the effects of the oxidative inhibition of these important signaling regulators on proximal and distal events in the insulin action cascade.
TABLE 1.
Potential cellular targets of insulin-induced ROS susceptible to biochemical regulation by oxidative inhibition
| Acronym | Enzyme |
|---|---|
| PTP1B | Protein-tyrosine phosphatase 1B |
| PTPs | Other PTPs potentially involved in insulin action (e.g., LAR) |
| PTEN | Phosphatase and tensin homolog deleted on chromosome 10 |
| PP2A | Protein serine/threonine phosphatase 2A |
| MKP-1 | MAP kinase phosphatase-1; dual-specificity (serine/tyrosine) phosphatase |
| Dual-specificity PTPs | Other dual-specificity regulatory PTPs |
See text for discussion.
Perspective. Reversible tyrosine phosphorylation plays an essential role in the regulation of transmission of the insulin signal at receptor and postreceptor sites in the insulin action pathway. PTPs, in particular PTP1B, and other thiol-sensitive signaling proteins are integral to the negative regulation of insulin signaling. A growing body of data over the past 3 decades has led to the appreciation that cellular stimulation with insulin generates ROS that can inhibit these negative regulators by oxidative biochemical alterations that, in turn, can facilitate the insulin signaling cascade. With the recognition that a small family of Nox homologs catalyzes the generation of ROS at the plasma membrane, we have recently provided evidence in the 3T3-L1 adipocyte system that Nox4, which is expressed in insulin-sensitive cell types, is a novel molecular target that may mediate this process (Fig. 2).
Much remains to be learned about the mechanism of Nox-generated ROS in response to insulin and the ROS targets that influence intracellular insulin signaling responses. Research in this area must account for observations related to the cellular itinerary of the insulin receptor, including the localization of receptor activation and deactivation, and how receptor internalization may be involved in this process. Nox4 is localized to the plasma membrane in adipose cells (M.K., B.J.G., unpublished observations), which may facilitate its interaction with the insulin receptor during the process of insulin-stimulated ROS production. If Nox is also involved in the receptor internalization process, the ROS could access oxidation-sensitive targets at the plasma membrane and at intracellular sites.
Understanding the subcellular localization of insulin receptor dephosphorylation might provide clues as to how the oxidative regulation of PTP activity may enhance receptor signal transduction. PTP1B and LAR both interact with autophosphorylated insulin receptors (140-142). Insulin receptor activation triggers its internalization through an endosomal compartment accompanied by dynamic changes in the receptor phosphorylation and activation state (143). Biochemical evidence in liver has suggested that intracellular PTP activity in an endosomal fraction, rather than PTPs localized to the plasma membrane, are responsible for insulin receptor dephosphorylation (144); however, receptor internalization may not be obligatory because blocking internalization in 3T3-L1 adipocytes does not diminish the ability of PTP1B to dephosphorylate the cell surface receptor (145). Recent studies using novel markers for fluorescence or luminescence microscopy in HEK-293 cells have revealed that the insulin receptor is dephosphorylated within minutes after internalization—interacting most effectively with the form of PTP1B targeted to the endoplasmic reticulum, where it normally resides (146), rather than a soluble cytoplasmic form (147)—and that the receptor interaction with PTP1B occurs in a perinuclear endosomal compartment (148). Similarly, work with the EGF receptor has shown it to be under tonic phosphatase suppression at the plasma membrane, but after ligand-mediated activation and endocytosis it undergoes dephosphorylation at specific sites on the surface of the endoplasmic reticulum (149,150). Thus, the sites of interaction between PTPs and the insulin receptor may include the cell surface, where the receptor is kept inactive in a tonically dephosphorylated state, and PTP inhibition by ROS may help facilitate the initial receptor activation process. The major interaction appears to occur after internalization, when the autophosphorylated receptors undergo the bulk of dephosphorylation, at sites involving endosomes and the endoplasmic reticulum. One hypothesis is that Nox4, for example, may be internalized during the course of insulin receptor signaling and transiently inhibit PTPs in a process that facilitates the initial phases of signal transduction.
Insight into the disposition of growth factor-induced ROS has recently been gleaned from elegant studies by Reynolds et al. (151), who have modeled EGF receptor activation and signal propagation with PTP inhibition by ROS. ROS generated in response to EGF stimulation in MCF7 cells were spatially constrained to a layer below the plasma membrane, and they did not appear to occur at endosomal membranes. Using reaction constants gleaned from published experimental work, including PTP inhibition by H2O2 and related effects, a model was developed that was most consistent with a bistable activation state for PTP and receptor tyrosine kinase activity. The formation of a reaction “wavefront” is postulated that involves local cycles of EGF receptor activation, hydrogen peroxide production, and PTP inhibition, which propagates along the plasma membrane. The model proposes that signal initiation involving oxidative inhibition of PTPs adds a feedback control loop to a reaction network that responds in an amplified and switch-like manner, especially at low levels of ligand stimulus. Consistent with this model, they also showed experimentally that blocking ligand-dependent H2O2 generation with the Nox inhibitor DPI abolished the propagation of receptor phosphorylation as well as the amplification of receptor activation at low concentrations of EGF, converting the system to a “stable” steady state with a more linear phosphorylation response to ligand stimulation (151). Diminishing PTP inactivation with DPI also suppresses insulin receptor activation and several aspects of the downstream insulin signaling cascade (26,152). Thus, it would be of interest to use these types of novel imaging techniques to evaluate the role of similar regulatory networks in insulin-sensitive cells.
ROS production after ligand stimulation, including insulin, generates only a fraction of the ROS concentration observed in phagocytic cells and follows a brief time course, on the order of minutes (25). These features apparently account for the signaling role of insulin-induced ROS compared with the chronic exposure to ROS in patients with hyperglycemia that is associated with organ dysfunction and chronic complications of diabetes (1). The low levels of ROS in insulin signaling implies that there must be specific cellular protein targets that are particularly susceptible to oxidative modification. Importantly, using novel protein labeling techniques, Rhee and colleagues (153,154) have shown that even in a cellular milieu containing millimolar concentrations of slowly reactive thiols like glutathione, only a limited set of proteins are rapidly oxidized by growth factor-stimulated ROS, including PTP1B and a few other proteins with reactive cysteines, including protein disulfide isomerases, thioredoxin reductase, and creatine kinase. We have confirmed similar findings after cellular insulin stimulation of insulin target cell types (X.W., B.J.G., unpublished observations). The biochemical evidence, therefore, also supports the notion of a discrete network of “redox circuitry” (51,59,155) with temporal and spatial influences that are likely to correspond to other regulatory aspects of the insulin action pathway (156).
Further work will help define the regulatory components and mechanism of Nox activation by insulin in various insulin-sensitive cell types and the effects of insulin-stimulated ROS on the insulin action cascade, with the identification of specific cellular targets susceptible to oxidative modification by insulin-stimulated ROS. Elucidation of these processes will determine how they are involved in the normal physiology of insulin signaling, if they contribute to insulin-resistant disease states, and whether elements of this system may emerge as novel targets for pharmaceutical intervention.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grant RO1 DK43396 (to B.J.G.). K.M. is supported by a postdoctoral fellowship training grant from the National Institute of Diabetes and Digestive and Kidney Diseases.
We are grateful to Dr. David Lambeth (Emory University, Atlanta, GA) and his laboratory group for their collaboration in our studies identifying a role for Nox4 in the insulin-induced generation of ROS.
Footnotes
- DPI
- diphenyleneiodonium
- DTT
- dithiothreitol
- EGF
- epidermal growth factor
- IRS
- insulin receptor substrate
- LAR
- leukocyte antigen-related protein tyrosine phosphatase
- MAP
- mitogen-activated protein
- Nox
- NADPH oxidase
- PDGF
- platelet-derived growth factor
- PI
- phosphatidylinositol
- PTP
- protein-tyrosine phosphatase
- ROS
- reactive oxygen species.
REFERENCES
- 1.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 2.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 3.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 4.Kido Y, Nakae J, Accili D. The insulin receptor and its cellular targets. J Clin Endocrinol Metab. 2001;86:972–979. doi: 10.1210/jcem.86.3.7306. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein BJ. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol. 2002;90:3–10. doi: 10.1016/s0002-9149(02)02553-5. [DOI] [PubMed] [Google Scholar]
- 6.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 7.White MF. IRS proteins and the common path to diabetes. Am J Physiol. 2002;283:E413–E422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein BJ. Protein-tyrosine phosphatases: emerging targets for therapeutic intervention in type 2 diabetes and related states of insulin resistance. J Clin Endocrinol Metab. 2002;87:2474–2480. doi: 10.1210/jcem.87.6.8641. [DOI] [PubMed] [Google Scholar]
- 9.Haring HU, Kasuga M, White MF, Crettaz M, Kahn CR. Phosphorylation and dephosphorylation of the insulin receptor: evidence against an intrinsic phosphatase activity. Biochemistry. 1984;23:3298–3306. doi: 10.1021/bi00309a028. [DOI] [PubMed] [Google Scholar]
- 10.Mooney RA, Anderson DL. Phosphorylation of the insulin receptor in permeabilized adipocytes is coupled to a rapid dephosphorylation reaction. J Biol Chem. 1989;264:6850–6857. [PubMed] [Google Scholar]
- 11.Bernier M, Liotta AS, Kole HK, Shock DD, Roth J. Dynamic regulation of intact and C-terminal truncated insulin receptor phosphorylation in permeabilized cells. Biochemistry. 1994;33:4343–4351. doi: 10.1021/bi00180a031. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein BJ. Protein-tyrosine phosphatase 1B (PTP1B): a novel therapeutic target for type 2 diabetes mellitus, obesity and related states of insulin resistance. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:265–275. doi: 10.2174/1568008013341163. [DOI] [PubMed] [Google Scholar]
- 13.Cheng A, Dube N, Gu F, Tremblay ML. Coordinated action of protein tyrosine phosphatases in insulin signal transduction. Eur J Biochem. 2002;269:1050–1059. doi: 10.1046/j.0014-2956.2002.02756.x. [DOI] [PubMed] [Google Scholar]
- 14.Asante-Appiah E, Kennedy BP. Protein tyrosine phosphatases: the quest for negative regulators of insulin action. Am J Physiol. 2003;284:E663–E670. doi: 10.1152/ajpendo.00462.2002. [DOI] [PubMed] [Google Scholar]
- 15.Tonks NK. PTP1B: from the sidelines to the front lines. FEBS Lett. 2003;546:140–148. doi: 10.1016/s0014-5793(03)00603-3. [DOI] [PubMed] [Google Scholar]
- 16.Tonks NK, Neel BG. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr Opin Cell Biol. 2001;13:182–195. doi: 10.1016/s0955-0674(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 17.Andersen JN, Mortensen OH, Peters GH, Drake PG, Iversen LF, Olsen OH, Jansen PG, Andersen HS, Tonks NK, Moller NP. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol Cell Biol. 2001;21:7117–7136. doi: 10.1128/MCB.21.21.7117-7136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 19.Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci USA. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maehama T, Dixon JE. The tumor suppressor, pten/mmac1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 21.Zhang ZY. Protein tyrosine phosphatases: structure and function, substrate specificity, and inhibitor development. Ann Rev Pharmacol Toxicol. 2002;42:209–234. doi: 10.1146/annurev.pharmtox.42.083001.144616. [DOI] [PubMed] [Google Scholar]
- 22.Denu JM, Dixon JE. Protein tyrosine phosphatases: mechanisms of catalysis and regulation. Curr Opin Chem Biol. 1998;2:633–641. doi: 10.1016/s1367-5931(98)80095-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhang ZY. Mechanistic studies on protein tyrosine phosphatases. Prog Nucleic Acid Res Mol Biol. 2003;73:171–220. doi: 10.1016/s0079-6603(03)01006-7. [DOI] [PubMed] [Google Scholar]
- 24.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1b in a431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 25.Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1B in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 26.Mahadev K, Wu X, Zilbering A, Zhu L, Lawrence JTR, Goldstein BJ. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J Biol Chem. 2001;276:48662–48669. doi: 10.1074/jbc.M105061200. [DOI] [PubMed] [Google Scholar]
- 27.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Molecular Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 28.Elchebly M, Cheng A, Tremblay ML. Modulation of insulin signaling by protein tyrosine phosphatases. J Mol Med. 2000;78:473–482. doi: 10.1007/s001090000141. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein BJ. Protein-tyrosine phosphatases and the regulation of insulin action. In: LeRoith D, Taylor SI, Olefsky JM, editors. Diabetes Mellitus: A Fundamental and Clinical Text. 3rd ed. Philadelphia, PA; Lippincott: 2003. pp. 255–268. [Google Scholar]
- 30.Hashimoto N, Zhang WR, Goldstein BJ. Insulin receptor and epidermal growth factor receptor dephosphorylation by three major rat liver protein-tyrosine phosphatases expressed in a recombinant bacterial system. Biochem J. 1992;284:569–576. doi: 10.1042/bj2840569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walchli S, Curchod ML, Gobert RP, Arkinstall S, van Huijsduijnen RH. Identification of tyrosine phosphatases that dephosphorylate the insulin receptor: a brute force approach based on “substrate-trapping” mutants. J Biol Chem. 2000;275:9792–9796. doi: 10.1074/jbc.275.13.9792. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein BJ, Bittner-Kowalczyk A, White MF, Harbeck M. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B: possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J Biol Chem. 2000;275:4283–4289. doi: 10.1074/jbc.275.6.4283. [DOI] [PubMed] [Google Scholar]
- 33.Calera MR, Vallega G, Pilch PF. Dynamics of protein-tyrosine phosphatases in rat adipocytes. J Biol Chem. 2000;275:6308–6312. doi: 10.1074/jbc.275.9.6308. [DOI] [PubMed] [Google Scholar]
- 34.Puius YA, Zhao Y, Sullivan M, Lawrence DS, Almo SC, Zhang ZY. Identification of a second aryl phosphate-binding site in protein-tyrosine phosphatase 1b: a paradigm for inhibitor design. Proc Natl Acad Sci U S A. 1997;94:13420–13425. doi: 10.1073/pnas.94.25.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salmeen A, Andersen JN, Myers MP, Tonks NK, Barford D. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Mol Cell. 2000;6:1401–1412. doi: 10.1016/s1097-2765(00)00137-4. [DOI] [PubMed] [Google Scholar]
- 36.Dadke S, Chernoff J. Interaction of protein tyrosine phosphatase (PTP) 1B with its substrates is influenced by two distinct binding domains. Biochem J. 2002;364:377–383. doi: 10.1042/BJ20011372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 38.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, Mcglade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 40.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 41.Haj FG, Markova B, Klaman LD, Bohmer FD, Neel BG. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B. J Biol Chem. 2003;278:739–744. doi: 10.1074/jbc.M210194200. [DOI] [PubMed] [Google Scholar]
- 42.Gu F, Dube N, Kim JW, Cheng A, Ibarra-Sanchez Mde J, Tremblay ML, Boisclair YR. Protein tyrosine phosphatase 1B attenuates growth hormone-mediated JAK2-STAT signaling. Mol Cell Biol. 2003;23:3753–3762. doi: 10.1128/MCB.23.11.3753-3762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan SG, Chiu DT, Errasfa M, Wang JM, Qi JS, Stern A. Effects of H2O2 on protein tyrosine phosphatase activity in HER14 cells. Free Radic Biol Med. 1994;16:399–403. doi: 10.1016/0891-5849(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 44.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 45.Rao GN. Hydrogen peroxide induces complex formation of shc-grb2-sos with receptor tyrosine kinase and activates ras and extracellular signal-regulated protein kinases group of mitogen-activated protein kinases. Oncogene. 1996;13:713–719. [PubMed] [Google Scholar]
- 46.Monteiro HP, Stern A. Redox modulation of tyrosine phosphorylation-dependent signal transduction pathways. Free Radic Biol Med. 1996;21:323–333. doi: 10.1016/0891-5849(96)00051-2. [DOI] [PubMed] [Google Scholar]
- 47.Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2: role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 49.Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–124. [PubMed] [Google Scholar]
- 50.Herrlich P, Bohmer FD. Redox regulation of signal transduction in mammalian cells. Biochem Pharm. 2000;59:35–41. doi: 10.1016/s0006-2952(99)00298-1. [DOI] [PubMed] [Google Scholar]
- 51.Rhee SG, Chang TS, Bae YS, Lee SR, Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol. 2003;14:S211–S215. doi: 10.1097/01.asn.0000077404.45564.7e. [DOI] [PubMed] [Google Scholar]
- 52.Grisham MB. Reactive Metabolites of Oxygen and Nitrogen in Biology and Medicine. RG Landes; Austin, TX: 1992. [Google Scholar]
- 53.Schroder E, Ponting CP. Evidence that peroxiredoxins are novel members of the thioredoxin fold superfamily. Protein Sci. 1998;7:2465–2468. doi: 10.1002/pro.5560071125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi HJ, Kang SW, Yang CH, Rhee SG, Ryu SE. Crystal structure of a novel human peroxidase enzyme at 2.0 A resolution. Nat Struct Biol. 1998;5:400–406. doi: 10.1038/nsb0598-400. [DOI] [PubMed] [Google Scholar]
- 55.Zhang ZY, Thiemesefler AM, Maclean D, Mcnamara DJ, Dobrusin EM, Sawyer TK, Dixon JE. Substrate specificity of the protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 1993;90:4446–4450. doi: 10.1073/pnas.90.10.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirotsu S, Abe Y, Okada K, Nagahara N, Hori H, Nishino T, Hakoshima T. Crystal structure of a multifunctional 2-Cys peroxiredoxin heme-binding protein 23 kDa/proliferation-associated gene product. Proc Natl Acad Sci U S A. 1999;96:12333–12338. doi: 10.1073/pnas.96.22.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide: role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 58.Bae GU, Seo DW, Kwon HK, Lee HY, Hong S, Lee ZW, Ha KS, Lee HW, Han JW. Hydrogen peroxide activates p70(S6k) signaling pathway. J Biol Chem. 1999;274:32596–32602. doi: 10.1074/jbc.274.46.32596. [DOI] [PubMed] [Google Scholar]
- 59.Go YM, Gipp JJ, Mulcahy RT, Jones DP. H2O2-dependent activation of GCLC-ARE4 reporter occurs by mitogen-activated protein kinase pathways without oxidation of cellular glutathione or thioredoxin-1. J Biol Chem. 2004;279:5837–5845. doi: 10.1074/jbc.M307547200. [DOI] [PubMed] [Google Scholar]
- 60.DeGnore JP, Konig S, Barrett WC, Chock PB, Fales HM. Identification of the oxidation states of the active site cysteine in a recombinant protein tyrosine phosphatase by electrospray mass spectrometry using on-line desalting. Rapid Commun Mass Spectrom. 1998;12:1457–1462. doi: 10.1002/(SICI)1097-0231(19981030)12:20<1457::AID-RCM346>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 61.Meng TC, Buckley DA, Galic S, Tiganis T, Tonks NK. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J Biol Chem. 2004;279:37716–37725. doi: 10.1074/jbc.M404606200. [DOI] [PubMed] [Google Scholar]
- 62.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 63.Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 64.Claiborne A, Yeh JI, Mallett TC, Luba J, Crane EJ, Charrier V, Parsonage D. Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry. 1999;38:15407–15416. doi: 10.1021/bi992025k. [DOI] [PubMed] [Google Scholar]
- 65.Claiborne A, Miller H, Parsonage D, Ross RP. Protein-sulfenic acid stabilization and function in enzyme catalysis and gene regulation. FASEB J. 1993;7:1483–1490. doi: 10.1096/fasebj.7.15.8262333. [DOI] [PubMed] [Google Scholar]
- 66.Skorey K, Ly HD, Kelly J, Hammond M, Ramachandran C, Huang Z, Gresser MJ, Wang QP. How does alendronate inhibit protein-tyrosine phosphatases. J Biol Chem. 1997;272:22472–22480. doi: 10.1074/jbc.272.36.22472. [DOI] [PubMed] [Google Scholar]
- 67.Barrett WC, DeGnore JP, Keng YF, Zhang ZY, Yim MB, Chock PB. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J Biol Chem. 1999;274:34543–34546. doi: 10.1074/jbc.274.49.34543. [DOI] [PubMed] [Google Scholar]
- 68.van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 69.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 70.Czech MP, Fain JN. Cu++-dependent thiol stimulation of glucose metabolism in white fat cells. J Biol Chem. 1972;247:6218–6223. [PubMed] [Google Scholar]
- 71.Czech MP, Lawrence JC, Jr, Lynn WS. Evidence for electron transfer reactions involved in the Cu2+-dependent thiol activation of fat cell glucose utilization. J Biol Chem. 1974;249:1001–1006. [PubMed] [Google Scholar]
- 72.May JM. The insulin-like effects of low molecular weight thiols: role of trace metal contamination of commercial thiols. Horm Metab Res. 1980;12:587–590. doi: 10.1055/s-2007-999206. [DOI] [PubMed] [Google Scholar]
- 73.Livingston JN, Gurny PA, Lockwood DH. Insulin-like effects of polyamines in fat cells: mediation by H2O2 formation. J Biol Chem. 1977;252:560–562. [PubMed] [Google Scholar]
- 74.May JM, de Haen C. The insulin-like effect of hydrogen peroxide on pathways of lipid synthesis in rat adipocytes. J Biol Chem. 1979;254:9017–9021. [PubMed] [Google Scholar]
- 75.Czech MP, Lawrence JC, Jr, Lynn WS. Evidence for the involvement of sulfhydryl oxidation in the regulation of fat cell hexose transport by insulin. Proc Natl Acad Sci U S A. 1974;71:4173–4177. doi: 10.1073/pnas.71.10.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mukherjee SP, Lynn WS. Role of cellular redox state and glutathione in adenylate cyclase activity in rat adipocytes. Biochim Biophys Acta. 1979;568:224–233. doi: 10.1016/0005-2744(79)90289-4. [DOI] [PubMed] [Google Scholar]
- 77.Li CH, Moule ML, Yip CC. Insulin receptors prepared with iodoacetamide show enhanced autophosphorylation and receptor kinase activity. J Biol Chem. 1991;266:7051–7057. [PubMed] [Google Scholar]
- 78.Heffetz D, Bushkin I, Dror R, Zick Y. The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. J Biol Chem. 1990;265:2896–2902. [PubMed] [Google Scholar]
- 79.Bevan AP, Drake PG, Yale JF, Shaver A, Posner BI. Peroxovanadium compounds: biological actions and mechanism of insulin-mimesis. Mol Cell Biochem. 1995;153:49–58. doi: 10.1007/BF01075918. [DOI] [PubMed] [Google Scholar]
- 80.Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser MJ, Ramachandran C. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J Biol Chem. 1997;272:843–851. doi: 10.1074/jbc.272.2.843. [DOI] [PubMed] [Google Scholar]
- 81.May JM, de Haen C. Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. J Biol Chem. 1979;254:2214–2220. [PubMed] [Google Scholar]
- 82.Mukherjee SP, Lynn WS. Reduced nicotinamide adenine dinucleotide phosphate oxidase in adipocyte plasma membrane and its activation by insulin: possible role in the hormone’s effects on adenylate cyclase and the hexose monophosphate shunt. Arch Biochem Biophys. 1977;184:69–76. doi: 10.1016/0003-9861(77)90327-7. [DOI] [PubMed] [Google Scholar]
- 83.Mukherjee SP, Lane RH, Lynn WS. Endogenous hydrogen peroxide and peroxidative metabolism in adipocytes in response to insulin and sulfhydryl reagents. Biochem Pharmacol. 1978;27:2589–2594. doi: 10.1016/0006-2952(78)90332-5. [DOI] [PubMed] [Google Scholar]
- 84.Krieger-Brauer HI, Kather H. Human fat cells possess a plasma membrane-bound H2O2-generating system that is activated by insulin via a mechanism bypassing the receptor kinase. J Clin Invest. 1992;89:1006–1013. doi: 10.1172/JCI115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krieger-Brauer HI, Kather H. The stimulus-sensitive H2O2-generating system present in human fat-cell plasma membranes is multireceptor-linked and under antagonistic control by hormones and cytokines. Biochem J. 1995;307:543–548. doi: 10.1042/bj3070543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krieger-Brauer HI, Kather H. Antagonistic effects of different members of the fibroblast and platelet-derived growth factor families on adipose conversion and NADPH-dependent H2O2 generation in 3T3 L1-cells. Biochem J. 1995;307:549–556. doi: 10.1042/bj3070549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goldstein BJ, Mahadev K, Wu X, Zhu L, Motoshima H.Role of insulin-induced reactive oxygen species in the insulin signaling pathway Antioxid Redox SignalIn press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu L, Zilbering A, Wu X, Mahadev K, Joseph JI, Jabbour S, Deeb W, Goldstein BJ. Use of an anaerobic environment to preserve the endogenous activity of protein-tyrosine phosphatases isolated from intact cells. FASEB J. 2001;15:1637–1639. doi: 10.1096/fj.00-0795fje. [DOI] [PubMed] [Google Scholar]
- 89.Shaw M, Cohen P, Alessi DR. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J. 1998;336:241–246. doi: 10.1042/bj3360241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ushio-Fukai M, Alexander RW, Akers M, Yin QQ, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- 91.Salsman S, Felts N, Pye QN, Floyd RA, Hensley K. Induction of Akt phosphorylation in rat primary astrocytes by H2O2 occurs upstream of phosphatidylinositol 3-kinase: no evidence for oxidative inhibition of PTEN. Arch Biochem Biophys. 2001;386:275–280. doi: 10.1006/abbi.2000.2202. [DOI] [PubMed] [Google Scholar]
- 92.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 93.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 94.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng G, Cao Z, Xu X, Van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 96.Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 97.Yang S, Zhang Y, Ries W, Key L. Expression of Nox4 in osteoclasts. J Cell Biochem. 2004;92:238–248. doi: 10.1002/jcb.20048. [DOI] [PubMed] [Google Scholar]
- 98.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang S, Madyastha P, Bingel S, Ries W, Key L. A new superoxide-generating oxidase in murine osteoclasts. J Biol Chem. 2001;276:5452–5458. doi: 10.1074/jbc.M001004200. [DOI] [PubMed] [Google Scholar]
- 100.Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol. 2003;285:F219–F229. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 101.Chamseddine AH, Miller FJ., Jr gp91phox contributes to NADPH oxidase activity in aortic fibroblasts, but not smooth muscle cells. Am J Physiol. 2003;285:H2284–H2289. doi: 10.1152/ajpheart.00459.2003. [DOI] [PubMed] [Google Scholar]
- 102.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 103.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 104.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 105.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 106.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 107.Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 108.Cheng G, Lambeth JD. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem. 2004;279:4737–4742. doi: 10.1074/jbc.M305968200. [DOI] [PubMed] [Google Scholar]
- 109.Cheng G, Ritsick D, Lambeth JD. Nox3 regulation by NOXO1, p47phox, and p67phox. J Biol Chem. 2004;279:34250–34255. doi: 10.1074/jbc.M400660200. [DOI] [PubMed] [Google Scholar]
- 110.Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 111.Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Krause KH, Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J Biol Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 112.Bae YS, Sung JY, Kim OS, Kim YJ, Hur KC, Kazlauskas A, Rhee SG. Platelet-derived growth factor-induced H(2)O(2) production requires the activation of phosphatidylinositol 3-kinase. J Biol Chem. 2000;275:10527–10531. doi: 10.1074/jbc.275.14.10527. [DOI] [PubMed] [Google Scholar]
- 113.Park HS, Lee SH, Park D, Lee JS, Ryu SH, Lee WJ, Rhee SG, Bae YS. Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol Cell Biol. 2004;24:4384–4394. doi: 10.1128/MCB.24.10.4384-4394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ceolotto G, Bevilacqua M, Papparella I, Baritono E, Franco L, Corvaja C, Mazzoni M, Semplicini A, Avogaro A. Insulin generates free radicals by an NAD(P)H, phosphatidylinositol 3′-kinase-dependent mechanism in human skin fibroblasts ex vivo. Diabetes. 2004;53:1344–1351. doi: 10.2337/diabetes.53.5.1344. [DOI] [PubMed] [Google Scholar]
- 115.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 116.Toporik A, Gorzalczany Y, Hirshberg M, Pick E, Lotan O. Mutational analysis of novel effector domains in Rac1 involved in the activation of nicotinamide adenine dinucleotide phosphate (reduced) oxidase. Biochemistry. 1998;37:7147–7156. doi: 10.1021/bi9800404. [DOI] [PubMed] [Google Scholar]
- 117.Alloul N, Gorzalczany Y, Itan M, Sigal N, Pick E. Activation of the superoxide-generating NADPH oxidase by chimeric proteins consisting of segments of the cytosolic component p67(phox) and the small GTPase Rac1. Biochemistry. 2001;40:14557–14566. doi: 10.1021/bi0117347. [DOI] [PubMed] [Google Scholar]
- 118.Sundaresan M, Yu ZX, Ferrans VJ, Sulciner DJ, Gutkind JS, Irani K, Goldschmidt-Clermont PJ, Finkel T. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem J. 1996;318:379–382. doi: 10.1042/bj3180379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.JeBailey L, Rudich A, Huang X, Ciano-Oliveira C, Kapus A, Klip A. Skeletal muscle cells and adipocytes differ in their reliance on TC10 and Rac for insulin-induced actin remodeling. Mol Endocrinol. 2004;18:359–372. doi: 10.1210/me.2003-0294. [DOI] [PubMed] [Google Scholar]
- 120.Marcusohn J, Isakoff SJ, Rose E, Symons M, Skolnik EY. The GTP-binding protein Rac does not couple PI 3-kinase to insulin-stimulated glucose transport in adipocytes. Curr Biol. 1995;5:1296–1302. doi: 10.1016/s0960-9822(95)00256-9. [DOI] [PubMed] [Google Scholar]
- 121.Sigal N, Gorzalczany Y, Sarfstein R, Weinbaum C, Zheng Y, Pick E. The guanine nucleotide exchange factor trio activates the phagocyte NADPH oxidase in the absence of GDP to GTP exchange on Rac. “The emperor’s new clothes.”. J Biol Chem. 2003;278:4854–4861. doi: 10.1074/jbc.M211011200. [DOI] [PubMed] [Google Scholar]
- 122.Debant A, Serra-Pages C, Seipel K, O’Brien S, Tang M, Park SH, Streuli M. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci U S A. 1996;93:5466–5471. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zabolotny JM, Kim YB, Peroni OD, Kim JK, Pani MA, Boss O, Klaman LD, Kamatkar S, Shulman GI, Kahn BB, Neel BG. Overexpression of the LAR (leukocyte antigen-related) protein-tyrosine phosphatase in muscle causes insulin resistance. Proc Natl Acad Sci U S A. 2001;98:5187–5192. doi: 10.1073/pnas.071050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mooney RA, LeVea CM. The leukocyte common antigen-related protein LAR: candidate PTP for inhibitory targeting. Curr Top Med Chem. 2003;3:809–819. doi: 10.2174/1568026033452294. [DOI] [PubMed] [Google Scholar]
- 125.Waters C, Pyne S, Pyne NJ. The role of G-protein coupled receptors and associated proteins in receptor tyrosine kinase signal transduction. Semin Cell Dev Biol. 2004;15:309–323. doi: 10.1016/j.semcdb.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 126.Malbon CC. Insulin signalling: putting the ‘G-’ in protein-protein interactions. Biochem J. 2004;380:e11–e12. doi: 10.1042/BJ20040619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Krieger-Brauer HI, Medda PK, Kather H. Insulin-induced activation of NADPH-dependent H2O2 generation in human adipocyte plasma membranes is mediated by Galphai2. J Biol Chem. 1997;272:10135–10143. doi: 10.1074/jbc.272.15.10135. [DOI] [PubMed] [Google Scholar]
- 128.Kreuzer J, Nurnberg B, Krieger-Brauer HI. Ligand-dependent autophosphorylation of the insulin receptor is positively regulated by Gi-proteins. Biochem J. 2004;380:831–836. doi: 10.1042/BJ20031659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Muller-Wieland D, White MF, Behnke B, Gebhardt A, Neumann S, Krone W, Kahn CR. Pertussis toxin inhibits autophosphorylation and activation of the insulin receptor kinase. Biochem Biophys Res Commun. 1991;181:1479–1485. doi: 10.1016/0006-291x(91)92106-t. [DOI] [PubMed] [Google Scholar]
- 130.Ferreira IA, Eybrechts KL, Mocking AI, Kroner C, Akkerman JW. IRS-1 mediates inhibition of Ca2+ mobilization by insulin via the inhibitory G-protein Gi. J Biol Chem. 2004;279:3254–3264. doi: 10.1074/jbc.M305474200. [DOI] [PubMed] [Google Scholar]
- 131.Moxham CM, Malbon CC. Insulin action impaired by deficiency of the G-protein subunit G ialpha2. Nature. 1996;379:840–844. doi: 10.1038/379840a0. [DOI] [PubMed] [Google Scholar]
- 132.Chen JF, Guo JH, Moxham CM, Wang HY, Malbon CC. Conditional, tissue-specific expression of Q205L G alpha i2 in vivo mimics insulin action. J Mol Med. 1997;75:283–289. doi: 10.1007/s001090050113. [DOI] [PubMed] [Google Scholar]
- 133.Song X, Zheng X, Malbon CC, Wang HH. Galpha i2 enhances in vivo activation of and insulin signaling to GLUT4. J Biol Chem. 2001;276:34651–34658. doi: 10.1074/jbc.M105894200. [DOI] [PubMed] [Google Scholar]
- 134.Zheng XL, Guo J, Wang H, Malbon CC. Expression of constitutively activated Gialpha2 in vivo ameliorates streptozotocin-induced diabetes. J Biol Chem. 1998;273:23649–23651. doi: 10.1074/jbc.273.37.23649. [DOI] [PubMed] [Google Scholar]
- 135.Guy GR, Cairns J, Ng SB, Tan YH. Inactivation of a redox-sensitive protein phosphatase during the early events of tumor necrosis factor/interleukin-1 signal transduction. J Biol Chem. 1993;268:2141–2148. [PubMed] [Google Scholar]
- 136.Kusari AB, Byon J, Bandyopadhyay D, Kenner KA, Kusari J. Insulin-induced mitogen-activated protein (MAP) kinase phosphatase-1 (MKP-1) attenuates insulin-stimulated MAP kinase activity: a mechanism for the feedback inhibition of insulin signaling. Mol Endocrinol. 1997;11:1532–1543. doi: 10.1210/mend.11.10.9998. [DOI] [PubMed] [Google Scholar]
- 137.Nakashima N, Sharma PM, Imamura T, Bookstein R, Olefsky JM. The tumor suppressor PTEN negatively regulates insulin signaling in 3T3-L1 adipocytes. J Biol Chem. 2000;275:12889–12895. doi: 10.1074/jbc.275.17.12889. [DOI] [PubMed] [Google Scholar]
- 138.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 139.Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Seely BL, Staubs PA, Reichart DR, Berhanu P, Milarski KL, Saltiel AR, Kusari J, Olefsky JM. Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes. 1996;45:1379–1385. doi: 10.2337/diab.45.10.1379. [DOI] [PubMed] [Google Scholar]
- 141.Bandyopadhyay D, Kusari A, Kenner KA, Liu F, Chernoff J, Gustafson TA, Kusari J. Protein-tyrosine phosphatase 1B complexes with the insulin receptor in vivo and is tyrosine-phosphorylated in the presence of insulin. J Biol Chem. 1997;272:1639–1645. doi: 10.1074/jbc.272.3.1639. [DOI] [PubMed] [Google Scholar]
- 142.Mooney RA, Kulas DT, Bleyle LA, Novak JS. The protein tyrosine phosphatase LAR has a major impact on insulin receptor dephosphorylation. Biochem Biophys Res Commun. 1997;235:709–712. doi: 10.1006/bbrc.1997.6889. [DOI] [PubMed] [Google Scholar]
- 143.Posner BI. Regulation of insulin receptor kinase activity by endosomal processes: possible areas for therapeutic intervention. Curr Opin Investig Drugs. 2003;4:430–434. [PubMed] [Google Scholar]
- 144.Drake PG, Bevan AP, Burgess JW, Bergeron JJM, Posner BI. A role for tyrosine phosphorylation in both activation and inhibition of the insulin receptor tyrosine kinase in vivo. Clin Exper Pharmacol Physiol. 1996;137:4960–4968. doi: 10.1210/endo.137.11.8895369. [DOI] [PubMed] [Google Scholar]
- 145.Shi K, Egawa K, Maegawa H, Nakamura T, Ugi S, Nishio Y, Kashiwagi A. Protein-tyrosine phosphatase 1B associates with insulin receptor and negatively regulates insulin signaling without receptor internalization. J Biochem (Tokyo) 2004;136:89–96. doi: 10.1093/jb/mvh094. [DOI] [PubMed] [Google Scholar]
- 146.Frangioni JV, Beahm PH, Shifrin V, Jost CA, Neel BG. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992;68:545–560. doi: 10.1016/0092-8674(92)90190-n. [DOI] [PubMed] [Google Scholar]
- 147.Boute N, Boubekeur S, Lacasa D, Issad T. Dynamics of the interaction between the insulin receptor and protein tyrosine-phosphatase 1B in living cells. EMBO Rep. 2003;4:313–319. doi: 10.1038/sj.embor.embor767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Romsicki Y, Reece M, Gauthier JY, Asante-Appiah E, Kennedy BP. PTP-1B dephosphorylation of the insulin receptor occurs in a perinuclear endosome compartment in HEK 293 cells. J Biol Chem. 2004;279:12868–12875. doi: 10.1074/jbc.M309600200. [DOI] [PubMed] [Google Scholar]
- 149.Haj FG, Verveer PJ, Squire A, Neel BG, Bastiaens PIH. Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science. 2002;295:1708–1711. doi: 10.1126/science.1067566. [DOI] [PubMed] [Google Scholar]
- 150.Offterdinger M, Georget V, Girod A, Bastiaens PI. Imaging phosphorylation dynamics of the epidermal growth factor receptor. J Biol Chem. 2004;279:36972–36981. doi: 10.1074/jbc.M405830200. [DOI] [PubMed] [Google Scholar]
- 151.Reynolds AR, Tischer C, Verveer PJ, Rocks O, Bastiaens PIH. EGFR activation coupled to inhibition of tyrosine phosphatases causes lateral signal propagation. Nat Cell Biol. 2003;5:447–453. doi: 10.1038/ncb981. [DOI] [PubMed] [Google Scholar]
- 152.Mahadev K, Wu X, Motoshima H, Goldstein BJ. Integration of multiple downstream signals determines the net effect of insulin on MAP kinase vs. PI 3′-kinase activation: potential role of insulin-stimulated H2O2. Cell Signal. 2004;16:323–331. doi: 10.1016/j.cellsig.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 153.Wu Y, Kwon KS, Rhee SG. Probing cellular protein targets of H2O2 with fluorescein-conjugated iodoacetamide and antibodies to fluorescein. FEBS Lett. 1998;440:111–115. doi: 10.1016/s0014-5793(98)01415-x. [DOI] [PubMed] [Google Scholar]
- 154.Kim JR, Yoon HW, Kwon KS, Lee SR, Rhee SG. Identification of proteins containing cysteine residues that are sensitive to oxidation by hydrogen peroxide at neutral pH. Anal Biochem. 2000;283:214–221. doi: 10.1006/abio.2000.4623. [DOI] [PubMed] [Google Scholar]
- 155.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 156.Saltiel AR, Pessin JE. Insulin signaling in microdomains of the plasma membrane. Traffic. 2003;4:711–716. doi: 10.1034/j.1600-0854.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 157.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]