Abstract
Cough initiated from the trachea and larynx in anaesthetized guinea-pigs is mediated by capsaicin-insensitive, mechanically sensitive vagal afferent neurones. Tachykinin-containing, capsaicin-sensitive C-fibres also innervate the airways and have been implicated in the cough reflex. Capsaicin-sensitive nerves act centrally and synergistically to modify reflex bronchospasm initiated by airway mechanoreceptor stimulation. The hypothesis that polymodal mechanoreceptors and capsaicin-sensitive afferent nerves similarly interact centrally to regulate coughing was addressed in this study. Cough was evoked from the tracheal mucosa either electrically (16 Hz, 10 s trains, 1–10 V) or by citric acid (0.001–2 m). Neither capsaicin nor bradykinin evoked a cough when applied to the trachea of anaesthetized guinea-pigs, but they substantially reduced the electrical threshold for initiating the cough reflex. The TRPV1 receptor antagonist capsazepine prevented the increased cough sensitivity induced by capsaicin. These effects of topically applied capsaicin and bradykinin were not due to interactions between afferent nerve subtypes within the tracheal wall or a direct effect on the cough receptors, as they were mimicked by nebulizing 1 mg ml−1 bradykinin into the lower airways and by microinjecting 0.5 nmol capsaicin into nucleus of the solitary tract (nTS). Citric acid-induced coughing was also potentiated by inhalation of bradykinin. The effects of tracheal capsaicin challenge on cough were mimicked by microinjecting substance P (0.5–5 nmol) into the nTS and prevented by intracerebroventricular administration (20 nmol h−1) of the neurokinin receptor antagonists CP99994 or SB223412. Tracheal application of these antagonists was without effect. C-fibre activation may thus sensitize the cough reflex via central mechanisms.
The relative role of bronchopulmonary C-fibres and rapidly adapting receptors in regulating cough remains controversial. It seems likely that the many species used to study cough and the state of consciousness or anaesthesia under which this reflex is studied contributes to the uncertainties associated with this issue. For example, the evidence in favour of a role for C-fibres in coughing is derived primarily from studies in conscious animals (primarily guinea-pigs) and in conscious human subjects (Karlsson & Fuller, 1999). In anaesthetized animals (cats, dogs and guinea-pigs), putatively selective stimulants of bronchopulmonary C-fibres have consistently failed to evoke coughing. On the contrary, C-fibre activation may inhibit coughing in anaesthetized animals (Tatar et al. 1988, 1994; Canning et al. 2004). This contrasts with the studies supporting a role for rapidly adapting stretch receptors (RARs) in coughing, most of which were carried out in anaesthetized cats and dogs (Widdicombe, 1954, 1998; Tatar et al. 1988, 1994). When studied in conscious animals, however, stimulants of RARs have been ineffective or only modestly effective at initiating cough (Barnes et al. 1984; Joos et al. 1987; Fujimura et al. 1992; Canning et al. 2004; El-Hashim & Amine, 2005). These conflicting observations indicate that either RARs and C-fibres regulate coughing independently and at different levels of consciousness, or that their mode of activation, synergistic interactions, or recruitment of a previously unrecognized subtype of airway afferent nerves is essential for initiating the cough reflex (Canning, 2002; Canning et al. 2004).
We recently reported that vagal afferent neurones arising from the nodose ganglia mediate coughing initiated by electrically, chemically or mechanically stimulating the trachea or larynx in anaesthetized guinea-pigs (Canning et al. 2004). These polymodal mechanoreceptors – the putative cough receptors – are not activated by bradykinin or capsaicin and possess physiological properties that are quite distinct from rapidly (RARs) and slowly (SARs) adapting stretch receptors innervating the intrapulmonary airways and lungs. Nociceptor activation with either capsaicin or bradykinin consistently failed to evoke cough in anaesthetized guinea-pigs. Nevertheless, it is likely that nociceptors play a role in regulating the cough reflex (Karlsson & Fuller, 1999; Canning, 2002). Coincident or prior nociceptor activation may alter sensitivity to tussive stimuli. Our previous studies in guinea-pigs indicate that mechanically sensitive and capsaicin-sensitive airway afferent nerves may interact synergistically at the level of the brainstem to mediate reflex bronchospasm (Mazzone & Canning, 2002a). This synergistic interaction is analogous to the process of central sensitization described in the somatic nervous system (Yaksh et al. 1999; Woolf & Salter, 2000; Mazzone & Canning, 2002c), with the tachykinin-containing nociceptor-like airway afferent nerves converging with and sensitizing the pathways receiving input from airway mechanoreceptors (Mazzone & Canning, 2002a; Undem et al. 2004). Based on the precedence established in our previous experiments and the evidence that C-fibre as well as airway mechanoreceptor activation can initiate coughing, we speculated that similar interactions between airway afferent nerve subtypes regulate cough. We show in the present study that capsaicin-sensitive nerve activation increases cough reflex sensitivity in anaesthetized guinea-pigs through central mechanisms.
Methods
Surgery and animal preparation
The Johns Hopkins Medical Institutions Animal Care and Use Committee approved all experiments described in this study. Male Hartley guinea-pigs (300–400 g, Hilltop, Scottdale, PA, USA) were anaesthetized with urethane (1 g kg−1, intraperitoneally, i.p.) and secured supine on a heated pad. This dose of urethane provides deep, stable anaesthesia for up to 9 h, although experiments rarely lasted longer than 3 h. The adequacy of the anaesthesia was assessed throughout the course of these experiments by checking for headshakes and avoidance responses following a sharp pinch of the hindlimb or during the surgical procedures. Although no animals required more anaesthetic during the course of an experiment, supplemental urethane would have been provided if responses to these noxious stimuli had been noted.
The methods for studying cough have been described elsewhere (Canning et al. 2004). Briefly, upon anaesthesia, the extrathoracic trachea was exposed by a midline incision in the neck and cannulated at its caudal-most end with a bent 15 gauge luer stub adaptor. Care was taken not to damage the tracheal vasculature or the recurrent laryngeal nerves, as these nerves carry the afferents that regulate the cough reflex evoked from the trachea and larynx (Canning et al. 2004). The tracheal cannula was attached to a small length of tubing that terminated inside a water-jacketed organ bath that was continuously filled with warmed (37°C) and humidified room air at a rate of 50 l h−1. The tracheal mucosa (rostral to the cannula) was then exposed by a midline incision in the ventral tracheal wall. In all of the experiments described, this segment of trachea was superfused (3 ml min−1) continuously with warmed (37°C), oxygenated Krebs–bicarbonate buffer composed as follows (mm): 118 NaCl; 5.4 KCl; 1 NaHPO4; 1.2 MgSO4; 1.9 CaCl2; 25 NaHCO3; 11.1 dextrose), containing 3 μm indomethacin and either each 0.1 μm of the neurokinin receptor antagonists CP99994 (NK1), SR48968 (NK2) and SB223412 (NK3), or 0.3 μm of the neurokinin receptor antagonist ZD6021 (NK1, NK2 and NK3). Indomethacin and the neurokinin receptor antagonists were included in the buffer to prevent formation of neuromodulatory prostanoids and to prevent the local actions of tachykinins released from airway C-fibres upon stimulation, respectively. The appropriateness of these drug concentrations has been shown previously (Mazzone & Canning, 2002a, b). The buffer was recovered from the trachea using a gentle suction source positioned at the level of the larynx.
Respiratory activity was monitored with a pressure transducer attached to a side port of the tracheal cannula and was recorded digitally using a Biopac data acquisition system. Respiratory rate was calculated online and expressed as the number of breaths per minute. Coughs were identified by a characteristic large expiratory effort following a brief, enhanced inspiratory effort, and were confirmed visually by the experimenter. At the end of each experiment, animals were asphyxiated with 100% carbon dioxide followed by exsanguination. The American Veterinary Medical Association recommends this method for killing guinea-pigs.
Experimental design
Cough evoked by electrical or chemical stimulation of the tracheal mucosa
Cough was evoked by electrical stimulation of the tracheal mucosa as previously described (Canning et al. 2004). Using great precision (to limit variability amongst the treatment groups), a custom-made platinum electrode was carefully placed onto the exposed mucosa adjacent to tracheal rings 5 and 6 caudal to the larynx and held in this position only by its own weight. Electrical stimuli (1–10 V, 16 Hz, 1 ms pulse duration, 10 s train) were delivered using an S88 Grass stimulator. We showed previously that cough evoked using this method is mediated by vagal afferent nerves arising from the nodose ganglia (Canning et al. 2004).
The electrical threshold for evoking cough was estimated in each animal by varying the stimulation voltage (1, 2, 4, 6, 8, 10, 12 V) while maintaining constant train durations and frequency. Stimuli were delivered at 5 min intervals. Voltage–response curves were constructed in an unpaired fashion in the absence or presence of sensitizing stimuli (see below). Voltage–response curves were terminated when stimulation at consecutive voltages (e.g. 2 and 4 V, or 8 and 10 V) evoked coughing. Unpaired designs were necessary as we found that prior stimulation caused a leftward shift in subsequently produced voltage–response curves.
We also evoked cough by applying 100 μl aliquots of citric acid (0.01–2 m) to the tracheal mucosa. The citric acid aliquots were administered over a 3–5 s period, adjacent to the tracheal cannula and directly into the Krebs buffer perfusing the trachea. Concentration–response curves were constructed, with aliquots of citric acid added in increasing concentrations at 1 min intervals. The concentration–response curves were displayed graphically and the cumulative number of coughs was determined. From the graphs, the concentration (C) of citric acid evoking two and five coughs was estimated for each experiment.
At the conclusion of each experiment, cough was evoked mechanically by probing the tracheal mucosa with a von Frey filament that delivered a punctate displacement force that is suprathreshold for all identified tracheal afferent nerves (4.7 mn; see Riccio et al. 1996).
Synergistic interactions between airway afferent subtypes regulating cough
To assess whether interactions between airway afferent neuronal subtypes are involved in modulating the cough reflex, we constructed voltage–coughing-response curves in the absence and presence of selective airway nociceptor stimulants. In initial experiments, we determined the effect of tracheal nociceptor stimulation with capsaicin and bradykinin on the voltage threshold for evoking the cough reflex. In these experiments, capsaicin (3 μm) or bradykinin (3 μm) was added to the tracheal perfusion buffer 5 min prior to electrical stimulation. The role of TRPV1 receptor activation in the sensitizing effects of capsaicin was determined by adding the TRPV1 receptor antagonist capsazepine (10 μm) to the tracheal perfusion buffer 10 min prior to the capsaicin challenge.
In subsequent studies, we tried to limit potential peripheral interactions between airway nociceptors and mechanoreceptors. Specifically, we assessed the ability of bronchopulmonary nociceptors to sensitize the cough reflex evoked by electrical or citric acid stimulation of the trachea. We constructed voltage–cough-response curves and citric acid concentration–cough-response curves following inhalation of bradykinin or vehicle (saline). Bradykinin (1 mg ml−1) was used in these studies instead of capsaicin because inhaled capsaicin, unlike bradykinin, evokes profound airways obstruction in guinea-pigs, a confounding variable that would complicate interpretation of the results (Canning et al. 2001; Canning, 2002). Solutions were nebulized into the breathing chamber via an ultrasonic nebulizer (Mystique, AirSep, Buffalo, New York ∼5 μm particle size) attached in series with the air inlet. Animals were allowed to inhale bradykinin or vehicle passively. For the experiments with citric acid, the nebulizer was turned on 1 min prior to constructing the concentration–response curve. Bradykinin or vehicle was delivered throughout the experiment. For the electrically evoked cough, bradykinin or vehicle were nebulized for 30 s prior to, and throughout, each 10 s period of electrical stimulation of the trachea. Following each of these 10 s periods of stimulation, animals were allowed to breathe room air until 30 s prior to the next period of stimulation. Previous studies indicate that inhalation of this concentration of bradykinin reliably evokes reflexes in either conscious or urethane-anaesthetized guinea-pigs, but produces minimal lower airways obstruction and no coughing (Farmer, 1997; Canning et al. 2001; our unpublished observations). In all experiments with nebulized bradykinin (or vehicle), 0.1 μm FR173657, a bradykinin B2 receptor antagonist, was added to the tracheal perfusate to block potential (but unlikely) local effects of systemically absorbed bradykinin on the trachea.
To provide further evidence for a central site of interaction between airway nociceptors and mechanoreceptors, we assessed the ability of centrally administered capsaicin to modulate cough evoked by electrical stimulation of the trachea. Animals were first placed in a Kopf stereotaxic frame. Using the midline of the occipital suture as a reference point, a borosilicate glass micropipette (30–40 μm tip diameter and bent to 90° midway along the shaft) was stereotaxically placed into the commissural nTS (cnTS) via a small hole in the skull (midline, 6.0 mm caudal from occipital suture, 13.0 mm ventral from the surface of the skull). The pipette was secured to the surrounding bone with dental cement and the animal was removed from the frame and prepared as described above. Before constructing the cough voltage–response curves, capsaicin (0.5 nmol in 500 nl) or an equivalent volume of vehicle (20% ethanol in saline) was microinjected over 3 min into the commissural nTS (Mazzone & Geraghty, 1999). We elected to microinject in the cnTS, based on our previous studies showing that this is a primary site of airway afferent nerve termination, particularly airway C-fibres (Mazzone & Canning, 2002a). The concentration of capsaicin utilized in these studies was near threshold for evoking reflexes in our previous studies, and the volume injected the minimum attainable, given the poor water solubility of the vanilloid (Mazzone & Geraghty, 1999).
Microinjections were performed as previously described (Mazzone & Canning, 2002b). A custom-made flexible cannula (30 gauge ‘Microfil’, World Precision Instruments, Sarasota, FL, USA) was fed into the glass pipette such that the tip of the cannula fit snugly into the tip of the pipette. The distal end of the cannula was attached to a 100 μl Hamilton glass syringe via a luer connection. Solutions were ejected from the syringe with a Razel syringe pump (Model A-99) at a speed of 166 nl min−1. Injection sites were identified in sections (50 μm) of the brainstem, which was recovered postmortem, fixed in 4% paraformaldehyde, frozen and sectioned. Injection sites were marked postmortem by microinjecting Evans blue dye (2% solution, 50 nl). Although precise localizations of the injection sites was not always possible due to tissue damage that occurred upon extracting the guide cannula (which had to be glued to the surface of the skull so the animal could be removed from the stereotaxic frame and placed supine), all cnTS microinjections were at or near midline, in the area of obex (defined as the caudal-most end of area postrema). The data were excluded from further analysis if this location had been missed by the microinjection, or if reasonably accurate determinations for the site of microinjection could not be provided. Negative control experiments in which capsaicin was microinjected into an adjacent (rostral and lateral) region of the brainstem not associated with vagal regulation of respiration or cough (the caudal, interpolar, trigeminal nucleus) were carried out in parallel.
We also studied the effects of capsaicin desensitization on citric acid-evoked coughing. Capsaicin was administered at a supramaximal concentration (10 μm), resulting in a transient, occasionally profound, slowing of respiration followed eventually by recovery of the normal respiratory pattern. Thirty minutes later, in the continued presence of capsaicin (or vehicle), citric acid concentration–response curves were constructed. The potency of citric acid and the total number of coughs evoked were determined and compared between the two treatment groups.
The role of tachykinins in cough evoked by electrical stimulation of the tracheal mucosa
We assessed the role of tachykinins in mediating the capsaicin-induced sensitization of the cough reflex by evaluating the ability of substance P (0.5 and 5 nmol) microinjected into the cnTS to mimic the effects of capsaicin, and by administering CP99994 (NK1, 20 nmol h−1) or SB223412 (NK3, 20 nmol h−1) intracerebroventricularly (i.c.v.) via a stainless steel cannula that was stereotaxically cemented into the right lateral cerebral ventricle (2 mm caudal and 1.8 mm lateral to bregma, and 4.8 mm below the surface of the skull). We also studied the effects of microinjecting the potent NK1, NK2 and NK3 antagonist ZD6021 (1 nmol in 100 nl over 3 min) into the cnTS as described above. Antagonist doses were based on previous studies (Canning et al. 2001; Mazzone & Canning, 2002a; Joad et al. 2004). The cannula for i.c.v. drug administration was attached to a Hamilton microsyringe via PE-tubing, and drugs were infused using a Razel A-99 syringe pump at a speed of 20 μl h−1. Infusions were initiated 15 min prior to adding capsaicin to the tracheal perfusate, and continued for the duration of the experiment (about 60 min). Cough was evoked electrically as described above. Injection sites were confirmed with Evans blue dye at the end of the experiments. Control studies were performed in parallel in which vehicle (dimethyl sulfoxide) was administered instead of the antagonist. In separate studies, we also assessed the effects of systemically administered neurokinin receptor antagonists (CP99994, SR48968 and SB223412) on cough evoked electrically or by citric acid in control animals (i.e. animals that had not first been challenged with either capsaicin or bradykinin). The antagonists were administered in combination at doses (1 mg kg−1 each, i.p. injection) that markedly inhibit or abolish (presumably C-fibre-mediated) reflexes while having no effect on reflexes associated with airway mechanoreceptor activation (Bolser et al. 1997; Canning et al. 2001; Mazzone & Canning, 2002a).
Data analysis
Cough was defined visually and based on rigid criteria for pressure changes associated with the effort (≥ 500% increase in peak expiratory pressure preceded by an enhanced inspiratory effort, with the whole cycle occurring in < 1 s). Data are presented as the percentage of animals coughing (one or more coughs) to a given electrical stimulus intensity or dose of citric acid. Changes in respiratory rate and peak expiratory pressures during cough (expressed as a percentage of tidal expiratory pressures) were determined and expressed as a mean ± s.e.m. Citric acid-evoked coughing data are presented as a mean ± s.e.m. coughs, and as the negative logarithm of citric acid concentration evoking two and five coughs (data is log-transformed for normalization and subsequent statistical analysis). When appropriate, statistical differences between the mean data of treatment groups are compared using analysis of variance. In experiments assessing thresholds for coughing, Chi square analysis is performed. P < 0.05 was considered significant. Occasionally (< 10% of experiments), the baseline respiratory rate was very slow (< 40 breaths min−1). Such preparations rarely coughed to any stimuli and were thus excluded from subsequent analyses.
Coughing occurred upon 1 or 2 V stimulation in 13 out of 26 animals first challenged with either capsaicin or bradykinin and in 0 out of 29 control animals. When comparing peak expiratory pressures during cough, we found that increasing the stimulation voltage from 1 to 2 V or from 2 to 4 V consistently increased the peak expiratory pressures during cough (912 ± 109%versus 1225 ± 299% of tidal breathing at the lower (1 or 2 V) and higher (2 or 4 V) stimulation voltages, respectively; P < 0.05). By contrast, in 33 instances where cough was evoked at consecutive voltages ≥ 4 V (e.g. 4 and 6 V, or 6 and 8 V), the peak expiratory pressures of the subsequently evoked cough were essentially unchanged (1156 ± 126%versus 1137 ± 103% of tidal breathing at the lower (4, 6, or 8 V) and higher (6, 8 or 10 V) stimulation voltages, respectively; P > 0.1). Thus, for statistical analyses, we only compared the peak expiratory pressures of coughs evoked by ≥ 4 V stimulation.
Drugs
Tocris provided capsazepine (Ellisville, MO, USA). Capsaicin, bradykinin, indomethacin, citric acid and urethane (ethyl carbamate) were purchased from Sigma (St Louis, MO, USA). GlaxoSmithKline (King of Prussia, PA, USA), AstraZeneca (Wilmington, DE, USA), and Schering-Plough (Kenilworth, NJ, USA) provided CP99994, SR48968, SB223412 and ZD6021. FR173657 was provided by Fujisawa (Osaka, Japan). Concentrated stock solutions were made (10–100 mm) of all drugs added to the tracheal perfusate: capsaicin (10 mm) and indomethacin (30 mm) were dissolved in ethanol; FR173657, CP99994, SR48968, SB223412, and ZD6021 (each 1 mm) and in some studies capsaicin (0.1 m) were dissolved in dimethyl sulfoxide. Bradykinin 1 mg ml−1 was dissolved in saline for inhalation. For CNS injections, capsaicin and ZD6021 were dissolved in 20% ethanol in saline, while CP99994 and SB223412 (each 1 nmol μl−1) were dissolved in dimethyl sulfoxide.
Results
Effects of tracheal capsaicin and bradykinin on electrically evoked cough
Electrical stimulation of the trachea at frequencies ≤ 4 Hz evoked no coughs in anaesthetized guinea-pigs regardless of the stimulation voltage (1–12 V) or train duration (5–20 s). Few of the animals studied coughed when the train duration was < 5 s, no matter the stimulus frequency or intensity. At optimal stimulation frequencies (16 Hz), and optimal pulse (1 ms) and train (10 s) durations, electrical stimulation of the trachea at 1, 2 or 4 V still failed to evoke coughing in control animals, although occasionally augmented breaths or sighs were induced. Cough was observed in 1 out of 7 control animals at a stimulus voltage of 6 V, and in 3 out of 7 control animals at 8 V, suggesting that this stimulus intensity was near threshold for evoking cough in anaesthetized guinea-pigs. Further increases in stimulation intensity to 10 V, probably resulting in more current spread and thus recruitment of more cough receptors and/or fewer activation failures in the stimulated cough receptors, evoked coughing in all control animals (Fig. 1).
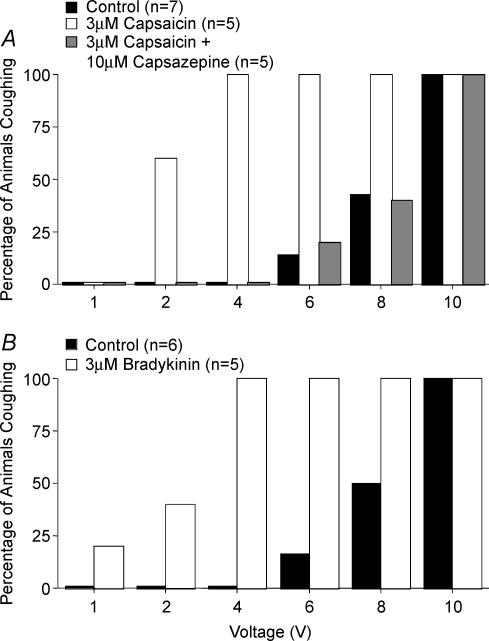
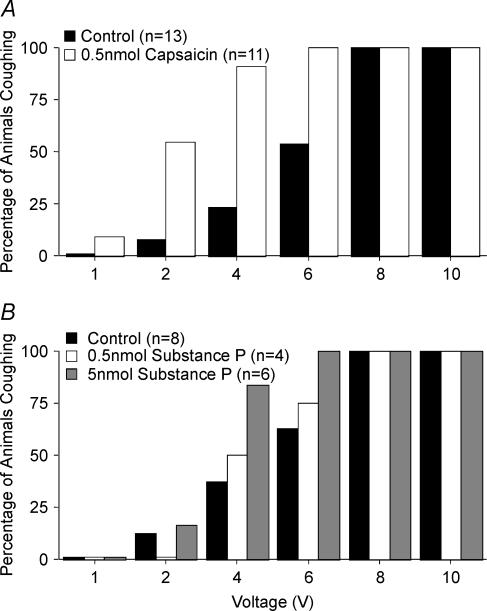
Figure 1. Capsaicin and bradykinin-induced sensitization of the cough reflex initiated by electrical stimulation of the tracheal mucosa of anaesthetized guinea-pigs.
The voltage threshold for evoking cough was estimated by electrically stimulating the tracheal mucosa at varying stimulation voltages (16 Hz, 10 s train, 1 ms pulse duration). Capsaicin (A) bradykinin (B) or vehicle were added to the tracheal perfusate prior to constructing the voltage–response curves. Capsazepine or its vehicle was added to the tracheal perfusate 10 min prior to the addition of capsaicin. The results are presented as the percentage of animals coughing in response to a given stimulation voltage. Challenge with either capsaicin or bradykinin increased the likelihood of coughing at lower stimulation intensities (2–4 V) relative to control (P < 0.001).
Applying capsaicin to the tracheal mucosa had variable effects on respiratory rate. As reported previously (Canning et al. 2004), high concentrations (≥ 10 μm) of capsaicin slowed respiration, with the rate recovering gradually over the ensuing 30 min. At lower concentrations (≤ 3 μm), capsaicin had modest and variable effects on respiration, tending to increase the rate (15 ± 5%) about 3 min after application. However, by the end of the 5 min leading up to the electrical stimuli used to evoke cough, the respiratory rate in 3 μm capsaicin-treated animals was similar to pretreatment values and similar to that in vehicle control animals.
As reported previously (Canning et al. 2004), tracheal capsaicin challenge did not evoke cough in anaesthetized guinea-pigs. In the presence of capsaicin, however, the electrical threshold for evoking cough was greatly reduced (Fig. 1A). Whereas no control animals coughed in response to 2 or 4 V stimulation, 3 of 5 animals treated with capsaicin coughed in response to 2 V stimulation, and all 5 animals challenged with capsaicin coughed in response to 4 V stimulation. Like that seen with capsaicin, tracheal bradykinin challenge also had variable and modest effects on respiratory rate, but still substantially reduced the electrical threshold for cough (Fig. 1B). Bradykinin and capsaicin also increased the peak expiratory pressures during cough evoked by electrical stimulation of the tracheal mucosa even though the voltage required to evoke cough following capsaicin or bradykinin challenge was considerably reduced (Table 1).
Table 1.
The effect of capsaicin and bradykinin on the peak expiratory pressures associated with coughing in anaesthetized guinea-pigs
| Treatment | Voltage (V) | Peak expiratory pressure (mmHg) | n |
|---|---|---|---|
| Control for tracheal bradykinin | 8.7 ± 0.6 | 1094 ± 103 | 10/5 |
| Tracheal bradykinin | 5.0 ± 0.4* | 1261 ± 143 | 6/3 |
| Control for tracheal capsaicin | 8.8 ± 0.6 | 1073 ± 176 | 11/5 |
| Tracheal capsaicin | 5.1 ± 0.5* | 2068 ± 366* | 9/5 |
| Control for inhaled bradykinin | 9.1 ± 0.6 | 777 ± 81 | 11/6 |
| Inhaled bradykinin | 5.1 ± 0.5* | 1059 ± 108* | 9/6 |
| Control for cnTS capsaicin | 7.7 ± 0.4 | 1010 ± 86 | 22/12 |
| cnTS capsaicin | 5.2 ± 0.4* | 1126 ± 102 | 15/9 |
| Control for cnTS substance P | 7.4 ± 0.6 | 1141 ± 94 | 13/7 |
| cnTS substance P (5 nmol) | 5.8 ± 0.6 | 1214 ± 117 | 8/4 |
Data are presented as the mean ± s.e.m. percentage of the peak expiratory pressures associated with eupneic breathing. The n values indicate the number of coughs measured/number of animals in which cough was studied. Cough was evoked by electrically stimulating the tracheal mucosa at 16 Hz, 1 ms pulse duration, 10 s train, 4–10 V. At stimulation voltages ≥ 4 V, the peak expiratory pressures during cough did not increase when the voltage was increased within any of the treatment groups and thus the expiratory pressures of coughs evoked by 4–12 V stimulation were pooled for statistical analysis. No control animals (n = 29) coughed in response to ≤ 4 V stimulation while 25 out of 26 animals first challenged with either capsaicin or bradykinin coughed in response to ≤ 4 V electrical stimulation (P < 0.0001). The peak expiratory pressures of coughs evoked at 1 or 2 V were considerably smaller in magnitude than that of coughs evoked at higher stimulation voltages, so they were excluded from the analyses (see Methods for further discussion).
P < 0.05 compared with vehicle control.
The TRPV1 receptor antagonist capsazepine prevented the capsaicin-induced sensitization of the cough response (Fig. 1A). Capsazepine did not reduce the peak expiratory pressures attained during cough evoked by electrical stimulation of the trachea (852 ± 78% and 1384 ± 257% of tidal expiration pressure for control and capsazepine-treated animals, respectively).
Effect of inhaled bradykinin and microinjection of capsaicin into the commissural nTS on electrically evoked and acid-induced cough
C-fibre activation may induce cough secondary to the peripheral release of tachykinins which in turn act on structural cells (e.g. smooth muscle, glands), resulting sequentially in airways obstruction, RAR activation and then cough (Bonham et al. 1996; Bergren, 1997; Matsumoto et al. 1997; Widdicombe, 1998; Canning, 2002). Although all of the experiments described here were carried out with neurokinin receptor antagonists in the tracheal perfusate, we tried to further limit peripheral interactions between the cough receptors and airway nociceptors by stimulating these afferent nerve subtypes at different, distant sites within the airways. Bradykinin or vehicle was delivered selectively to the lower airways to modulate cough evoked from the extrathoracic trachea of anaesthetized guinea-pigs. Following saline inhalation, which did not evoke cough, stimulation voltage intensities ≤ 4 V induced no coughs, while 16% (1 out of 6) and 50% (3 out of 6) of saline challenged animals coughed in response to 6 and 8 V stimulation, respectively, and all coughed in response to 10 V stimulation (Fig. 2). Inhalation of bradykinin also failed to evoke cough but substantially lowered the electrical threshold for cough. Most (5 out of 6) animals inhaling bradykinin coughed in response to 4 V stimulation, and all 6 animals inhaling the autacoid coughed during the 6 V stimulation (Fig. 2). Bradykinin also increased by ∼5-fold the potency of citric acid for evoking cough, increased the total number of coughs evoked by the acid over an entire concentration–response curve (0.001–0.1 m), and increased peak expiratory pressures associated with coughing evoked by citric acid (Fig. 3, Tables 1 and 2).
Figure 2. Inhalation of bradykinin sensitizes the cough reflex initiated by electrical stimulation of the tracheal mucosa of anaesthetized guinea-pigs.
Bradykinin (1 mg ml−1) or vehicle (saline) was nebulized into the air inspired by the animals 30 s prior to and during the electrical stimulation used to initiate coughing. No animals coughed in response to inhalation of either bradykinin or vehicle, but bradykinin challenge increased respiratory rate (see Fig. 3). The results are presented as the percentage of animals coughing in response to a given stimulation voltage. Inhalation of bradykinin increased the likelihood of coughing at lower stimulation intensities (2–4 V) relative to control (P < 0.001).
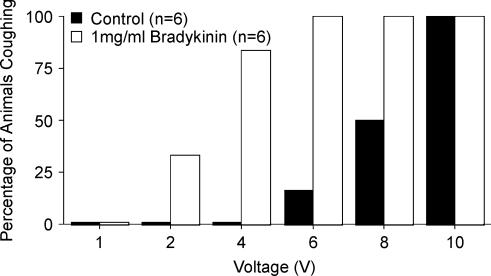
Figure 3. Inhalation of bradykinin sensitizes the cough reflex initiated by citric acid applied topically to the tracheal mucosa of anaesthetized guinea-pigs.
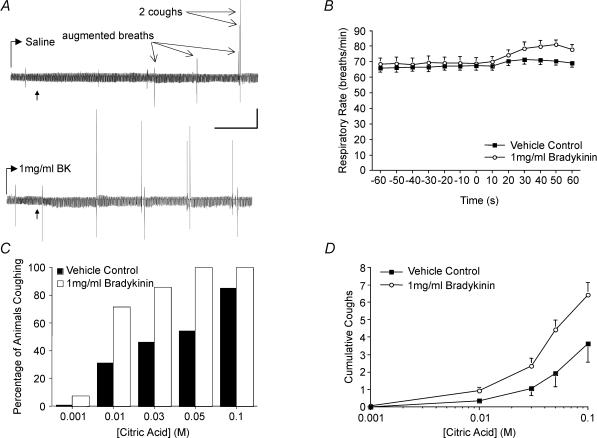
Bradykinin (1 mg ml−1) or vehicle (saline) was nebulized into the air inspired by the guinea-pigs 60 s prior to and throughout the entire citric acid concentration–response curve. A, representative traces (∼5 min) of citric acid-evoked cough during inhalation of saline (top) and bradykinin (bottom). Bradykinin and saline inhalation evoked augmented breaths but no coughing. Bradykinin evoked more augmented breaths than saline. Citric acid, delivered in 100 μl aliquots in increasing concentrations from 0.001 to 0.1 m (the time of first dose administration is marked by an arrow on each trace) were administered at 1 min intervals and evoked augmented breaths or coughing, usually followed by a brief slowing of respiration, particularly upon challenge with higher concentrations of citric acid (≥ 0.1 m). Vertical and horizontal bars denote 5 cmH2O and 1 min, respectively. B, effects of saline and 1 mg ml−1 bradykinin inhalation (beginning at time 0) on respiratory rate. Respiratory rate increased more during bradykinin inhalation (18 ± 3% peak increase, n = 14) than during inhalation of saline (6 ± 1% peak increase, n = 13; P < 0.01). C, percentage of animals coughing in response to increasing concentrations of citric acid following inhalation of saline (filled bars) or 1 mg ml−1 bradykinin (open bars). Bradykinin significantly increased the probability of 0.01 m citric acid challenge to evoke cough in the anaesthetized guinea-pigs (P < 0.05). D, mean ± s.e.m. cumulative coughs evoked by increasing concentrations of citric acid during inhalation of saline (open circles) and 1 mg ml−1 bradykinin (filled circles). Inhalation of bradykinin significantly increased the total number of coughs evoked cumulatively by 0.001–0.1 m citric acid and increased the potency of citric acid for inducing cough (see Table 2).
Table 2.
Inhalation of bradykinin sensitizes the cough reflex evoked by citric acid in anaesthetized guinea-pigs
| Saline control | Bradykinin (1 mg ml−1) | |
|---|---|---|
| Expiratory pressures during cough (%) | 1168 ± 57 | 1357 ± 53* |
| Citric acid concentration for 2 coughs (mm) | 125 ± 41 | 44 ± 20* |
| Citric acid concentration for 5 coughs (mm) | 367 ± 107 | 162 ± 107* |
| Animals with ≤ 5 cumulative coughs (%) | 23 | 0* |
| Animals with ≤ 5 cumulative coughs (n) | 3/13 | 0/14* |
| Animals with ≥ 10 cumulative coughs (%) | 54 | 93* |
| Animals with ≥ 10 cumulative coughs (n) | 7/13 | 13/14* |
Aerosols of bradykinin or its vehicle (saline) were given 1 min prior to and throughout construction of the concentration–response curves to citric acid (0.001–2 m). Citric acid was applied topically to the tracheal mucosa in 100 μl aliquots at 1 min intervals. The cumulative number of coughs evoked by citric acid was determined and from a ‘best fit’ graph of the results, the concentration of citric acid evoking 2 and 5 coughs was estimated for each experiment; for statistical analyses, the individual values of concentrations evoking 2 and 5 coughs were log-transformed for normalization of the data. Estimating the concentration of citric acid evoking 5 coughs was not possible in 2 of the 13 control (saline-challenged) experiments, as they failed to cough 5 times cumulatively.
P < 0.05, compared with saline control.
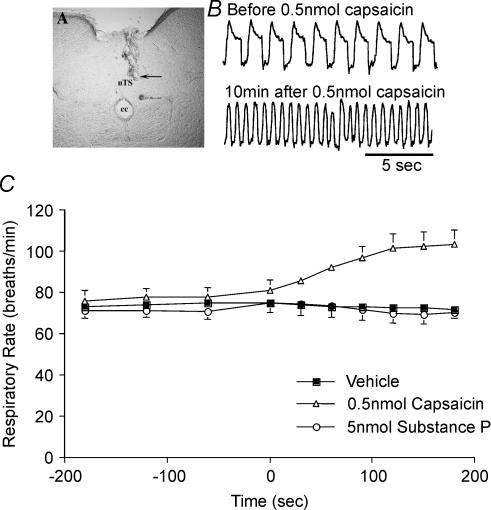
In subsequent experiments we determined the effect of activating the central terminals of nociceptors on the voltage–response curve for cough. Neither capsaicin nor its vehicle evoked cough when microinjected into the commissural nTS. Capsaicin but not its vehicle did, however, markedly increase (61 ± 5% increase; P < 0.05) respiratory rate upon microinjection, which peaked about 5 min after the injection. Respiratory rate tended to return to preinjection values within 15–20 min (Fig. 4). The microinjected capsaicin but not its vehicle also greatly reduced the electrical threshold for evoking cough from the trachea (Fig. 5A). Microinjecting the same dose and volume of capsaicin into the interpolar trigeminal subnucleus, which is not associated with vagal regulation of respiration or cough, was without effect on respiration or electrically induced coughing (0 out of 4 animals coughed in response to 6 V stimulation, 4 out of 4 coughed in response to 10 V stimulation; compare with results summarized in Fig. 5). By contrast, microinjecting capsaicin into adjacent locations in nTS (the intermediate and ventrolateral subnuclei) had no effect on respiration but still seemed to sensitize the cough reflex (2 out of 3 animals coughed in response to 4 V stimulation, 3 out of 3 animals coughed in response to 6 V stimulation; compare with results from control preparations in Figs 1, 2, 5 and 6).
Figure 4. Effect of microinjecting capsaicin and substance P into the cnTS on respiratory rate in anaesthetized guinea-pigs.
Capsaicin (0.5 nmol), substance P (0.5–5 nmol) or vehicle (20% ethanol in saline) was microinjected (0.5 μl) into the cnTS. A, representative injection site, indicated by arrow (∼0.5 mm caudal to obex). CC, central canal. B, representative traces showing respiration before and 10 min after microinjection of capsaicin. C, mean ± s.e.m. effects of cnTS microinjection of capsaicin, substance P and vehicle (0.5 μl over 3 min beginning at time 0) on respiratory rate. Microinjecting 0.5 nmol capsaicin into brainstem subnuclei rostral and lateral to cnTS, including the interpolar trigeminal nucleus (n = 4) and the intermediate and ventrolateral nTS (n = 3) was without effect on respiration (not shown).
Figure 5. Microinjection of capsaicin or substance P into the cnTS sensitizes the cough reflex in anaesthetized guinea pigs.
Cough was evoked electrically from the trachea (16 Hz, 10 s train, 1–10 V). The effects of capsaicin (A) and substance P (B) microinjection on the voltage–coughing-response curve are depicted. The results are presented as the percentage of animals coughing in response to a given stimulation voltage. Microinjection of 0.5 nmol capsaicin or 5 nmol substance P into the cnTS increased the likelihood of coughing at lower stimulation intensities (2–6 V) relative to control (P < 0.001).
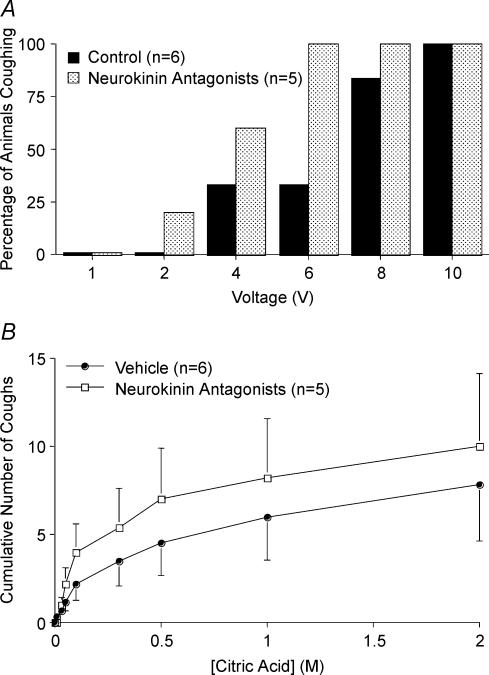
Figure 6. Neurokinin receptor antagonists have no effect on cough evoked electrically or by citric acid in control animals.
Cough was evoked from the tracheal mucosa electrically (16 Hz, 10 s train, 1 ms pulse duration, 1–10 V) (A) and subsequently by citric acid (0.001–2 m) applied topically to the tracheal mucosa (B). Animals were first pretreated intraperitoneally either with vehicle (dimethyl sulfoxide; n = 6) or with a combination of three neurokinin receptor antagonists CP99994, SR48968 and SB223412 (1 mg kg−1 each, n = 5). The antagonists (administered at doses known to abolish C-fibre-mediated reflexes in guinea pigs; Bolser et al. 1997; Canning et al. 2001; Mazzone & Canning, 2002a) had no effect on the percentage of animals coughing in response to any stimulation voltage or citric acid challenge and had no effect on the tussigenic potency or efficacy of citric acid (P > 0.1). Moreover, in these animals, which were not challenged with capsaicin prior to evoking cough (see Figs 7 and 8), the antagonists had no effect on the peak expiratory pressures during cough evoked either electrically or by citric acid (the peak expiratory pressures of coughs evoked following antagonist administration averaged 106 ± 13% of that evoked in stimulation (voltage or citric acid concentration) matched controls; P > 0.1).
As reported previously (Canning et al. 2004), capsaicin desensitization of the tracheal mucosa failed to prevent citric acid-evoked cough. Induced by continuously perfusing the vanilloid over the tracheal mucosa (beginning 30 min prior to and then throughout the citric acid challenge), capsaicin desensitization did, however, substantially reduce the number of coughs evoked by nine doses of citric acid (0.01–2 m), applied topically to the trachea in ascending concentrations (11.0 ± 1.8 coughs in vehicle control experiments versus 4.8 ± 0.7 coughs following capsaicin desensitization; n = 4–5, P < 0.05).
Effect of centrally administered substance P and neurokinin receptor antagonists on cough
Microinjecting substance P (0.5–5 nmol) into the cnTS mimicked the effects of capsaicin on cough, dose-dependently decreasing the electrical threshold for cough evoked from the trachea. These doses of substance P had no effect on respiratory rate or pattern (Figs 4C and 5B).
Without prior nociceptor stimulation by capsaicin or bradykinin, the neurokinin receptor antagonists CP99994, SR48968 and SB223412, simultaneously administered at doses that prevent C-fibre mediated reflexes (1 mg kg−1i.p.; see Bolser et al. 1997; Canning et al. 2001; Mazzone & Canning, 2002a), had no effect on tidal breathing pattern or cough evoked from the trachea by electrical stimulation or by citric acid (Fig. 6). Upon i.c.v. adminstration, CP99994 and SB223412, (administered individually in separate experiments) still failed to prevent coughing evoked by electrical stimulation of the tracheal mucosa, but prevented capsaicin-induced sensitization of electrically evoked cough. Microinjecting the potent but non-selective neurokinin receptor antagonist ZD6021 (1 nmol) into cnTS did not prevent electrically evoked coughing in control preparations (6 out of 6 animals coughed in response to 8 V electrical stimulation). In addition, it did not prevent tracheal capsaicin-induced sensitization of coughing; 4 out of 4 animals coughed in response to 6 V stimulation after microinjection of ZD6021 into the cnTS and intratracheal administration of capsaicin (compare results in Figs 1, 5 and 6, 7).
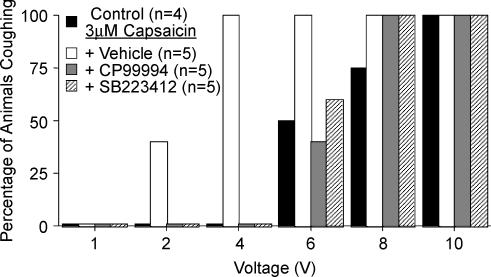
Figure 7. Intracerebroventricular administration of CP99994 or SB223412 prevents tracheal capsaicin-induced sensitization of cough in anaesthetized guinea-pigs.
Cough was evoked electrically from the trachea (16 Hz, 10 s train, 1–10 V). Antagonists (1 nmol μl−1) or vehicle (dimethyl sulfoxide) were administered into the lateral ventricles by continuous infusion (20 μl h−1) beginning 10 min prior to adding 3 μm capsaicin to the tracheal perfusate. The results are depicted as the percentage of animals coughing in response to a given stimulation voltage. Tracheal capsaicin increased the likelihood of coughing at lower stimulation intensities (2–4 V) relative to control (P < 0.001), an effect that was prevented by CP99994 and by SB223412.
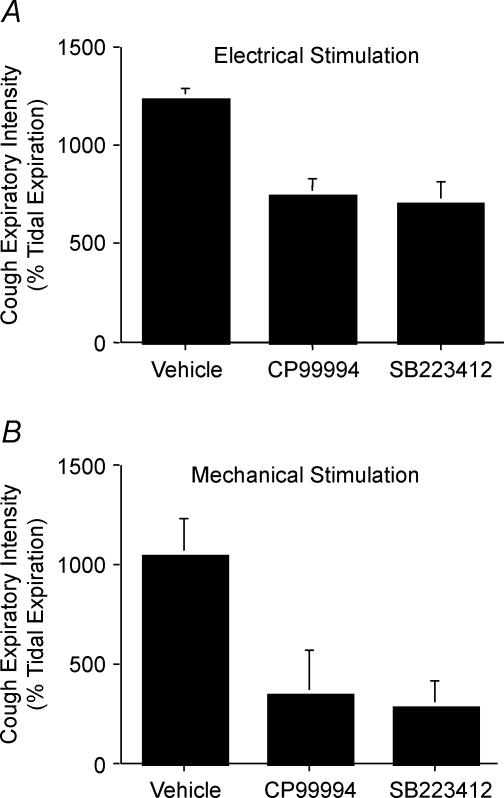
In animals challenged intratracheally with capsaicin, i.c.v. pretreatment with CP99994 or SB223412, but not vehicle, induced abnormal respiratory patterns characterized by alternating 30 s intervals of slow and fast breathing, which were terminated following an induced or spontaneous augmented breath (sigh) or an induced cough. The i.c.v. infusion of either CP99994 or SB223412 also significantly (P < 0.05) reduced the intensity of individual cough expiratory efforts (Fig. 8). For electrically evoked cough following capsaicin challenge, peak expiratory pressures during cough were reduced from 1234 ± 58% of tidal expiration in vehicle-treated animals to 743 ± 88 and 708 ± 108% of tidal expiration in animals treated with CP99994 and SB223412, respectively. Similarly, for expiratory reflexes and coughs evoked by mechanical stimulation of the trachea, peak expiratory pressures were reduced from 1046 ± 188% tidal expiration in vehicle-treated animals to 341 ± 227 and 282 ± 130% of tidal expiration in animals treated with CP99994 and SB223412, respectively.
Figure 8. CP99994 and SB223412 reduce peak expiratory pressures during cough initiated from the tracheal or laryngeal mucosa.
Data are presented as the mean ± s.e.m. expiratory pressure during cough relative to peak tidal expiratory pressures. All animals were challenged with 3 μm capsaicin administered to the tracheal perfusate. At least one cough evoked electrically (A) or mechanically (B) in each of 5 animals in each treatment group was analysed.
Discussion
The results of the present study confirm that airway nociceptor activation fails to result directly in coughing in anaesthetized guinea-pigs (Canning et al. 2004). While neither bradykinin nor capsaicin evoked cough following anaesthesia, both compounds sensitized the cough reflex. This sensitizing effect probably depends on central interactions between cough receptors and nociceptors and is analogous to the afferent nerve interactions regulating reflex bronchospasm we previously described (Mazzone & Canning, 2002a). The data also provide further evidence that tachykinins acting in the CNS regulate airway defensive reflexes in guinea-pigs.
Central synergistic interactions between airway afferent nerve subtypes
We recently reported that reflex-mediated tracheal contractions evoked by laryngeal capsaicin challenge are absolutely dependent upon ongoing mechanoreceptor activity arising from the intrapulmonary airways and lungs. Disrupting the intrapulmonary mechanoreceptor activity while preserving entirely laryngeal afferent and tracheal efferent innervation essentially abolished the tracheal reflex evoked by the laryngeal capsaicin challenge. This interaction between the tonically active intrapulmonary mechanoreceptors and the acutely activated laryngeal nociceptors is analogous to the process of central sensitization described in somatosensory pathways (Woolf & Salter, 2000; Mazzone & Canning, 2002a).
In the present study we observed that bradykinin and capsaicin failed to evoke cough in anaesthetized guinea-pigs but consistently and reliably reduced the threshold for cough evoked by electrical or acid stimulation of the trachea. Conversely, we showed that prior capsaicin desensitization did not prevent coughing evoked by citric acid but reduced the number of coughs evoked cumulatively by the acid. The sensitizing effect of acute bradykinin and capsaicin challenge on cough and the sensitizing effects of acid-induced activation of capsaicin-sensitive nerves on cough cannot be due to a peripheral sensitization of the cough receptors, as previous studies indicate that cough receptors do not express capsaicin (TRPV1) or bradykinin receptors and are neither activated nor sensitized by these agents (Riccio et al. 1996; Kajekar et al. 1999; Myers et al. 2002; Canning et al. 2004). As the reduced threshold for cough produced by capsaicin and bradykinin occurred with neurokinin receptor antagonists in the tracheal perfusate, and was evident even when the nociceptors were activated at sites distal to the tracheal locations from where cough was evoked, we conclude that the interaction between these afferent nerve subtypes must occur in the CNS. In fact, cnTS microinjection of capsaicin (thereby selectively activating the central endings of nociceptors (including airway nociceptors) terminating in this region; Mazzone & Geraghty, 1999; Mazzone & Canning, 2002a; Patterson et al. 2003) similarly reduced the electrical threshold for evoking cough from the trachea.
In our view, the most interesting and important experiments completed in this study were those in which we found that bradykinin nebulized into the lower airways sensitized the cough reflex evoked from the trachea. Given the selectivity of bradykinin (and capsaicin) for nociceptors and the insensitivity of the cough receptors to these agents, there would seem to be no viable alternative hypothesis to the notion that these afferent nerve subtypes interact centrally and synergistically to regulate cough. In fact, in these experiments the trachea was treated topically with a bradykinin receptor antagonist via the tracheal perfusate, thereby eliminating any remote chance that these two stimuli might interact peripherally. Similar approaches were used recently to document central interactions of afferent nerves regulating pain sensation in the oesophagus (Sarkar et al. 2001; Hobson et al. 2004).
As a third line of evidence, albeit the least compelling, that sensitization of cough by airway nociceptor activation occurs through central sites of interaction, we studied the effects of cnTS microinjection of capsaicin on the cough reflex. There are limitations to this approach to addressing our hypothesis. For example, we cannot achieve selectivity for activating airway nociceptors with this method, nor can we know with any certainty what synapses are sensitized by this stimulus. Indeed, neither the CNS terminations of cough receptors in any species nor the CNS terminations of airway C-fibres in guinea-pigs have been clearly defined. But a likely site of termination of and interaction between these afferents is the nTS (Kubin & Davies, 1995; Mazzone & Canning, 2002a; Joad et al. 2004). Microinjecting capsaicin (but not its vehicle) into or adjacent to the commissural, ventrolateral or intermediate subnuclei of nTS sensitized the cough reflex, whereas microinjecting an identical concentration and volume of capsaicin into the adjacent trigeminal nucleus was without effect. Each of these nTS subnuclei is a potential site of airway C-fibre termination, but only cnTS microinjection of capsaicin mimicked the effects of peripherally administered capsaicin and bradykinin on respiration. Combined with our tracing studies, suggesting cnTS convergence of airway nociceptors and mechanoreceptors (probably including the cough receptors; Mazzone & Canning, 2002a), these data demonstrate selectivity of our microinjection techniques using this threshold concentration of capsaicin (Mazzone & Geraghty, 1999), and suggest that the cnTS may be at least one location for central integration of cough receptor and airway nociceptor input.
The sensitization of cough without any change in respiratory pattern or rate induced by microinjecting capsaicin in and adjacent to the intermediate and ventrolateral subnuclei of nTS is interesting. Esophageal afferent nerves may terminate in these subnuclei (Wank & Neuhuber, 2001; Suwanprathes et al. 2003; Lang et al. 2004). In unpublished studies, we have shown that intraesophageal capsaicin administration (but not vehicle) has no effect on respiration but sensitizes subsequently evoked coughing evoked by electrically stimulating the tracheal mucosa. This may be relevant to the excessive coughing commonly reported in patients with gastroesophageal reflux disease (Mazzone & Canning, 2002d). It is worth noting, however, that sensitization of cough may not be a consequence of nociceptor activation in general. Capsacin challenge of the nose or larynx induces a profound slowing of respiration but no sensitization of coughing (B. J. Canning and N. Mori, unpublished observations).
Mechanistically, the simplest explanation for the results of the present study is that cough receptors and nociceptors regulate a common subset of nTS relay neurones. Many of the sensitizing stimuli used in this study also increased respiratory rate. As the brainstem networks regulating breathing and cough also probably overlap (Shannon et al. 1998), enhanced respiratory circuit activity could also increase the likelihood of cough. Extensive convergence of visceral afferents in the nTS has been reported (Mifflin, 1996; Paton, 1998; Silva-Carvalho et al. 1998), and neuronal tracing studies indicate that tracheal mechanoreceptors and nociceptors may converge centrally in the nTS of guinea-pigs (Mazzone & Canning, 2002a). Although electrophysiological studies carried out in other species suggest that intrapulmonary nociceptors may not converge centrally with airway mechanoreceptors (Kubin & Davies, 1995; Paton, 1998; Doyle et al. 2002), airway afferent nerve subtype interactions in the nTS are still possible, perhaps limited to the extrapulmonary airway afferents (which have been poorly characterized in terms of CNS terminations), or perhaps through volume transmission (Duggan et al. 1990; Zoli et al. 1998; Baude & Shigemoto, 1998), interneurones, or at distal sites along the cough reflex pathway.
Consequences of airway nociceptor stimulation on cough
We expected and observed that sensitization of cough would manifest as an increased sensitivity to tussive stimuli and an increased intensity of the cough efforts (as measured by peak expiratory pressures). Increased expiratory pressures during cough could be due to recruitment of additional afferent nerves or enhanced synaptic efficacy along the reflex pathway. Topical citric acid, perfusing most of the extrathoracic trachea and probably activating many afferent nerves, produced coughs of greater intensity than the coughs evoked by electrical stimulation (which probably activates only a small, concentrated subset of tracheal afferents). Similarly, coughing evoked by stimulation voltages of ≤ 2 V in capsaicin- or bradykinin-challenged guinea-pigs were consistently smaller in magnitude than those evoked by stimulation voltages of ≥ 4 V. These differences could probably be attributed to recruitment of additional cough receptors and thus a greater afferent drive for cough. Also, with electrical stimulation, stimulus intensities required to evoke cough following sensitization were reduced 2–5-fold and yet peak expiratory pressures during cough were still increased. Peak expiratory pressures during cough induced by threshold concentrations of citric acid were also increased after bradykinin inhalation. These data suggest that CNS facilitation of cough receptor input occurs upon nociceptor stimulation.
Studies in cats and in dogs indicate that C-fibres may be inhibitory to the cough reflex (Tatar et al. 1988, 1994). These data contradict the present findings and could reflect species differences in regulation of cough or may reveal distinct and opposing roles for pulmonary and bronchial C-fibres in coughing. But it seems likely that the apparently conflicting results reflect important differences in experimental design. Tatar et al. (1988, 1994) gave capsaicin intravenously in a bolus, probably targeting pulmonary C-fibres, resulting in short but profound interruptions in respiration (apnoea). At the peak of these apneic episodes, subsequently evoked coughs were attenuated in both frequency and intensity. Under conditions where central respiratory drive is inhibited, it may not be surprising that cough is coincidentally suppressed. In contrast, we used capsaicin and bradykinin challenges that were near threshold for C-fibre activation and targeted primarily nociceptors of the large airways. Moreover, in most experiments we evoked cough 5–10 min after nociceptor stimulation, when respiratory rates had stabilized. That sensitization as opposed to inhibition of cough occurs several minutes after the initial nociceptor stimulation may indicate that a fundamental change in the excitability of nTS reflex pathway neurones (e.g. recruitment of NMDA receptors) occurs over time. This would be similar to the changes in reflex sensitivity observed in somatosensory pathways (Woolf & Thompson, 1991; Ma & Woolf, 1995; Yaksh et al. 1999; Svensson et al. 2003).
Although the results of the present study shed some light on potential mechanisms of enhanced cough sensitivity, it remains unclear why C-fibre-selective stimulants readily evoke cough in conscious animals and humans but consistently fail to do so following anaesthesia (Canning, 2002; Canning et al. 2004). Many C-fibre-mediated bronchopulmonary reflexes and C-fibre activation are readily evoked following anaesthesia, as is cough receptor-dependent cough. Perhaps C-fibres initiate the urge to cough and not the uncontrollable drive to coughing as might happen with aspiration. Alternatively, cough receptor activation may provide suprathreshold input to the cough reflex pathway whereas C-fibre activation is just threshold for coughing in conscious animals, and subthreshold during anaesthesia.
Role of tachykinins in the central processing of the cough reflex
We found that neurokinin receptor antagonists (CP99994, SR48968, SB223412, or ZD6021) administered by microinjection to the nTS, systemically (i.v.), centrally (i.c.v.) or directly to the tracheal perfusate had no effect on coughing evoked in control animals. This is not surprising, as coughing evoked from the tracheal mucosa of anaesthetized guinea-pigs is dependent upon activation of capsaicin-insensitive cough receptors (Canning et al. 2004). These vagal afferents do not express tachykinins. Joad et al. (2004) reported similar results. In contrast to the cough receptors, however, airway C-fibres do express the tachykinins (Riccio et al. 1996; Myers et al. 2002; Undem et al. 2004). Also not surprisingly, then, airway defensive reflexes in guinea-pigs initiated by C-fibre activation with capsaicin or bradykinin are mediated in large part by central release of these tachykinins (Bolser et al. 1997; Canning et al. 2001; Canning, 2002; Mazzone & Canning, 2002a; Myers et al. 2002; El-Hashim & Amine, 2005). Consistent with these previous studies of C-fibre-dependent reflexes in the airways and with many studies of central sensitization of somatic reflexes (Xu et al. 1992; Nagy et al. 1994; Ma & Woolf, 1995; Houghton et al. 2000), we found that neurokinin receptor antagonists prevent capsaicin-induced sensitization of coughing. We also found that nTS microinjection of substance P mimicked the effects of capsaicin on cough. Tachykinins induce sustained reductions in the activation threshold of spinal integrative neurones, leading to heightened reflex responses to mechanoreceptor activation (Krivoy et al. 1980; Ma & Woolf, 1995; Barbieri & Nistri, 2001). Tachykinins play a similar role in potentiating airway reflexes in guinea-pigs at the level of the brainstem (Mutoh et al. 2000; Mazzone & Canning, 2002a; Joad et al. 2004).
Although the results of the present study further support the hypothesis that tachykinins are uniquely involved in regulating reflexes initiated by airway C-fibre activation, they are by no means definitive. Subsets of airway C-fibres and capsaicin-sensitive nerves do not express tachykinins (Riccio et al. 1996; Undem et al. 2004) and some airway C-fibre-dependent effects in the CNS are mediated by glutamate (Mutoh et al. 2000). CP99994 or SB223412 administered i.c.v. prevented capsaicin-induced sensitization of coughing, but cnTS microinjection of ZD6021, a compound with nanomolar potencies at NK1, NK2 and NK3 receptors, failed to prevent sensitization of cough produced by tracheal capsaicin administration. ZD6021 mimics the effects of CP99994 and SB223412 on reflex bronchospasm, essentially abolishing the C-fibre-dependent parasympathetic reflexes evoked by capsaicin and bradykinin when administered i.v. or i.c.v., while having no effect on baseline cholinergic tone or histamine-evoked reflex bronchospasm (probably RAR-dependent reflexes; Canning et al. 2001; Mazzone & Canning, 2002a). Provided the cnTS is the primary site of tracheal C-fibre termination in the brainstem, the amount (1 nmol) of ZD6021 microinjected into cnTS in this study was probably sufficient, as it was 10 times the amount of SR140333, an NK1-selective antagonist, microinjected into nTS by Joad et al. (2004) to prevent cigarette smoke-induced sensitization of cough. Locations either within or outside of nTS in addition to cnTS probably regulate capsaicin-induced sensitization of cough. Alternatively, CP99994 and SB223412 may modulate the effects of tachykinin containing brainstem neurones that regulate respiration and/or C-fibre-dependent reflexes that are not dependent upon the nTS (Gray et al. 2001; Morgado-Valle & Feldman, 2004). Consistent with the latter scenario, i.c.v. CP99994 and SB223412 administered prior to capsaicin challenge induced abnormal respiratory patterns and reduced the peak expiratory pressures attained during coughing, but only following sensitization of cough with capsaicin. Centrally administered neurokinin receptor antagonists have similar effects on coughing in cats (Bolser et al. 1997). Regardless, the results of these experiments in no way diminish the strength of our conclusions regarding central synergistic interactions between airway afferent nerves in regulating reflex bronchospasm and cough. A better understanding of the neurochemistry and CNS termination sites of airway afferent nerve subtypes combined with parallel electrophysiological analyses will facilitate the design of hypothesis-driven experiments aimed at better defining the actions and sites of actions of tachykinins in mediating nociceptor-dependent sensitization of cough and reflex bronchospasm.
Implications of afferent nerve interactions in airways disease
The results presented may be relevant to chronic cough in disease. In healthy airways, coughing due to aspiration, inhaled particulates and accumulated mucus may be initiated directly by activation of the cough receptors. Under these circumstances the cough reflex is defensive in nature. But in airway diseases such as COPD and asthma and in extrapulmonary diseases like gastroesophageal reflux disease and allergic rhinitis, activation of normally quiescent nociceptors by acid or inflammatory mediators may sensitize the cough reflex via central synergistic interactions with cough receptors and perhaps RARs. These afferent nerve interactions and the resulting sensitization may increase coughing responses to tussigenic stimuli (Mazzone & Canning, 2002d). Therapeutics that can prevent nociceptor activation may thus prove useful in treating chronic cough.
Acknowledgments
This research was funded by grants from the National Institutes of Health, Bethesda, Maryland. Stuart Mazzone is a National Health and Medical Research Council of Australia CJ Martin Fellow (007188).
References
- Barbieri M, Nistri A. Depression of windup of spinal neurons in the neonatal rat spinal cord in vitro by an NK3 tachykinin receptor antagonist. J Neurophysiol. 2001;85:1502–1511. doi: 10.1152/jn.2001.85.4.1502. [DOI] [PubMed] [Google Scholar]
- Barnes NC, Piper PJ, Costello JF. Comparative effects of inhaled leukotriene C4, leukotriene D4, and histamine in normal human subjects. Thorax. 1984;39:500–504. doi: 10.1136/thx.39.7.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A, Shigemoto R. Cellular and subcellular distribution of substance P receptor immunoreactivity in the dorsal vagal complex of the rat and cat: a light and electron microscope study. J Comp Neurol. 1998;402:181–196. [PubMed] [Google Scholar]
- Bergren DR. Sensory receptor activation by mediators of defense reflexes in guinea-pig lungs. Respir Physiol. 1997;108:195–204. doi: 10.1016/s0034-5687(97)00030-3. [DOI] [PubMed] [Google Scholar]
- Bolser DC, DeGennaro FC, O'Reilly S, McLeod RL, Hey JA. Central antitussive activity of the NK1 and NK2 tachykinin receptor antagonists, CP-99,994 and SR 48968 in the guinea-pig and cat. Br J Pharmacol. 1997;121:165–170. doi: 10.1038/sj.bjp.0701111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham AC, Kott KS, Ravi K, Kappagoda CT, Joad JP. Substance P contributes to rapidly adapting receptor responses to pulmonary venous congestion in rabbits. J Physiol. 1996;493:229–238. doi: 10.1113/jphysiol.1996.sp021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ. Interactions between vagal afferent nerve subtypes mediating cough. Pulm Pharmacol Ther. 2002;15:187–192. doi: 10.1006/pupt.2002.0363. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557:543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Reynolds SM, Mazzone SB. Multiple mechanisms of reflex bronchospasm in guinea pigs. J Appl Physiol. 2001;91:2642–2653. doi: 10.1152/jappl.2001.91.6.2642. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin YH, Andresen MC. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J Neurosci. 2002;22:8222–8229. doi: 10.1523/JNEUROSCI.22-18-08222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan AW, Hope PJ, Jarrott B, Schaible HG, Fleetwood-Walker SM. Release, spread and persistence of immunoreactive neurokinin A in the dorsal horn of the cat following noxious cutaneous stimulation. Studies with antibody microprobes. Neuroscience. 1990;35:195–202. doi: 10.1016/0306-4522(90)90134-p. [DOI] [PubMed] [Google Scholar]
- El-Hashim AZ, Amine SA. The role of substance P and bradykinin in the cough reflex and bronchoconstriction in guinea-pigs. Eur J Pharmacol. 2005;513:125–133. doi: 10.1016/j.ejphar.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Farmer SG. The kallikrein-kinin system in asthma and acute respiratory distress syndrome. In: Farmer SG, editor. The Kinin System. New York: Academic; 1997. pp. 249–263. [Google Scholar]
- Fujimura M, Sakamoto S, Kamio Y, Matsuda T. Effects of methacholine induced bronchoconstriction and procaterol induced bronchodilation on cough receptor sensitivity to inhaled capsaicin and tartaric acid. Thorax. 1992;47:441–445. doi: 10.1136/thx.47.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson AR, Khan RW, Sarkar S, Furlong PL, Aziz Q. Development of esophageal hypersensitivity following experimental duodenal acidification. Am J Gastroenterol. 2004;99:813–820. doi: 10.1111/j.1572-0241.2004.04167.x. [DOI] [PubMed] [Google Scholar]
- Houghton AK, Ogilvie J, Clarke RW. The involvement of tachykinin NK2 and NK3 receptors in central sensitization of a spinal withdrawal reflex in the decerebrated, spinalized rabbit. Neuropharmacol. 2000;39:133–140. doi: 10.1016/s0028-3908(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Joad JP, Munch PA, Bric JM, Evans SJ, Pinkerton KE, Chen CY, Bonham AC. Passive smoke effects on cough and airways in young guinea pigs: role of brainstem substance P. Am J Respir Crit Care Med. 2004;169:499–504. doi: 10.1164/rccm.200308-1139OC. [DOI] [PubMed] [Google Scholar]
- Joos GF, Pauwels RA, Van Der Straeten ME. Effect of inhaled substance P and neurokinin A on the airways of normal and asthmatic subjects. Thorax. 1987;42:779–783. doi: 10.1136/thx.42.10.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajekar R, Proud D, Myers AC, Meeker SN, Undem BJ. Characterization of vagal afferent subtypes stimulated by bradykinin in guinea pig trachea. J Pharmacol Exp Ther. 1999;289:682–687. [PubMed] [Google Scholar]
- Karlsson JA, Fuller RW. Pharmacological regulation of the cough reflex – from experimental models to antitussive effects in Man. Pulm Pharmacol Ther. 1999;12:215–228. doi: 10.1006/pupt.1999.0207. [DOI] [PubMed] [Google Scholar]
- Krivoy WA, Couch JR, Stewart JM, Zimmermann E. Modulation of cat monosynaptic reflexes by substance P. Brain Res. 1980;202:365–372. doi: 10.1016/0006-8993(80)90148-1. [DOI] [PubMed] [Google Scholar]
- Kubin L, Davies RO. Central pathways of pulmonary and airway vagal afferents. In: Hornbein TF, editor. Regulation of Breathing. Vol. 79. New York: Marcel Dekker; 1995. pp. 219–284. [Google Scholar]
- Lang IM, Dean C, Medda BK, Aslam M, Shaker R. Differential activation of medullary vagal nuclei during different phases of swallowing in the cat. Brain Res. 2004;1014:145–163. doi: 10.1016/j.brainres.2004.03.061. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Involvement of neurokinin receptors in the induction but not the maintenance of mechanical allodynia in rat flexor motoneurones. J Physiol. 1995;486:769–777. doi: 10.1113/jphysiol.1995.sp020852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Takeda M, Saiki C, Takahashi T, Ojima K. Effects of tachykinins on rapidly adapting pulmonary stretch receptors and total lung resistance in anesthetized, artificially ventilated rabbits. J Pharmacol Exp Ther. 1997;283:1026–1031. [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ. Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am J Physiol Regulatory Integrative Comp Physiol. 2002a;283:R86–R98. doi: 10.1152/ajpregu.00007.2002. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ. An in vivo guinea pig preparation for studying the autonomic regulation of airway smooth muscle tone. Auton Neurosci. 2002b;99:91–101. doi: 10.1016/s1566-0702(02)00053-x. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ. Central nervous system control of the airways: pharmacological implications. Curr Opin Pharmacol. 2002c;2:220–228. doi: 10.1016/s1471-4892(02)00151-0. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ. Plasticity of the cough reflex. European Respiratory Rev. 2002d;12:236–242. [Google Scholar]
- Mazzone SB, Geraghty DP. Respiratory action of capsaicin microinjected into the nucleus of the solitary tract: involvement of vanilloid and tachykinin receptors. Br J Pharmacol. 1999;127:473–481. doi: 10.1038/sj.bjp.0702522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin SW. Convergent carotid sinus nerve and superior laryngeal nerve afferent inputs to neurons in the NTS. Am J Physiol. 1996;271:R870–R880. doi: 10.1152/ajpregu.1996.271.4.R870. [DOI] [PubMed] [Google Scholar]
- Morgado-Valle C, Feldman JL. Depletion of substance P and glutamate by capsaicin blocks respiratory rhythm in neonatal rat in vitro. J Physiol. 2004;555:783–792. doi: 10.1113/jphysiol.2003.060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh T, Bonham AC, Joad JP. Substance P in the nucleus of the solitary tract augments bronchopulmonary C fiber reflex output. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1215–R1223. doi: 10.1152/ajpregu.2000.279.4.R1215. [DOI] [PubMed] [Google Scholar]
- Myers AC, Kajekar R, Undem BJ. Allergic inflammation-induced neuropeptide production in rapidly adapting afferent nerves in guinea pig airways. Am J Physiol Lung Cell Mol Physiol. 2002;282:L775–L781. doi: 10.1152/ajplung.00353.2001. [DOI] [PubMed] [Google Scholar]
- Nagy I, Miller BA, Woolf CJ. NK1 and NK2 receptors contribute to C-fibre evoked slow potentials in the rat spinal cord. Neuroreport. 1994;5:2105–2108. doi: 10.1097/00001756-199410270-00029. [DOI] [PubMed] [Google Scholar]
- Paton JF. Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fiber stimulation in the mouse. J Neurophysiol. 1998;79:2365–2373. doi: 10.1152/jn.1998.79.5.2365. [DOI] [PubMed] [Google Scholar]
- Patterson LM, Zheng H, Ward SM, Berthoud HR. Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res. 2003;311:277–287. doi: 10.1007/s00441-002-0682-0. [DOI] [PubMed] [Google Scholar]
- Riccio MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segre-gation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol. 1996;496:521–530. doi: 10.1113/jphysiol.1996.sp021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Hobson AR, Furlong PL, Woolf CJ, Thompson DG, Aziz Q. Central neural mechanisms mediating human visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1196–G1202. doi: 10.1152/ajpgi.2001.281.5.G1196. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary res-piratory network and a model of cough motor pattern generation. J Appl Physiol. 1998;84:2020–2035. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- Silva-Carvalho L, Paton JF, Rocha I, Goldsmith GE, Spyer KM. Convergence properties of solitary tract neurons responsive to cardiac receptor stimulation in the anesthetized cat. J Neurophysiol. 1998;79:2374–2382. doi: 10.1152/jn.1998.79.5.2374. [DOI] [PubMed] [Google Scholar]
- Suwanprathes P, Ngu M, Ing A, Hunt G, Seow F. c-Fos immunoreactivity in the brain after esophageal acid stimulation. Am J Med. 2003;115(Suppl. 3A):31S–38S. doi: 10.1016/s0002-9343(03)00190-6. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- Tatar M, Sant'Ambrogio G, Sant'Ambrogio FB. Laryngeal and tracheobronchial cough in anesthetized dogs. J Appl Physiol. 1994;76:2672–2679. doi: 10.1152/jappl.1994.76.6.2672. [DOI] [PubMed] [Google Scholar]
- Tatar M, Webber SE, Widdicombe JG. Lung C-fibre receptor activation and defensive reflexes in anaesthetized cats. J Physiol. 1988;402:411–420. doi: 10.1113/jphysiol.1988.sp017212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wank M, Neuhuber WL. Local differences in vagal afferent innervation of the rat esophagus are reflected by neurochemical differences at the level of the sensory ganglia and by different brainstem projections. J Comp Neurol. 2001;435:41–59. doi: 10.1002/cne.1192. [DOI] [PubMed] [Google Scholar]
- Widdicombe JG. Respiratory reflexes from the trachea and bronchi of the cat. J Physiol. 1954;123:55–70. doi: 10.1113/jphysiol.1954.sp005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JG. Afferent receptors in the airways and cough. Respir Physiol. 1998;114:5–15. doi: 10.1016/s0034-5687(98)00076-0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-d-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Dalsgaard CJ, Wiesenfeld-Hallin Z. Spinal substance P and N-methyl-d-aspartate receptors are coactivated in the induction of central sensitization of the nociceptive flexor reflex. Neuroscience. 1992;51:641–648. doi: 10.1016/0306-4522(92)90303-j. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Hua X-Y, Kalcheva I, Nozaki-Taguchi N, Marsala M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc Nat Acad Sci U S A. 1999;96:7680–7686. doi: 10.1073/pnas.96.14.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Torri C, Ferrari R, Jansson A, Zini I, Fuxe K, Agnati LF. The emergence of the volume transmission concept. Brain Res Brain Res Rev. 1998;26:136–147. doi: 10.1016/s0165-0173(97)00048-9. [DOI] [PubMed] [Google Scholar]