Abstract
We investigated exocytosis of PC12 cells using two-photon excitation imaging and extracellular polar tracers (TEP imaging) at the basal region of PC12 cells adjacent to the glass cover slip. TEPIQ (two-photon extracellular polar-tracer imaging-based quantification) analysis revealed that most exocytosis was mediated by large dense-core vesicles (LVs) with a mean diameter of 220 nm, and that exocytosis of LVs occurred slowly with a mean latency of ∼7 s even though exocytosis was induced with large increases in cytosolic Ca2+ concentration by uncaging of a caged-Ca2+ compound. We also found that 97% of exocytic LVs remained poised at the plasma membrane, 72% maintained their fusion pores in an open conformation for more than 30 s, and 76% triggered sequential compound exocytosis of vesicles that were located deeper in the cytosol. Sequential compound exocytosis by PC12 cells was confirmed by electron microscopic investigation with photoconversion of diaminobenzidine by FM1-43 (a polar membrane tracer). Our data suggest that pre-stimulus docking of LVs to the plasma membrane does not necessarily hasten the fusion reaction, while docking and resulting stability of exocytic LVs facilitates sequential compound exocytosis, and thereby allowing mobilization of deep vesicles.
Exocytic vesicles are docked to the plasma membrane before stimulation in many secretory cells (Steyer et al. 1997; Avery et al. 2000; Tsuboi et al. 2002). This pre-stimulus docking is thought to facilitate fusion and be a preparatory step for exocytosis (Parsons et al. 1995; Neher, 1998). However, it has been noticed that when exocytosis is triggered at the resting level of cytosolic Ca2+, docked large dense-core vesicles (LVs) in secretory cells undergo considerably slower fusion (Ninomiya et al. 1997; Haller et al. 1998; Voets, 2000; Ashery et al. 2000) than synaptic vesicles in the active zone (Augustine et al. 1985; Sabatini & Regehr, 1996; Bollmann et al. 2000; Schneggenburger & Neher, 2000). Thus, the role of pre-stimulus docking of vesicles in preparations lacking the active zone is not fully understood.
To obtain new insight into the processes of exocytosis and endocytosis, we developed two-photon excitation imaging of preparations immersed in polar tracers (TEP imaging), where vesicles are labelled after the fusion reaction (Nemoto et al. 2001; Takahashi et al. 2002; Kasai et al. 2005). Such post-fusion labelling is superior to pre-fusion labelling for tracking the fates of vesicles after fusion, especially when the intercellular space is narrow, and the background fluorescence is low (Kasai et al. 2005). Post-fusion labelling shows no selection bias, and exocytosis and endocytosis can be studied in a fully quantitative manner similar to membrane capacitance measurements.
In the current study, we applied TEP imaging and TEPIQ analyses (Kasai et al. 2005) to the base of the rat pheochromocytoma line, PC12. We succeeded in estimating the diameters of exocytic vesicles as 220 nm. These large dense-core vesicles (LVs) underwent slow exocytosis even though large increases in cytosolic Ca2+ were applied by photolysis of a caged-Ca2+ compound. We found that LVs remained stably attached to the plasma membrane with an open fusion pore and frequently gave rise to sequential compound exocytosis. We confirmed these observations by electron microscopy (EM).
Methods
Cell preparations
A subclone of PC12 cells (B4) was grown in a Dulbecco's modified Eagle's medium-based culture medium in the absence of NGF (nerve growth factor) as previously described (Kishimoto et al. 2001). PC12 cells were examined in a recording chamber containing 0.1 mm glass coverslips (Matsunami-glass, Osaka, Japan). The bathing solution for the experiments (SolA) consisted of 140 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm glucose, and 10 mm Hepes-NaOH (pH 7.4) (320 mosmolar). Imaging experiments were performed at room temperature (24–25°C).
Two-photon extracellular polar-tracer (TEP) imaging
TEP imaging was performed as described previously (Kasai et al. 2005). The PC12 cells were loaded with 30 μm nitrophenyl-EGTA (NPE)-acetoxymethyl ester (AM) (Molecular Probes) and, in some experiments, with 10 μm fura-2FF-AM (TEF Laboratories, Austin, TX, USA). Using a glass pipette one or two of the following fluorescent tracers were applied locally: FM1-43 (25 μm), sulforhodamine B (SRB; 0.5 mm), or 10 kDa fluorescein dextrans (FD; 2 mm) (Molecular Probes). For washout of dyes, the recording chamber was rapidly superfused with solution lacking dye. Photolysis of NPE was induced with a mercury lamp (U-ULS100HG; Olympus) through a 360 nm band pass filter (Kasai et al. 2005). The radiation of the mercury lamp was gated with an electric shutter (IX-ESU; Olympus) with a 125 ms opening duration. The fluorescence of SRB was measured at 570–650 nm (red channel), whereas those of FM1-43, fura-2FF and FD were measured at 400–550 nm (blue channel). The laser power at the specimen was typically 10 mW, and the wavelength was 830 or 850 nm for single- or double-staining, respectively. The control voltages of the photomultipliers were set at 550 and 600 V for the red and blue channels, respectively.
The calibration constants for TEPIQ analyses, FE and FM, were obtained as described previously (Kasai et al. 2005) in the experimental conditions for PC12 cells. In the presence of only SRB, FE was 223 432 ± 4888 AU μm−2 (mean ±s.d.). In the presence of both SRB and FM1-43, FE and FM were 209 570 ± 2500 AU μm−2 and 3136 ± 504 AU μm−2, respectively. For the estimation of FM, we used β-cells, since FM1-43 fluorescence intensity of PC12 cells was about three times greater than predicted by their diameters, as in the case of the membrane capacitance measurement, possibly due to the presence of microvilli in the plasma membrane. For reference, the conversion coefficient (mC) (Kasai et al. 2005) was 0.297 ± 0.048 (5 β-cells) in the experimental conditions of this study.
Photoconversion of DAB and EM analysis
To rapidly fix the cells, the recording chamber (0.5 ml) was superfused (0.2 ml s−1) with PBS containing 2% glutaraldehyde within ∼5 s of NPE photolysis. The preparations were therefore fixed within 10 s after photolysis. Photoconversion of diaminobenzidine (DAB) by FM1-43 was performed as described previously (Henkel et al. 1996; Harata et al. 2001). In brief, cells were exposed to the fixative for 20 min and then washed first for 1 h with 100 mm glycine in PBS and then for 5 min with 100 mm ammonium chloride. After an additional brief wash with PBS, the cells were incubated for 20 min with PBS containing DAB (1 mg ml−1; Wako, Tokyo, Japan) at pH 7.9. Fluorescence excitation (100 W mercury lamp, 475 nm) was then performed for 8–10 min with the cells in the DAB solution. The cells were subsequently washed for between 1 h and overnight with 100 mm sodium cacodylate buffer (pH 7.4), exposed to 2% osmium tetroxide for 3 h, dehydrated in a series of graded ethanol solutions, and embedded in Epon (Epon 812, TAAB, UK). After incubation for 2 days at 60°C, the coverslip was removed from the Epon with hydrogen flouride, and thin sections (50–60 nm) were cut vertically relative to the surface of the coverslip and mounted on copper grids. The grids were incubated with 2% uranyl acetate for 10 min and with lead solution for 5 min and were then observed by EM (2000EX; Jeol, Tokyo, Japan).
Results
Ca2+-dependent exocytosis at the base of PC12 cells
We triggered exocytosis in PC12 cells by photolysis of the caged-Ca2+ compound NPE-AM, which releases NPE inside of the cells following degradation by intracellular esterases. The rapid increase in the intracellular free Ca2+ concentration ([Ca2+]i) caused by NPE photolysis was examined using the indicator fura-2FF. In agreement with previous findings (Nemoto et al. 2004), the [Ca2+]i was estimated to 10–25 μm in magnitude and > 10 s in duration. We performed TEP imaging with sulforhodamine B (SRB), a fluid-phase polar fluorescent tracer (Nemoto et al. 2001; Takahashi et al. 2002) that neither stains nor permeates the plasma membrane. We chose to investigate the basal region of PC12 cells adjacent to the glass coverslip (Fig. 1A), which was narrow (20–40 nm; see Fig. 7A) and gave rise to low levels of background fluorescence and noise.
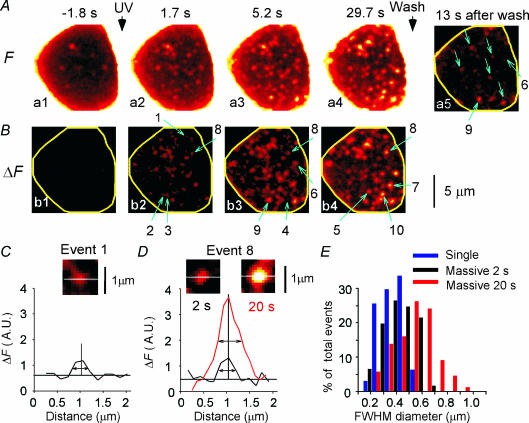
Figure 1. Two-photon imaging of exocytosis with SRB at the base of a PC12 cell.
A, sequential images of SRB fluorescence (F) obtained from the base of a cell loaded with NPE-AM and immersed in a solution containing SRB. Photolysis by UV exposure of NPE was induced at a time between frames a1 and a2. The dye was washed out 50 s after stimulation, and frame a5 was obtained 13 s after the wash. Blue arrows in a5 indicate spots left after washout. B, the difference images (ΔF) shown in frames b1 to b4 were obtained by subtracting the resting image (frame a1) in A from frames a1 to a4, respectively. Fluorescent spots often became brighter over time. Blue arrows in b2–b4 labelled 1–10 represent the events that are referred in other figures. C and D, FWHM diameters of single event 1 in B, and massive event 8 at 2 s (black) and 20 s after (red) the UV stimulation, respectively. E, distributions of FWHM diameters for single (blue), and massive events at 2 s (black) and 20 s (red) after the UV stimulation, respectively.
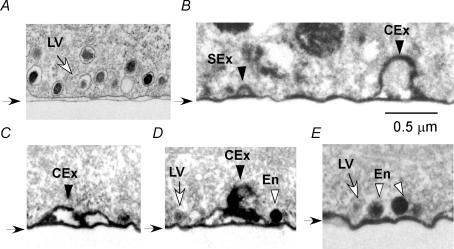
Figure 7. Ultrastructural identification of exocytic and endocytic vesicles in PC12 cells.
Images were obtained at the base of the cells perpendicular to glass coverslips (black arrows). The surface of the glass appeared irregular due to deformation by tissue processing. A, a control cell without stimulation and photoconversion. B–E, cells immersed in FM1-43 for 20 s and stimulated by photolysis of NPE 10 s after the initial exposure to the tracer and fixed 10 s after photolysis. Photoconversion of DAB was induced by FM1-43 molecules remaining after tracer washout. LV and SEx indicate a single LV before and after exocytosis, respectively. CEx represents compound exocytosis by LVs. En denotes direct endocytic vesicles. The external scale bar (0.5 μm) in B applies to all panels.
Photolysis of NPE resulted in the appearance of many discrete fluorescent spots at the base of the cell (Fig. 1B). Three types of events were distinguished from the time courses of fluorescence intensity. In 24% of the events (total of 217 events), the fluorescence intensity showed a single stepwise increase (single events; Figs 1B and 2A, event 1). The occurrence of single events may be underestimated because their signals were small. In 36% of the events, the increases occurred in a stepwise manner more than once (2–5 times; multi-step events; Figs 1B and 2A, events 2–4). The full-width-at-half-maximal (FWHM) diameters of the single events (mean = 0.31 μm, n = 35) (Fig. 1C and E) and the multi-step events (data not shown) were close to the spatial resolution (0.32 μm) of our two-photon microscope (Kasai et al. 2005), and they did not represent the actual diameters of exocytic vesicles.
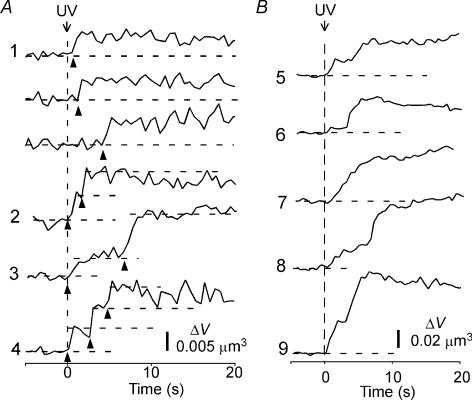
Figure 2. Analysis of single- and multi-step events.
Time courses of the fluorescence intensity of individual spots for single and multi-step events (A) and massive events (B). Traces labelled 1–9 correspond to events 1–9 in frames b2 to b4 in Fig. 3B. Arrow heads in A indicate individual exocytic events.
In the remaining 40% of the events, the fluorescence increases that occurred were not always associated with well-resolved steps (massive events; Figs 1B and 2B, events 5–10). The mean FWHM diameters of the massive events (0.57 μm, n = 42) were only 1.9-fold larger than those of the single events (Fig. 1D and E), but they were more than 10 times brighter (Figs 1D and 2B). The massive events could represent either swelling of vesicles or exocytosis of many vesicles whose individual steps were not well resolved with the 0.3- to 1.5-s time resolution of our imaging system. The following TEPIQ analyses indicated that the latter was the case.
TEPIQ analyses of exocytosis in PC12 cells
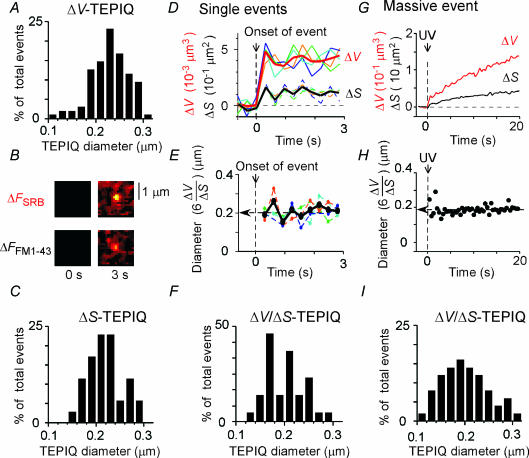
Using TEPIQ analyses, we estimated the diameters of exocytic vesicles associated with the stepwise events (Fig. 2A, arrow heads). TEPIQ analysis of ΔV predicted the diameters of individual vesicles in the single and multi-step events to be between 0.11 and 0.33 μm (0.23 ± 0.044 μm, mean ±s.d., n = 95) (Fig. 3A). This value was consistent with the diameter of PC12 cell LVs as determined by EM (see Fig. 7), supporting the idea that the stepwise events reflect the exocytosis of LVs. These findings also suggested that there was no significant binding of SRB to the granule contents.
Figure 3. TEPIQ analyses of LVs in PC12 cells.
A and C, histograms of vesicle diameter obtained by TEPIQ analyses of ΔV (A) and ΔS (C), respectively, in single events. B, multicolour TEP imaging of the exocytosis of a single LV. Shown are the background-subtracted images for SRB (ΔFSRB) and FM1-43 (ΔFFM1-43) fluorescence before and 3 s after NPE photolysis. D, time courses of ΔV and ΔS during single exocytic events simultaneously measured with SRB (continuous lines) and FM1-43 (dashed lines). Data for 6 vesicles are aligned at the onset of events. Thick lines show the mean time courses. E, time courses of vesicle diameter (6 ΔV/ΔS) for the data shown in D. F, a histogram of vesicle diameter obtained by TEPIQ analyses of ΔV/ΔS in single events. G and H, time courses of ΔV and ΔS (G) and of vesicle diameter (6 ΔV/ΔS) (H) for a massive event. I, a histogram of vesicle diameter obtained by TEPIQ analyses of ΔV/ΔS in massive events.
When exocytosis was visualized in the FM1-43-containing solution (Fig. 3B), TEPIQ analysis of ΔS yielded a vesicle diameter of 0.22 ± 0.043 μm (n = 36) for stepwise events (Fig. 3C), where ΔS is surface area. The fact that the vesicle diameter determined by TEPIQ analysis of ΔS was not significantly different from that determined by TEPIQ analysis of ΔV or from EM studies indicates the absence of staining of the granule matrix by FM1-43 (Angleson et al. 1999) in PC-12 cells. TEPIQ analysis of ΔV/ΔS yielded a similar estimate of the vesicle diameter (0.21 ± 0.059 μm, n = 39) (Fig. 3D–F), indicating that the vesicles were nearly spherical.
We next estimated the average diameters of vesicles involved in the massive events using ΔV/ΔS-TEPIQ analysis (Fig. 3G–I), in order to distinguish whether the massive events were due to many LVs or to swelling of vesicles. This method allows estimation of the diameter even though individual events are not resolved (Kasai et al. 2005). The ΔV/ΔS-TEPIQ diameter should be increased when vesicles swell, since swelling would cause larger influx of SRB in the vesicles relative to that of FM1-43 into the vesicle membranes. We found that the average diameter (6ΔV/ΔS) was 0.20 ± 0.049 μm (n = 50) (Fig. 3H and I) 10 s after the onset of exocytosis. The diameter was constant in 50% of events (Fig. 3H), although a slight increase (6–16%) or decrease (6–17%) was detected in 33% and 17% of events, respectively. This indicates that the massive events were primarily mediated by many vesicles with the diameter of LVs, but not by swelling of vesicles. By dividing the maximal volume of such spots by that of a single LV (0.005 μm3), TEPIQ analyses predicted the number of LVs involved in the multi-step and massive events to be 2–51 (12 ± 10, n = 89) (see Fig. 5B). When ΔV/ΔS-TEPIQ analysis was applied to a large region of interest at the base of the cells, it yielded a mean vesicle diameter of 0.19 ± 0.043 μm (8 cells; data not shown), indicating that vesicles at the base of cells were mostly LVs.
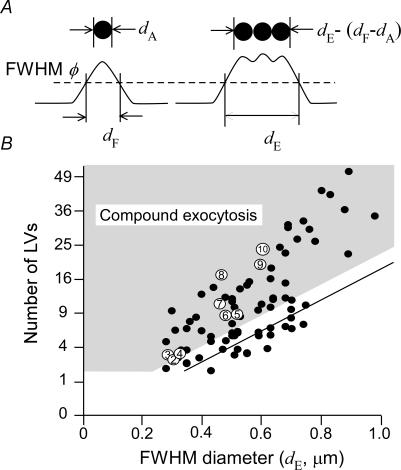
Figure 5. Relationship between numbers of LVs and FWHM diameters in multi-step and massive exocytic events in PC12 cells.
A, relationship between actual diameters of vesicles (upper panels) and their FWHM diameters of fluorescence profiles (lower panels). B, the number of LVs involved in each exocytic event plotted against its FWHM diameter, which was estimated by dividing the volume of massive events by that of single events (0.005 μm3). The straight line denotes the maximal number of LVs that can be explained by surface vesicles [(dE−dF+dA)/dA]2. The shaded area represents the events that cannot be accounted for by surface exocytosis at the plasma membrane, taking into account an error of 0.1 μm in the estimates of FWHM diameters. Open circles with a number denote data obtained from the events shown in Fig. 1B.
Stability of exocytic LVs in PC12 cells
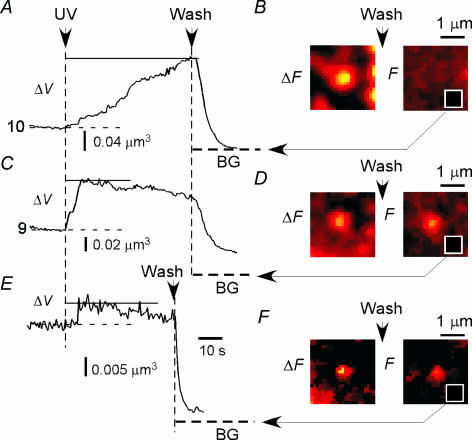
In striking contrast with insulin granules (Takahashi et al. 2002; Kasai et al. 2005), most exocytic events in PC12 cells (97%, n = 217) stayed at the site of appearance for more than 30 s after exocytosis in PC12 cells (Figs 1B and 2). Thus, full fusion events were rare in LV exocytosis (3% of the total) and thus were suppressed in PC12 cells. Moreover, fusion pores kept open for more than 30 s in 72% of vesicles because SRB fluorescence could be washed out (Figs 1A, and 4A and B). This was not because LV exocytosis was studied at the base of the cells on the glass surface, since we also have observed the same phenomenon at the lateral membrane (Fig. 2A and D of Liu et al. 2005). In 28% of the events, however, fluorescence remained after washout of SRB (Figs 1Aa5 (arrows) and 4C–F), indicating closure of fusion pores, which is often referred to as ‘kiss-and-run’ (Fesce et al. 1994) or direct endocytosis (Ceccarelli et al. 1973). The closure of the fusion pore was further supported by the fact that the exocytic vesicles that underwent direct endocytosis reduced their fluorescence (10–90%; mean = 23%, n = 49) even in the presence of SRB (Fig. 4C and E). This was probably due to bleaching of SRB in the endocytic vesicles. In fact, there was little reduction in the fluorescence for events where fusion pores were open (Fig. 1B). Direct endocytosis appeared to occur more frequently in single events (55%) than in multi-step or massive events (16%).
Figure 4. Behaviours of fusion pores of LVs in PC12 cells.
A, C and E, time courses of fluorescence intensity of massive (A and C) and single (E) events before and after washout of SRB. Fluorescence was either eliminated (A) or left unwashed (C and E). These results indicate that the fusion pore was open in A but closed in C and E by the time of washout. Traces 9 and 10 correspond to respective spots in frames b3 and b4 of Fig. 3B, respectively. Dashed lines labelled BG indicate background fluorescence obtained from white squares in B, D and F after washout of SRB. B, D and F, fluorescence images for the data shown in A, C and E, respectively. Images on the left (ΔF) were obtained by subtraction of baseline images before washout of SRB. Images on the right (F) show those after the washout.
Thus, in 72% of the events, exocytic vesicles were stably maintained with open fusion pores for more than 30 s. These stable exocytic vesicles may become a target for deep vesicle exocytosis. Indeed, we found that the fluorescence intensity of 76% of the exocytic spots increased with time (Fig. 1B, spots 2–10), often in a stepwise manner (Fig. 2A, traces 2–4), suggesting the occurrence of sequential compound exocytosis of LVs. Sequential exocytosis was directly visualized in pancreatic acinar and β-cells (Nemoto et al. 2001; Takahashi et al. 2004), which have larger vesicles (diameter of 0.4–1 μm). It was not straightforward for us to use this approach to demonstrate sequential exocytosis in PC12 cells, because the diameters of the LVs were smaller (0.22 μm) than the spatial resolution of the optical microscope. The following observations, however, indicated that the multi-step and massive events mostly reflected sequential compound exocytosis.
Sequential compound exocytosis of LVs in PC12 cells
The multi-step and massive events were brighter than would be predicted from their FWHM diameters, if they were all LVs that were attached to the plasma membrane at the base of cells. Assuming that an LV has an actual diameter of dA and a FWHM diameter of dF (Fig. 5A), and that a set of vesicles in a circle at the base of a cell has an apparent FWHM diameters of dE, the number of vesicles in the circle is predicted to be [(dE−dF+dA)/dA]2 or less (Fig. 5A). Therefore, the multi-step or massive events with dE of 0.53, 0.75 and 0.97 μm can accommodate at most 4, 9 and 16 LVs at the base of the cell, respectively (the straight line in Fig. 5B), given that dA and dF are 0.22 μm and 0.31 μm, respectively. The actual number of exocytic vesicles estimated from TEPIQ analyses, however, exceeded the theoretical maxima in most (76/89 = 85%) multi-step and massive events (Fig. 5B, circles). Even if FWHM diameters were overestimated by 0.1 μm, 73% of the events would still be outside the region that can be explained by exocytosis at the base of cells (the shaded region in Fig. 5B). Many events contained 2–4 times larger numbers of LVs than the theoretical maxima, suggesting that two to four layers of vesicles were involved. These findings indicate that the multi-step and massive events predominantly reflect compound exocytosis of LVs. In fact, EM investigations have detected the prevalence of compound exocytic vesicles (see Fig. 7 and Watanabe et al. 1983).
The ΔV/ΔS-TEPIQ diameters increase by 6–16% in 33% of the massive events, suggesting that the compound vesicles slightly swelled over time. In addition, ΔV/ΔS-TEPIQ analysis may underestimate the diameter of compound vesicles in the out-of-focal plane (Fig. 8bE of Kasai et al. 2005) and that, even though ΔV/ΔS-TEPIQ diameters appeared to be constant, the actual diameter of compound vesicles will increase by 4% when a compound vesicle with a diameter of 0.5 μm is centred at 0.25 μm above the focal plane (Fig. 8E of Kasai et al. 2005). Slight swelling was consistent with electron microscopic images of compound exocytosis in which original individual Ω-profiles were not readily identifiable (see Fig. 7).
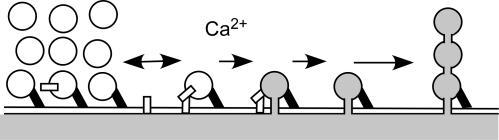
Figure 8. Pre-stimulus docking and sequential exocytosis.
Vesicles are clustered at the plasma membrane, and remained docked after fusion reaction, keeping their fusion pores open. Such primary exocytic vesicles become targets for subsequent exocytosis of LVs deep in the cytosol. Black bars denote ‘intervening strands’ connecting between docked vesicles and the plasma membrane. Open bars denote putative proteins involved in membrane fusions.
TEP imaging revealed that large endocytic vesicles were found after washout of SRB in 16% of the multi-step and massive events (Figs 1Aa5 and 4D). Such large vesicles were probably generated by compound exocytosis and by ‘en block’ endocytosis by closure of a fusion pore. Consistent with this possibility, membrane capacitance measurements in PC12 cells indicate the sudden formation of large endocytic vesicles. Specifically, a stepwise reduction of membrane area reflecting large endocytic vesicles (with a diameter of > 0.5 μm if they are spherical) is detected after massive exocytosis of LVs (Kasai et al. 1996). Thus, the formation of large compound vesicles is supported by both TEP imaging and membrane capacitance measurements.
We suspect that compound exocytosis in PC12 cells is strictly sequential, in that it does not involve vesicle-to-vesicle fusion events preceding plasma membrane fusion events (multigranular compound exocytosis). Indeed, large stepwise increases in fluorescence were infrequent in our images. Also, time-resolved capacitance measurements do not show large capacitance steps during exocytosis in PC12 cells (Kasai et al. 1996), even though they do detect large stepwise reductions in capacitance under the same experiments (Kasai et al. 1996). We therefore think that multigranular compound exocytosis is rare in PC12 cells, as in β-cells (Takahashi et al. 2004) and mast cells (Alvarez de Toledo & Fernandez, 1990). In contrast, this form of exocytosis is dominant in eosinophils (Scepek & Lindau, 1993; Hafez et al. 2003).
Time course of large dense-core vesicle exocytosis in PC12 cells
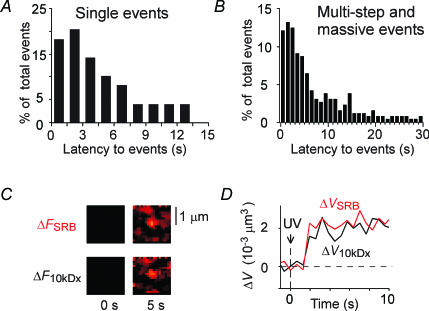
Exocytosis of LVs in single events exhibited a mean latency of 6.8 s (Fig. 6A), indicating that surface LVs undergo slow exocytosis in PC12 cells. Well-resolved LV exocytosis in multi-step (Fig. 2A, arrowheads) and massive events also showed a similar latency of about 7.4 s (Fig. 6B). The long latencies were not due to slow staining of LVs with SRB, because exocytic LVs in PC12 cells were similarly stained by 10 kDa FD (molecular diameter of ∼6 nm; n = 5; Fig. 6C and D). The fusion pore thus expanded rapidly to 6 nm, which should allow staining of PC12 LVs by SRB within 1 ms.
Figure 6. Time courses of LV exocytosis in PC12 cells.
A and B, latency histograms for exocytosis of well-resolved individual LV exocytosis for single (A) and multi-step and massive events (B). C, single exocytic events stained by both SRB and 10 kDa FD. The background-subtracted images for SRB (ΔFSRB) and 10 kDa FD (ΔF10kDax) fluorescence before and 5 s after NPE photolysis are shown. D, time course of ΔV estimated by TEPIQ analysis with SRB (ΔVSRB) and 10 kDa FD (ΔV10kDax).
Given that exocytic events of LVs were stably maintained after fusion, the time constants for exocytosis of LVs could be obtained from the increase in fluorescence. For the massive events, this value was 7.2 ± 4.6 s (n = 13). The mean time constant of all LV exocytosis based on the time course of total fluorescence increases was 6.7 s (range, 3–15 s; s.d.= 5.6 s, n = 8) at the cell base (data not shown), and 5.7 s (range, 2–10 s; s.d.= 2.6 s, n = 8) in the region facing intercellular space (Fig. 3A and 5B of Liu et al. 2005). Thus, all approaches estimated the overall time constants for LV exocytosis to be between 6 and 8 s, in agreement with the results of amperometric measurements (Ninomiya et al. 1997; Grishanin et al. 2004). We speculate that sequential compound exocytosis proceeded in the cytosol no slower than exocytosis of surface LVs, as in pancreatic acinar cells (Nemoto et al. 2001, 2004).
Ultrastructural identification of exocytic and endocytic vesicles
We examined the ultrastructural correlates of exocytosis at the base of the PC12 cells adjacent to the glass coverslip. The PC12 cell preparations were the same as used for TEP imaging. The sizes of LVs (Fig. 7A) were distributed between 0.14 and 0.29 μm (0.20 ± 0.04 μm, n = 95), in agreement with previous observations (Lowe et al. 1988; Tooze et al. 1991; Kasai et al. 1999). To label Ca2+-induced exocytic and endocytic vesicles, we immersed the cells in a FM1-43-containing solution during stimulation, fixed within 10 s after stimulation, and photoconverted the DAB using FM1-43 fluorescence as previously described (Henkel et al. 1996; Harata et al. 2001). Cells exposed to FM1-43 for only 20 s before fixation had only their plasma membrane stained, while cytosolic organelles were not stained (data not shown).
When the cells were stimulated with photolysis of NP-EGTA, many traces of LV exocytosis were apparent at the base of the cell on the glass coverslips (Fig. 7B–E). Some vesicles still contained dense matrix, probably reflecting single (Fig. 7B, SEx) or compound exocytic events of LVs (Fig. 7B–D, CEx), which were not found in unstimulated control cells (data not shown). These exocytic structures had an opening to the extracellular space, and were found with the spatial frequency of 0.3 μm−1 at the base of cells (the total length of 78 μm). In addition to exocytic LVs, we found strongly DAB-positive round vesicles (En in Fig. 7D and E) with diameters similar to those of LVs (0.20 ± 0.035 μm, n = 65), which were just attached to the plasma membrane without opening to the extracellular space. The fusion pore of these vesicles must be closed, as FM1-43 was trapped in the vesicles. These structures reflected direct or ‘kiss-and-run’ endocytosis of LVs, as predicted from TEP images (Fig. 4E and F), and were found with a spatial frequency of 0.24 μm−1. The fact that the endocytic LVs were mostly found attached to the plasma membrane confirmed the persistence of docking after direct endocytosis (Fig. 1B). A clustering of LV exocytosis as depicted in Fig. 5A (right) has not been detected, supporting the idea that the majority of the massive events reflect compound exocytosis.
The compound exocytosis did not proceed at depths more than 0.8 μm into the cytosol, and such compound vesicles could not be readily discerned without functional labelling, which labelled excess membranes (Fig. 7B–D). The prevalence and diameters (0.4–0.6 μm) of the compound exocytosis in EM images were consistent with those found in TEP imaging (Figs 1 and 5B). Although the compound vesicles were not round, original individual Ω-shapes were not detected in such compound exocytic structures, possibly due to mild swelling of the vesicles or deformation by chemical fixation and photoconversion. Similar ultrastructural features of compound exocytosis were reported in PC12 cells treated with latrotoxin (Watanabe et al. 1983). Compound vesicles were always connected with the plasma membrane as indicated by staining of membranes with FM1-43, which supports the absence of multigranular compound exocytosis of LVs in PC12 cells.
Discussion
We have applied TEPIQ analyses to LV exocytosis of PC12 cells. The diameters of LVs of ∼0.22 μm calculated with TEPIQ were consistent with the EM studies. Furthermore, we found that many LVs in PC12 cells remained attached to the plasma membrane after fusion, kept their fusion pores open, and gave rise to sequential compound exocytosis (Fig. 8).
Stable attachment of exocytic LVs in PC12 cells
Many studies have shown that LVs dock at the plasma membrane prior to a stimulus in PC12 cells (Martin & Kowalchyk, 1997; Avery et al. 2000; Tsuboi et al. 2002). Our data further indicate that LVs stay at the site of exocytosis long after fusion and maintain intact vesicle cavities (Fig. 8). The fact that direct endocytic vesicles also remain attached to the site of exocytosis further supports the existence of mechanisms that keep LVs attached to the plasma membrane even after closure of the fusion pores. Persistent attachment of vesicles was also suggested in pre-fusion labelling studies of exocytosis (Taraska et al. 2003; Perrais et al. 2004; Tsuboi et al. 2004), wherein GFP-labelled proteins stayed at the docking site long after fusion events. Quick-freeze deep-etch EM analysis of chromaffin (Nakata et al. 1990) and pituitary cells (Senda et al. 1994) has revealed fine strands connecting LVs to the plasma membrane that are sustained after the fusion reaction, and that may reflect annexins (Nakata et al. 1990). These data suggest that the pre-stimulus docking reaction can persist even after the fusion reaction (Fig. 8).
Although LVs are tightly docked to the plasma membrane, they undergo relatively slow exocytosis in PC12 cells with a time constant of ∼7 s, suggesting that pre-stimulus docking does not necessarily hasten the fusion reaction. Similarly, the time constant of fusion is about 0.2–1 s even in chromaffin cells wherein exocytosis was induced at the resting [Ca2+]i level, and was not primed by submicromolar increases in [Ca2+]i for more than 3 min (Ninomiya et al. 1997; Haller et al. 1998; Voets et al. 1999; Ashery et al. 2000). In contrast, the time constant of fusion for synaptic vesicles in the active zone is a fraction of a millisecond even without such Ca2+ priming (Llinas et al. 1981; Sabatini & Regehr, 1996; Bollmann et al. 2000; Schneggenburger & Neher, 2000). Thus, active zone structures and proteins must be utilized for submillisecond fusion reaction of docked vesicles.
Sequential exocytosis of LVs
Although we found that pre-stimulus docking does not necessarily hasten the fusion reaction, such docking has been reported in many secretory cells (Steyer et al. 1997; Martin & Kowalchyk, 1997; Ohara-Imaizumi et al. 2004). Thus, it is worth considering the physiological role of pre-stimulus docking. One possibility is that docking persists after fusion reaction, and preserves the Ω-shaped profiles of fused vesicles at the site of exocytosis, thereby facilitating sequential compound exocytosis (Fig. 8). In fact, using TEP imaging in PC12 cells, we have, for the first time, found strong evidence for sequential exocytosis. Importantly, sequential compound exocytosis can easily escape detection by other approaches such as pre-fusion labelling studies, amperometry and membrane capacitance measurements. In contrast to LVs, most small vesicles of PC12 cells did not show pre-stimulus docking (Liu et al. 2005), readily detached from the plasma membrane after exocytosis, and show no sequential exocytosis (Fig. 9 of Liu et al. 2005).
Sequential compound exocytosis is the most efficient mechanism for the mobilization of vesicles deep in the cytosol (Nemoto et al. 2001; Takahashi et al. 2004), and it is found in many other secretory cells, including exocrine pancreas cells (Ichikawa, 1965; Nemoto et al. 2001, 2004; Thorn et al. 2004; Pickett et al. 2005), β-cells (Orci et al. 1973; Takahashi et al. 2004), nasal gland cells (Oshima et al. 2005), mast cells (Anderson et al. 1973; Alvarez de Toledo & Fernandez, 1990; Guo et al. 1998), and pituitary lactotrophs (Angleson et al. 1999). In all of these examples, primary vesicles were attached to the site of exocytosis to support secondary exocytosis. Thus, we propose that pre-stimulus docking of vesicles promotes mobilization of deep vesicles via sequential compound exocytosis. In contrast to PC12 cells, sequential exocytosis is relatively infrequent in β-cells (Takahashi et al. 2004), partly because Ω-profiles of insulin granules in β-cells are unstable and ready to collapse into the plasma membrane (Takahashi et al. 2002). The suppression of sequential exocytosis may be relevant to the regulation of insulin exocytosis, which is known to depend critically on cellular energy states (Takahashi et al. 2004).
Two-photon excitation imaging has been essential to reveal new features of exocytosis even at the base of PC12 cells, because two-photon excitation enables simultaneous multicolour imaging (Schapper et al. 2003), and greatly mitigates heat generation by extracellular tracers (Kasai et al. 2005). Since an evanescent wave microscope can only visualize a thin surface layer, it can neither investigate sequential exocytosis, nor estimate diameters of vesicles. TEP imaging can be further utilized to dissect intermediate steps of exocytosis.
Acknowledgments
We thank T. Kanaseki for helpful suggestions and T. Kise, T. Suzuki and N. Takahashi for technical assistance. This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by research grants from the Human Frontier Science Program Organization, NIH and the Takeda Science Foundation.
References
- Alvarez de Toledo G, Fernandez JM. Compound versus multigranular exocytosis in peritoneal mast cells. J General Physiol. 1990;95:397–409. doi: 10.1085/jgp.95.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Slorach SA, Uvnas B. Sequential exocytosis of storage granules during antigen-induced histamine release from sensitized rat mast cells in vitro. An electron microscopic study. Acta Physiol Scand. 1973;88:359–372. doi: 10.1111/j.1748-1716.1973.tb05465.x. [DOI] [PubMed] [Google Scholar]
- Angleson JK, Cochilla AJ, Kilic G, Nussinovitch I, Betz WJ. Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytic events. Nat Neurosci. 1999;2:440–446. doi: 10.1038/8107. [DOI] [PubMed] [Google Scholar]
- Ashery U, Varoqueaux F, Voets T, Betz A, Thakur P, Koch H, Neher E, Brose N, Rettig J. Munc13-1 acts as a priming factor for large dense-core vesicles in bovine chromaffin cells. EMBO J. 2000;19:3586–3596. doi: 10.1093/emboj/19.14.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ, Charlton MP, Smith SJ. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J Physiol. 1985;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery J, Ellis DJ, Lang T, Holroyd P, Riedel D, Henderson RM, Edwardson JM, Jahn R. A cell-free system for regulated exocytosis in PC12 cells. J Cell Biol. 2000;148:317–324. doi: 10.1083/jcb.148.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B, Borst JG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP, Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973;57:499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesce R, Grohovaz F, Valtorta F, Meldolesi J. Neurotransmitter release: fusion or ‘kiss-and-run’? Trends Cell Biol. 1994;4:1–4. doi: 10.1016/0962-8924(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Grishanin RN, Kowalchyk JA, Klenchin VA, Ann K, Earles CA, Chapman ER, Gerona RR, Martin TF. CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron. 2004;43:551–562. doi: 10.1016/j.neuron.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Guo Z, Turner C, Castle D. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998;94:537–548. doi: 10.1016/s0092-8674(00)81594-9. [DOI] [PubMed] [Google Scholar]
- Hafez I, Stolpe A, Lindau M. Compound exocytosis and cumulative fusion in eosinophils. J Biol Chem. 2003;278:44921–44928. doi: 10.1074/jbc.M306013200. [DOI] [PubMed] [Google Scholar]
- Haller M, Heinemann C, Chow RH, Heidelberger R, Neher E. Comparison of secretory responses as measured by membrane capacitance and by amperometry. Biophys J. 1998;74:2100–2113. doi: 10.1016/S0006-3495(98)77917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harata N, Ryan TA, Smith SJ, Buchanan J, Tsien RW. Visualizing recycling synaptic vesicles in hippocampal neurons by FM 1-43 photoconversion. Proc Natl Acad Sci U S A. 2001;98:12748–12753. doi: 10.1073/pnas.171442798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel AW, Lubke J, Betz WJ. FM1-43 dye ultrastructural localization in and release from frog motor nerve terminals. Proc Natl Acad Sci U S A. 1996;93:1918–1923. doi: 10.1073/pnas.93.5.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa A. Fine structural changes in response to hormonal stimulation of the perfused caine pancreas. J Cell Biol. 1965;24:369–385. doi: 10.1083/jcb.24.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Hatakeyama H, Kishimoto T, Liu T-T, Nemoto T, Takahashi N. A new quantitative (two-photon extracellular polar-tracer imaging-based quantification (TEPIQ)) analysis for diameters of exocytic vesicles and its application to pancreatic islets. J Physiol. 2005;568:891–903. doi: 10.1113/jphysiol.2005.093047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Kishimoto T, Liu T-T, Miyashita Y, Podini P, Grohovaz F, Meldolesi J. Multiple and diverse forms of regulated exocytosis in wild-type and defective PC12 cells. Proc Natl Acad Sci U S A. 1999;96:945–949. doi: 10.1073/pnas.96.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Takagi H, Ninomiya Y, Kishimoto T, Ito K, Yoshida A, Yoshioka T, Miyashita Y. Two components of exocytosis and endocytosis in PC12 cells studied using caged-Ca2+ compounds. J Physiol. 1996;494:53–65. doi: 10.1113/jphysiol.1996.sp021475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Liu T-T, Ninomiya Y, Takagi H, Yoshioka T, Ellis-Davies GC, Miyashita Y, Kasai H. Ion selectivities of the Ca2+ sensors for exocytosis in rat phaeochromocytoma cells. J Physiol. 2001;533:627–637. doi: 10.1111/j.1469-7793.2001.t01-1-00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T-T, Kishimoto T, Hatakeyama H, Nemoto T, Takahashi N, Kasai H. Exocytosis and endocytosis of small vesicle in PC12 cells studied with TEPIQ (two-photon extracellular polar-tracer imaging-based quantification) analysis. J Physiol. 2005;568:917–929. doi: 10.1113/jphysiol.2005.094011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Steinberg IZ, Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981;33:323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AW, Madeddu L, Kelly RB. Endocrine secretory granules and neuronal synaptic vesicles have three integral membrane proteins in common. J Cell Biol. 1988;106:51–59. doi: 10.1083/jcb.106.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TFJ, Kowalchyk JA. Docked secretory vesicles undergo Ca2+-activated exocytosis in a cell-free system. J Biol Chem. 1997;272:14447–14453. doi: 10.1074/jbc.272.22.14447. [DOI] [PubMed] [Google Scholar]
- Nakata T, Sobue K, Hirokawa N. Conformational change and localization of calpactin I complex involved in exocytosis as revealed by quick-freeze, deep-etch electron microscopy and immunocytochemistry. J Cell Biol. 1990;110:13–25. doi: 10.1083/jcb.110.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Nemoto T, Kimura R, Ito K, Tachikawa A, Miyashita Y, Iino M, Kasai H. Sequential-replenishment mechanism of exocytosis in pancreatic acini. Nat Cell Biol. 2001;3:253–258. doi: 10.1038/35060042. [DOI] [PubMed] [Google Scholar]
- Nemoto T, Kojima T, Oshima A, Bito H, Kasai H. Stabilization of exocytosis by dynamic F-actin coating of zymogen granules in pancreatic acini. J Biol Chem. 2004;279:37544–37550. doi: 10.1074/jbc.M403976200. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Kishimoto T, Yamazawa T, Ikeda H, Miyashita Y, Kasai H. Kinetic diversity in the fusion of exocytotic vesicles. EMBO J. 1997;16:929–934. doi: 10.1093/emboj/16.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Nishiwaki C, Kikuta T, Nagai S, Nakamichi Y, Nagamatsu S. TIRF imaging of docking and fusion of single insulin granule motion in primary rat pancreatic beta-cells: different behaviour of granule motion between normal and Goto-Kakizaki diabetic rat beta-cells. Biochem J. 2004;381:13–18. doi: 10.1042/BJ20040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Malaisse-Lagae F, Ravazzola M, Amherdt M, Renold AE. Exocytosis-endocytosis coupling in the pancreatic beta cell. Science. 1973;181:561–562. doi: 10.1126/science.181.4099.561. [DOI] [PubMed] [Google Scholar]
- Oshima A, Kojima T, Dejima K, Hisa I, Kasai H, Nemoto T. Two-photon microscopic analysis of acetylcholine-induced mucus secretion in guinea pig nasal glands. Cell Calcium. 2005;37:349–357. doi: 10.1016/j.ceca.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Coorssen JR, Horstmann H, Almers W. Docked granules, the exocytotic burst, and the need for ATP hydrolysis in endocrine cells. Neuron. 1995;15:1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- Perrais D, Kleppe IC, Taraska JW, Almers W. Recapture after exocytosis causes differential retention of protein in granules of bovine chromaffin cells. J Physiol. 2004;560:413–428. doi: 10.1113/jphysiol.2004.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett JA, Thorn P, Edwardson JM. The plasma membrane Q-SNARE syntaxin 2 enters the zymogen granule membrane during exocytosis in the pancreatic acinar cell. J Biol Chem. 2005;280:1506–1511. doi: 10.1074/jbc.M411967200. [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Timing of neurotransmission at fast synapses in the mammalian brain. Nature. 1996;384:170–172. doi: 10.1038/384170a0. [DOI] [PubMed] [Google Scholar]
- Scepek S, Lindau M. Focal exocytosis by eosinophils – compound exocytosis and cumulative fusion. EMBO J. 1993;12:1811–1817. doi: 10.1002/j.1460-2075.1993.tb05829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapper F, Goncalves JT, Oheim M. Fluorescence imaging with two-photon evanescent wave excitation. Eur Biophys J. 2003;32:635–643. doi: 10.1007/s00249-003-0326-7. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Senda T, Okabe T, Matsuda M, Fujita H. Quick-freeze, deep-etch visualization of exocytosis in anterior pituitary secretory cells: localization and possible roles of actin and annexin II. Cell Tissue Res. 1994;277:51–60. doi: 10.1007/BF00303080. [DOI] [PubMed] [Google Scholar]
- Steyer JA, Horstmann H, Almers W. Transport, docking and exocytosis of single secretory granules in live chromaffin cells. Nature. 1997;388:474–478. doi: 10.1038/41329. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Hatakeyama H, Okado H, Miwa A, Kishimoto T, Kojima T, Abe T, Kasai H. Sequential exocytosis of insulin granules is associated with redistribution of SNAP25. J Cell Biol. 2004;165:255–262. doi: 10.1083/jcb.200312033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kishimoto T, Nemoto T, Kadowaki T, Kasai H. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science. 2002;297:1349–1352. doi: 10.1126/science.1073806. [DOI] [PubMed] [Google Scholar]
- Taraska JW, Perrais D, Ohara-Imaizumi M, Nagamatsu S, Almers W. Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc Natl Acad Sci U S A. 2003;100:2070–2075. doi: 10.1073/pnas.0337526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P, Fogarty KE, Parker I. Zymogen granule exocytosis is characterized by long fusion pore openings and preservation of vesicle lipid identity. Proc Natl Acad Sci U S A. 2004;101:6774–6779. doi: 10.1073/pnas.0400336101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA, Flatmark T, Tooze J, Huttner WB. Characterization of the immature secretory granule, an intermediate in granule biogenesis. J Cell Biol. 1991;115:1491–1503. doi: 10.1083/jcb.115.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, McMahon HT, Rutter GA. Mechanisms of dense core vesicle recapture following ‘kiss and run’ (‘cavicapture’) exocytosis in insulin-secreting cells. J Biol Chem. 2004;279:47115–47124. doi: 10.1074/jbc.M408179200. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, Terakawa S, Scalettar BA, Fantus C, Roder J, Jeromin A. Sweeping model of dynamin activity. Visualization of coupling between exocytosis and endocytosis under an evanescent wave microscope with green fluorescent proteins. J Biol Chem. 2002;277:15957–15961. doi: 10.1074/jbc.C200051200. [DOI] [PubMed] [Google Scholar]
- Voets T. Dissection of three Ca2+-dependent steps leading to secretion in chromaffin cells from mouse adrenal slices. Neuron. 2000;28:537–545. doi: 10.1016/s0896-6273(00)00131-8. [DOI] [PubMed] [Google Scholar]
- Voets T, Neher E, Moser T. Mechanisms underlying phasic and sustained secretion in chromaffin cells from mouse adrenal slices. Neuron. 1999;23:607–615. doi: 10.1016/s0896-6273(00)80812-0. [DOI] [PubMed] [Google Scholar]
- Watanabe O, Torda M, Meldolesi J. The effect of α-latrotoxin on the neurosecretory PC12 cell line: electron microscopy and cytotocity studies. Neuroscience. 1983;3:1011–1024. doi: 10.1016/0306-4522(83)90239-7. [DOI] [PubMed] [Google Scholar]