Abstract
The main active-site loop of the copper-binding protein azurin (a cupredoxin) has been shortened from C112TFPGH117SALM121 to C112TPH115PFM118 (the native loop from the cupredoxin amicyanin) and also to C112TPH115PM117. The Cu(II) site structure is almost unaffected by shortening, as is that of the Cu(I) center at alkaline pH in the variant with the C112TPH115PM117 loop sequence. Subtle spectroscopic differences due to alterations in the spin density distribution at the Cu(II) site can be attributed mainly to changes in the hydrogen-bonding pattern. Electron transfer is almost unaffected by the introduction of the C112TPH115PFM118 loop, but removal of the Phe residue has a sizable effect on reactivity, probably because of diminished homodimer formation. At mildly acidic pH values, the His-115 ligand protonates and dissociates from the cuprous ion, an effect that has a dramatic influence on the reactivity of cupredoxins. These studies demonstrate that the amicyanin loop adopts a conformation identical to that found in the native protein when introduced into azurin, that a shorter than naturally occurring C-terminal active-site loop can support a functional T1 copper site, that CTPHPM is the minimal loop length required for binding this ubiquitous electron transfer center, and that the length and sequence of a metal-binding loop regulates a range of structural and functional features of the active site of a metalloprotein.
Keywords: copper proteins, electron transfer, metalloproteins, protein engineering
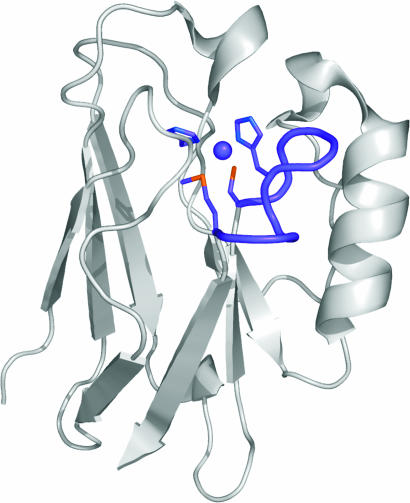
Numerous approaches are being used to design metal-binding sites in proteins (1), with many of these studies informed by an understanding of the basic structural requirements for biological metal centers. Metal-binding sites in proteins are commonly formed from loops, because these regions are reasonably tolerant to sequence modifications outside of the coordinating residues (1). Cupredoxins are copper-containing electron transfer (ET) proteins that provide a significant challenge for protein-design experiments (2–4) because their scaffold is thought to constrain the metal site structure (5). In the type 1 (T1) copper sites of cupredoxins (see Fig. 1), three of the four canonical ligands Cys, His, and, usually, Met are present on a loop linking the C-terminal strands of a rigid β-barrel (7, 8). The fourth ligand, a His, is donated from a β-strand more in the core of the fold (see Fig. 1). The lengths of the metal-binding loops in known cupredoxins range from 7 to 16 residues and have a variety of primary structures. These proteins, therefore, provide a suitable system for investigating the importance of loop length and structure for the active-site integrity of a metalloprotein. Loop-directed mutagenesis has been used to swap loops between different cupredoxins, giving sites with authentic T1 properties (8–12). In this work, we present studies that have been aimed at assessing the structural consequences of shortening the active-site loop of a cupredoxin and have determined the shortest C-terminal loop sequence required for a functional T1 copper site.
Fig. 1.
The structure of AZ, with the C-terminal ligand-containing loop and the copper ion shown in purple. The side chains of the Cys-112, His-117, and Met-121 ligands on this loop and also of His-46 are shown as stick models in purple. The backbone carbonyl oxygen of Gly-45, which provides the second weak axial interaction at the copper site, and the helical nature of the His-117-to-Met-121 sequence are omitted for clarity. The figure was prepared by using the program pymol (6).
The cupredoxin azurin (AZ) was carefully chosen for these studies because of its stability and ease of crystallization and its being a structurally well characterized member of this class of protein (13–16). The T1 copper site of AZ involves strong coordination by the thiolate sulfur of Cys-112 and the Nδ1 nitrogens of the His-46 and His-117 ligands (see Fig. 1). The metal is in the plane of these three equatorial ligands and has two additional axial interactions with the thioether sulfur of Met-121 and the backbone carbonyl oxygen of Gly-45, giving a trigonal bipyramidal active-site geometry. The Cys-112, His-117, and Met-121 ligands are situated on the loop (C112TFPGH117SALM121) linking β-strands 7 and 8. This loop can be replaced (11) with the sequence CTPHPFM [the shortest naturally occurring loop from amicyanin (AMI)], giving a T1 copper site with authentic properties [including a S(Cys) → Cu(II) ligand-to-metal charge transfer transition at ≈600 nm and a small hyperfine coupling constant in the EPR spectrum (17)]. The structure of the Cu(II) form of the AZAMI variant is presented here that has led to studies aimed at investigating the effect of further shortening the C-terminal ligand-containing loop. The properties and structure of an AZ variant possessing the loop sequence CTPHPM provide an intriguing insight into the basic requirements for a functional active site.
Results
Protein Purification.
The molecular mass of AZAMI-F (loop sequence C112TPH115PM117), determined by Fourier-transform ion cyclotron resonance mass spectrometry, is 13,564.6 Da (theoretical mass = 13,565.3 Da with disulfide). The AZAMI-T variant (loop sequence C112PH114PFM117) is expressed in Escherichia coli in reasonably high amounts. This variant does not bind copper and has a very broad shoulder at 292 nm in its UV/visible (vis) spectrum compared with AZ and AZAMI-F (see Fig. 5, which is published as supporting information on the PNAS web site). This distinctive feature arises from the environment of Trp-48, and its appearance in AZAMI-T indicates incorrect folding (18). MALDI-TOF mass spectrometry identified a mass of 13,615 Da for AZAMI-T (theoretical mass = 13,614 Da) under reducing conditions [by using Tris(2-carboxyethyl)phosphine hydrochloride], which was not present in the absence of reductant. AZAMI-T does not fold correctly, giving disulfide-linked multimeric species.
UV/vis, EPR, and Paramagnetic NMR.
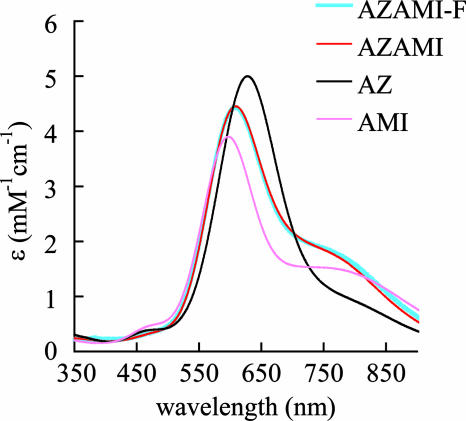
The UV/vis spectra of AZAMI-F, AZAMI, AZ, and AMI, are shown in Fig. 2. For AZAMI-F, the main S(Cys) → Cu(II) ligand-to-metal charge transfer transition is at 608 nm (ε = 4,400 M−1·cm−1, see Table 1), whereas this band is at 609 nm (ε = 4,500 M−1·cm−1), 628 nm (ε = 5,100 M−1·cm−1), and 596 nm (ε = 3,900 M−1·cm−1) in AZAMI, AZ, and AMI, respectively (11). The EPR spectra of AZAMI-F, AZAMI, AZ, and AMI are shown in Fig. 6, which is published as supporting information on the PNAS web site, and the parameters are listed in Table 1. The spectrum of AZAMI-F is very similar to that of AZAMI, which exhibits subtle alterations compared with AZ. The gz values of AZAMI-F, AZAMI, and AMI are lower than that for AZ, and the Az values of AZAMI-F and AZAMI are higher than those of AZ and AMI. The paramagnetic 1H NMR spectrum of AZAMI-F is compared with those of AZAMI (11) and AZ (24) in Fig. 8, which is published as supporting information on the PNAS web site, and has been assigned by using saturation-transfer difference experiments on 1:1 mixtures of oxidized and reduced protein (see Fig. 9 and Table 2, which are published as supporting information on the PNAS web site). In AZAMI-F, the CγH proton resonances from the axial Met ligand are shifted outside of the diamagnetic region of the spectrum, which is not the case in AZ (in AZAMI one of these is observed).
Fig. 2.
UV/vis spectra of AZAMI-F compared with those of AZAMI, AZ, and AMI. Spectra were obtained at 25°C in 20 mM Tris, pH 8.0, for AZAMI-F and in 10 mM phosphate, pH 8.0, for the other three proteins.
Table 1.
Properties of AZAMI-F compared with those of AZAMI, AZ, and AMI
| Parameter | AZAMI-F | AZAMI | AZ | AMI |
|---|---|---|---|---|
| UV/vis* | ||||
| λmax1, nm | 470 (sh)† | 470 (sh)† | 460 | 460 |
| λmax2, nm | 608 | 609 | 628 | 596 |
| ε≈600, mM−1·cm−1 | 4.4 | 4.5 | 5.1 | 3.9 |
| A≈460/A≈600 | 0.08 | 0.07 | 0.07 | 0.11 |
| EPR‡ | ||||
| gx | 2.041 | 2.033 | 2.035 | 2.032 |
| gy | 2.046 | 2.048 | 2.054 | 2.047 |
| gz | 2.232 | 2.225 | 2.261 | 2.235 |
| Ax, mT | 1.0 | 1.1 | 1.4 | 0.6 |
| Ay, mT | 0.8 | 1.0 | 1.4 | 0.8 |
| Az, mT | 6.1 | 6.9 | 5.3 | 5.4 |
| Other | ||||
| δobs Asn-47 CαH§ | 17.8 | 17.5 | 19.0 | 14.1 |
| pKared (CV)¶ | 5.9 | 5.5 | <2‖ | 6.4** |
| Em, mV (CV)†† | 306 | 261 | 295 | 255** |
| kese, M−1·s−1‡‡ | 1.4 × 104 | 5.5 × 105 | 2.0 × 106 | 1.3 × 105 |
*Measured in 20 mM Tris, pH 8.0 for AZAMI-F and 10 mM phosphate at pH 8.0 for the other proteins (all at 25°C).
†(sh), shoulder.
‡Recorded at −196°C in 25 mM Hepes at pH 7.6 plus 40% glycerol. All the EPR parameters were derived from simulations (for AZAMI-F, see Fig. 7, which is published as supporting information on the PNAS web site) using the program simfonia (Bruker).
§The observed chemical shift (in ppm) of the Asn-47 CαH proton resonance (Asn-55 CαH in AMI) (19), which exhibits a sizable paramagnetic shift as the backbone NH of this residue hydrogen bonds to the thiolate sulfur of the Cys ligand.
¶For the C-terminal His ligand in the Cu(I) proteins measured by cyclic voltammetry (CV).
‖An estimated upper limit from ref. 20.
**Taken from ref. 21.
††All measured at pH 7.5.
Reduction Potentials.
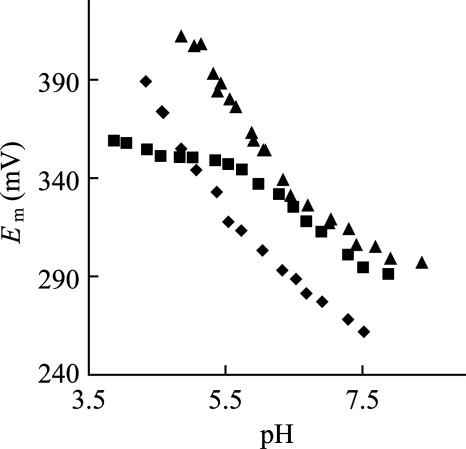
AZAMI-F yields good quasireversible responses on a modified gold electrode in the pH range ≈8.5–5. The reduction potential (Em) of AZAMI-F at pH 7.5 is 306 mV, which compares to values of 261 mV, 295 mV, and 255 mV (all at pH 7.5) for AZAMI, AZ, and AMI, respectively (see Table 1). The Em of AZAMI-F increases with decreasing pH, and, in the range 6.5–5, a feature with a slope of −60 mV/pH is present that does not occur in the WT protein (see Fig. 3). This observation is consistent with reduction in this pH range being accompanied by the uptake of a proton at the active site (protonation of the C-terminal His-115 ligand) (21, 22, 25–29). The pH-dependence of the difference in the Em values for AZAMI-F and AZ in the pH range 7–5 was fit (for the equation used and the fit obtained, see Fig. 10, which is published as supporting information on the PNAS web site), giving a pKa of 5.9 for His-115 in the Cu(I) protein. The His-117 ligand in AZ does not protonate in the accessible pH range [its pKa has been calculated to be <2 in the Cu(I) protein] (20). A pKa of 5.5 has been determined for His-115 in Cu(I) AZAMI (11).
Fig. 3.
Dependence on pH (I = 0.10 M, NaCl) of the Em of AZAMI-F (▴). Also shown are the data for AZ (■) and AZAMI (♦). All of the Em values are referenced to the NHE at 22°C. In AZ, it is known that the pH-dependence of Em in the 6–8 range is due to the protonation/deprotonation of the noncoordinated His-35 and His-83 residues, and AZAMI-F and AZAMI display a similar behavior (the pKa values of His-35 and His-83 are analogous to those in the WT protein).
Electron Self-Exchange Reactivity.
The electron self-exchange (ESE) rate constant (kese) of AZAMI-F has been determined at 40°C by using 1H NMR spectroscopy. The influence of oxidized protein concentration on the T2−1 values of active-site resonances arising from the Cu(I) protein in the WEFT spectra has been analyzed (see Fig. 11, which is published as supporting information on the PNAS web site), yielding kese values ranging from 1.2 × 104 M−1·s−1 to 1.8 × 104 M−1·s−1. The average kese value is 1.4 × 104 M−1·s−1, which is confirmed by the coalescence behavior of the Val-22 CγH3 resonance in a 1:1 mixture of Cu(II) and Cu(I) proteins. The kese value for AZ under comparable conditions (40°C, pH 9.0) is 2.0 × 106 M−1·s−1 (see Table 1) (23), with AZAMI having similar reactivity (see Table 1) (11).
Crystal Structures of Oxidized AZAMI and AZAMI-F.
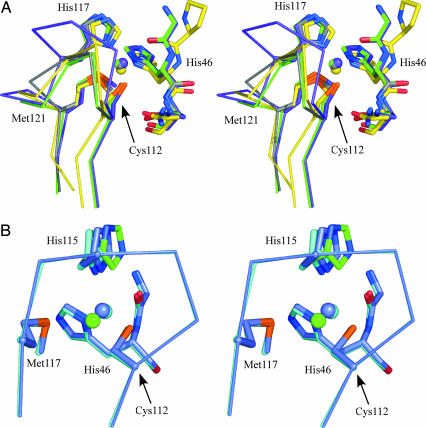
The overall structures of AZAMI and AZAMI-F are very similar to that of the WT protein, as demonstrated by the fact that the Cα atoms superimpose with rms deviations (rmsds) of 0.43 Å and 0.54 Å [when 4AZU (15) is used, with 118 and 117 equivalent Cα atoms considered, respectively] and 0.54 Å and 0.70 Å [with 1JZF (16), with the same overlaid residues as for the comparisons to 4AZU]. The Cα atoms of AZAMI and AZAMI-F superimpose with an rmsd of 0.29 Å, when 104 equivalent Cα atoms are used. The positions of the β-strands are virtually unchanged by the loop contractions and, of particular note, is the fact that β7 and β8 show remarkable homology. The loop linking these two β-strands (see Figs. 1 and 4A) runs from the Cys-112 ligand to the coordinating Met-121 in AZ, with the sequence C112TFPGH117SALM121 (the central His-117 is also coordinated to the metal). In AZ, the loop sequence from His-117 to Met-121 forms a single turn of α-helix. In AZAMI and AZAMI-F, the loop sequences are C112TPH115PFM118 (analogous to the sequence C92TPH95PFM98 found in AMI) (30) and C112TPH115PM117, respectively (see Fig. 4A), and display no helical content. The copper-binding loop of AZAMI is almost identical to that of AMI (see Fig. 4A), except that the orientation of the Thr on the loop is different because of a hydrogen-bonding interaction of the side chain of this residue with the backbone CO of Tyr-72 in AZAMI (and AZ and AZAMI-F), whereas, in AMI, the Oγ of the corresponding Thr-93 hydrogen bonds with the His-56 Nδ1 atom (see Table 3, which is published as supporting information on the PNAS web site).
Fig. 4.
Stereoview of active sites. (A) Overlay of the active sites of Cu(II) AZAMI (green), AZAMI-F (gray), AZ (purple), and AMI (yellow). The side chains of the coordinating residues and the amino acids on either side of the N-terminal His ligand are shown as stick models, copper atoms as spheres, and the backbone of the C-terminal ligand-containing loops as Cα traces. The residues are labeled as in AZ. (B) Overlay of the active sites of the Cu(I) form of AZAMI-F at pH 8 (slate) and pH 6 (cyan) is shown. The second conformation of the copper ion and His-115 at pH 6 are colored green. The figure was prepared by using the program pymol (6).
Significant alterations in the hydrogen-bonding pattern around the active site result from the AZAMI and AZAMI-F loop contractions (see Table 3). A number of key hydrogen bonds found in AZ are still present in AZAMI and AZAMI-F, such as those between the backbone CO of His-115/117 and the backbone NH of Met-117/118/121 and between the backbone NH of Cys-112 and the backbone CO of Met-117/118/121 and also the three interactions involving Thr-113. A hydrogen bond to the thiolate sulfur of the Cys ligand is made by the backbone amide of Asn-47 in AZ, which is preserved in AZAMI and AZAMI-F and is a conserved structural feature of all cupredoxins (7). In AZ, a hydrogen bond exists between the backbone amide of Phe-114 and the thiolate sulfur of the Cys-112 ligand. This hydrogen bond is missing in AZAMI and AZAMI-F (and AMI) because of a Pro residue being found at position 114. The backbone CO of Phe-114 makes a hydrogen bond to the backbone NH of His-117 in AZ, which is absent in the loop contraction variants but is replaced by a weak hydrogen bond (3.3 Å) between the backbone CO of Cys-112 and the backbone NH of His-115 in AZAMI [that is even weaker in AZAMI-F (3.7 Å)]. The NH of His-115 may form a weak hydrogen bond with the thiolate sulfur of Cys-112 in AZAMI (3.7 Å) that is even weaker in AZAMI-F (4.1 Å) and is not present in AZ. In summary, the number of hydrogen bonds around the active-site loop is decreased by making the AZAMI mutation and is almost identical to the number found in AMI. The hydrogen-bonding pattern is similar in AZAMI-F and AZAMI.
Considering the dramatic loop contractions performed and their impact on the active-site hydrogen-bonding pattern, the Cu(II) centers of AZAMI and AZAMI-F are both remarkably similar to that found in AZ (Fig. 4A; and see Table 4, which is published as supporting information on the PNAS web site). The most significant effect is an increase in the distance to the copper from the backbone carbonyl oxygen of Gly-45 in both AZAMI and AZAMI-F. In AZ, the imidazole ring of the His-117 ligand is flanked on one side by Phe-114 and on the other by Met-13. In AZAMI and AZAMI-F, the interaction of the Met-13 side chain with His-115 is retained, but the phenyl ring on the opposite side is absent, as Phe-114 is replaced by a Pro. Although the position of the metal-coordinating Nδ1 atom is almost identical in all of the proteins, the overall orientation of the side chain of His-115 is quite different in AZAMI and AZAMI-F compared with AZ. There are also subtle differences between AZAMI and AZAMI-F. These alterations are most likely imposed by the different structures of the loops. The copper in the AZAMI structure is more solvent-exposed than in AZ (13–16), to a similar extent as in AMI (30). The active site of AZAMI-F appears to be slightly more buried than those of AZAMI and AMI. A water molecule occupies the space created by the removal of Phe-114 in AZAMI and AZAMI-F, which hydrogen bonds to the backbone carbonyl oxygen of Gly-45. A similar interaction between a water and the backbone carbonyl oxygen of Pro-52 is found in AMI (30). In AZ, AZAMI, and AZAMI-F, the Nε2 of the exposed His ligand is hydrogen bonded to a water molecule (that bridges with the backbone carbonyl O of Val-43). In AZ, the hydrophobic side chain of Leu-120 sits on one side of the Met-121 ligand (the phenyl ring of Phe-15 is located on the opposite side of the thioether moiety). In AZAMI, the phenyl group of Phe-117 replaces the side chain of the Leu residue (Phe-97 in AMI), and the Met ligand is positioned between two phenyl groups. Removal of this Phe residue results in the Pro-116 side chain of AZAMI-F, partly filling the cavity left by the deleted residue (the position of this Pro is completely different from that of Pro-96 in AMI).
The Structure of Cu(I) AZAMI-F at pH 8 and 6.
Reduction of AZAMI-F at pH 8 results in very little change in structure [the Cα atoms superimpose on those of the Cu(II) protein with an rmsd of 0.12 Å when all equivalent Cα atoms are used]. At the copper site, there is very little difference, with the only significant alteration being a 0.19-Å increase in the Cu–N(His-115) bond length (see Table 4). These active-site changes upon redox interconversion are only slightly larger than those observed in AZ. The loop structure is virtually identical in both the oxidized and reduced forms.
The overall structure of the reduced protein at pH 6 shows no differences from that at pH 8 (the Cα atoms superimpose, with an rmsd of 0.08 Å when equivalent Cα atoms are used). However, at the active site, dramatic alterations are observed (see Fig. 4B and Table 4). The best fit of the electron density for the copper and His-115 involves modeling two conformations for each (with 50% occupancy). One of these conformations gives an active-site geometry very similar to that for the high-pH Cu(I) form. The second conformation involves movement of His-115 away from the cuprous ion and an ≈180° rotation of its imidazole ring. The metal to Gly-45(O) distance increases, whereas the Cu(I)–S(Met-117) distance decreases, giving a trigonal planar Cu(I) site.
Discussion
The overall structures of AZAMI and AZAMI-F are remarkably similar to that of AZ. Shortening the loop linking β7 and β8 modifies the active-site environment and dramatically decreases the number of hydrogen bonds. Key hydrogen bonds remain, and a couple of new ones, reflecting those found in AMI, are introduced. The loop structure in AZAMI is almost identical to that of AMI, indicating that the cupredoxin scaffold does not influence the arrangement of the loop, probably a consequence of the short AMI loop and the presence of Pro residues on either side of the central His ligand that constrain the structure. The active-site geometries of Cu(II) AZAMI and AZAMI-F are remarkably similar to that of AZ. The only minor modification is a lengthening of the Cu-to-Gly-45(O) distance, which is mainly due to movement of the copper away from this residue, resulting in its being further displaced from the plane of the three equatorial ligands. The limited, and almost identical, influence of the AZAMI and AZAMI-F mutations on the spectroscopic properties of AZ (see Table 1) indicates that these changes are not directly related to loop length and are more likely caused by alterations in the hydrogen-bonding arrangement around the active site. In particular, the AZAMI and AZAMI-F mutations both result in the removal of one of the two hydrogen bonds to the thiolate sulfur of the Cys ligand (from the NH of Phe-114, see Table 3). Similar effects are observed in the Phe114Pro AZ variant and can be attributed to increased spin density on the copper and also the axial Met ligand (the CγH proton resonances exhibit larger δc values in both AZAMI and AZAMI-F). The shorter Cu–S(Cys-112) distance and the removal of one of the hydrogen bonds to the coordinating thiolate would be expected to increase spin density on the Cys ligand (31). The smaller gz value in AZAMI-F (and AZAMI) is more similar to that of AMI and could result from the decreased interaction of Cu(II) with the backbone carbonyl of Gly-45.
The relatively small influence on the active-site structure of the introduction of the shortest naturally occurring ligand-containing loop of AMI into AZ demonstrates that a large proportion of the native loop is not actually required for a stable (and functional, see below) active site. Further shortening gives rise to smaller loop sequences than those that have evolved in cupredoxins. The properties of the AZAMI-T variant demonstrate that the Thr residue in the AZAMI loop is essential for stabilizing the active-site structure (this observation is not restricted to only AZ, because shortening the loop in the cupredoxin plastocyanin to CPHPM failed to produce a T1 copper-binding variant), whereas the properties of the AZAMI-F show that the Phe is not required, most likely a result of the strong hydrogen bonds around the active site contributed by the backbone and side chain of the Thr residue that stabilize part of the loop structure in AZ, AMI, AZAMI, and AZAMI-F (Table 3). In contrast, the main-chain atoms of Phe-117 in AZAMI (which are removed in the AZAMI-F variant) and Phe-97 in AMI are not involved in any strong hydrogen bonds (the main chain of the corresponding Leu-120 residue in AZ is involved in two hydrogen bonds in the loop region). Thus, it appears that the Thr is essential but that the Phe is dispensable. The two Pro residues in the AMI loop appear to be necessary to enable connection of the β-strands while placing the His residue in the correct location (along with the Cys and Met) to bind copper (a residue between the Thr and the His ligand is also essential for the hydrogen-bonding pattern of the former). These experiments demonstrate that CTPHPM is probably the shortest loop sequence that can both link β7 and β8 of a cupredoxin domain while also accommodating and stabilizing a T1 copper site. The question then arises as to why nature has chosen to use longer loops in all cupredoxins, and, in some cases, many more residues (up to 16) are found. This feature can be appreciated if the functional characteristics of AZAMI-F and AZAMI are considered.
It is quite remarkable that the Em value, one of the most important features of an ET protein, is almost unaltered by making the AZAMI-F mutation (a slightly larger effect is seen in the AZAMI variant). The subtle geometric alterations and the different solvent accessibilities of the copper sites observed upon loop contraction would be expected to exert some influence on Em. Furthermore, the dramatic hydrogen-bonding differences should give rise to sizable alterations in Em (32). A number of other factors are important for tuning the Em of a T1 copper site, including additional attributes of their constrained active sites and the electrostatic environment of the protein (5, 32, 33). Many active-site features have been altered by the drastic loop contractions performed, and work is underway to assess their relative influence on Em. Regardless of the reasons for AZAMI-F and AZ having almost identical Ems, these studies demonstrate that the long C-terminal active-site loop of AZ is not required to give a value of ≈300 mV, which is of paramount importance for the redox function of this protein.
The intrinsic ET reactivity of AZAMI-F is two orders of magnitude smaller than that of AZ and AZAMI. The limited changes in the active-site structure of the Cu(II) and Cu(I) forms (the latter at pH 8) indicate that an increased inner-sphere reorganization energy, which is usually very low for T1 copper sites (5, 34, 35), is not the main cause of this effect (the total reorganization energy may have increased). The diminished kese is probably due to decreased homodimer formation resulting from shortening the surface-exposed C-terminal loop (the electronic-coupling matrix element for electron self-exchange (ESE) could have decreased in AZAMI-F). The C-terminal His ligand of AZ is surrounded by a large hydrophobic patch that is the recognition site for ESE (36), and this region is influenced by the loop contractions. The ET reactivity of AZAMI is similar to that of AZ (11), and, thus, the diminished loop in this variant does not influence protein association. This observation could be due to the introduction of the Phe-117 residue in place of the hydrophobic side chains of Ala-119 and Leu-120. The removal of this Phe residue in AZAMI-F seems to have a significant impact on homodimer formation. The lower intrinsic reactivity of AZAMI-F will also influence interactions with physiological partners, although these are not known for AZ (37).
Reduction under mildly acidic conditions results in the His-115 ligand of AZAMI-F protonating and dissociating from the copper. The cuprous ion moves away from the backbone carbonyl oxygen of Gly-45 toward the thioether sulfur of Met-117. The copper ion lies almost in the plane of the Cys, Met, and His-46 ligands, i.e., has a trigonal planar geometry. This effect influences the Em of AZAMI-F [the trigonal planar geometry favors Cu(I)], and, from the pH-dependence of this parameter, a pKa of 5.9 is obtained for the His-115 ligand in the Cu(I) protein. Similar behavior has been observed in plastocyanin (25, 26), pseudoazurin (27, 28), and AMI (21, 22, 29) and is thought to have physiological relevance (26, 29). Loop mutation experiments have shown that, in most cases, lengthening the ligand-containing loop lowers the His ligand pKa (9, 10, 12, 38), whereas loop contraction increases the value (8, 11, 12). In AZ, the His-117 ligand does not protonate in the accessible pH range, and a pKa of <2 has been calculated (20). In the Cu(I) AZAMI loop-contraction variant, the pKa of His-115 is 5.5 [this pKa is lower than the value of 6.4 for His-96 in Cu(I) AMI] (21, 38). Further shortening of the loop leads to an additional increase in the pKa. The structures of AZAMI and AZAMI-F demonstrate that their active-site structures, including hydrogen-bonding patterns, are very similar, and, thus, His ligand protonation must be influenced by the length of the loop [probably an entropic effect (38, 39)]. The increase in the pKa of the His ligand upon shortening this loop results in a value closer to physiological pH and is, thus, more likely to have a functional role.
Conclusions
When the shorter loop of AMI is introduced into AZ, this region adopts a conformation almost identical to that found in AMI. A smaller than naturally occurring ligand-containing loop can be used to link the C-terminal β-strands of a cupredoxin domain with retention of a functional T1 copper site (AZAMI-F). Surprisingly, these loop contractions have very little effect on Em. The intrinsic ET reactivity is almost unaffected by introducing the AMI loop into AZ but is decreased by two orders of magnitude when a nonnative loop is used. This lower reactivity is most likely due to alterations in the protein's hydrophobic patch that disfavor homodimer formation. Loop-shortening results in an increase in the pKa of the C-terminal His ligand and, thus, provides a way to tune this physiologically relevant effect. The sequence CTPHPM seems to be the shortest loop that can maintain both the structural integrity and the functionality of an electron-transferring biological copper site.
Materials and Methods
Loop-Contraction Mutagenesis.
Loop-contraction mutagenesis was carried out by using the QuikChange (Stratagene) site-directed mutagenesis kit. pTrcAZ, a pTrc99a derivative harboring the gene from Pseudomonas aeruginosa AZ, including the signal peptide, was used as the template (11). The Cys-112-to-Met-121 loop of AZ was mutated from C112TFPGH117SALM121 to C112TPH115PM117 by using the following primers: catgttcttctgcaccccgcacccgatgaagggcacc (forward) and ggtgcccttcatcgggtgcggggtgcagaagaacatg (reverse), to give AZAMI-F. The Cys-112-to-Met-121 loop of AZ was also mutated to C112PH114PFM117 by using the following primers: catgttcttctgcccgcacccgttcatgaagggcacc (forward) and ggtgcccttcatgaacgggtgcgggcagaagaacatg (reverse), to give the AZAMI-T variant. Both strands of the mutated plasmids (pTrcAZAMI-F and pTrcAZAMI-T) were sequenced to verify the mutations.
Cell Growth, Protein Isolation, and Purification.
E. coli JM101 (pTrcAZAMI, pTrcAZAMI-F, and pTrcAZAMI-T) were grown and the periplasmic proteins obtained as described in ref. 11. Isolation and purification of AZAMI-F were carried out as described for AZAMI (11). Pure oxidized AZAMI-F (≈10 mg/liter of cell culture) with an A280/A608 ratio of ≤2.0 gave a single band on a 15% SDS/PAGE gel.
UV/vis Spectrophotometry and Extinction Coefficients.
UV/vis spectra were measured at 25°C on a PerkinElmer λ 35 spectrophotometer. To establish molar absorption coefficients (ε values), the copper concentration was determined by using an M Series Atomic Absorption spectrometer (AAS) (Thermo Electron, Cambridge, U.K.). The protein was fully oxidized and washed with 0.5 mM EDTA to remove any adventitiously bound metal. After the excess oxidant and EDTA were removed by using ultrafiltration, a UV/vis spectrum was acquired and the copper concentration determined by AAS using copper standards in the range of 0.5–2 ppm Cu(II) made from a stock standard (Aldrich). The absence of Zn(II) was verified by AAS using standards (Aldrich) in the range 0.25–1 ppm.
EPR Spectroscopy.
X-band EPR spectra were recorded at −196°C on a Bruker EMX spectrometer, as described in ref. 11.
Protein Electrochemistry.
The direct measurement of the Em of AZAMI-F was carried out at ambient temperature (22 ± 1°C) by using an electrochemical approach described in ref. 11. The gold working electrode was modified by using either a 1 M solution of 2-diethylaminoethanethiol for the pH range 7.0–8.5 or a saturated solution of 4,4-dithiodipyridine (Aldrithiol-4) for pH <7.0.
Protein Samples for 1H NMR Spectroscopy.
The protein was usually either fully reduced, fully oxidized, or contained ≈1:1 Cu(II):Cu(I) protein (40). The excess reductant/oxidant was removed and the protein exchanged into 10, 20, or 100 mM phosphate, typically at pH* 8.0 (99.9% D2O), by using ultrafiltration (pH values quoted for deuterated solutions are uncorrected for the deuterium isotope effect and, thus, are indicated by pH*).
NMR Spectroscopy.
1H NMR spectra were acquired on a JEOL λ 500 spectrometer as described in ref. 40. kese values were determined at 40°C by measuring the influence of increasing concentrations of Cu(II) protein on the T2−1 values of resonances in the Cu(I) protein (40). The kese values were verified by analyzing the coalescence of the Val-22 CγH3 and Val-31 CγH3 resonances in 1:1 mixtures of the Cu(I):Cu(II) protein, which are upfield-shifted and, therefore, clearly resolved in the NMR spectrum and have different chemical shifts in the reduced and oxidized forms.
Crystallization and Analysis.
Crystallization conditions for Cu(II) AZAMI, Cu(II) AZAMI-F, and the reduction of Cu(II) AZAMI-F crystals are described in Table 5, which is published as supporting information on the PNAS web site. Detailed data-collection and processing statistics are given in Table 5. In summary, the Rmerge values for each data set were Cu(II) AZAMI, 3.9%; Cu(II) AZAMI-F, 6.0%; Cu(I) AZAMI-F (pH 6), 6.0%; and Cu(I) AZAMI-F (pH 8), 6.4%. The final refinement statistics for the structures are Cu(II) AZAMI, Rfactor = 23.3% (Rfree = 26.9%) for all data (29 − 1.61 Å), with bond length and angle rmsds of 0.006 Å and 1.35°, respectively; Cu (II) AZAMI-F, Rfactor = 12.7% (Rfree = 15.5%) for all data (33 − 1.25 Å), with bond length and angle rmsds of 0.018 Å and 1.75°, respectively; Cu (I) AZAMI-F, pH 6, Rfactor = 13.9% (Rfree = 17.8%) for all data (43 − 1.4 Å), with bond length and angle rmsds of 0.018 Å and 1.71°, respectively; and Cu (I) AZAMI-F, pH 8, Rfactor = 14.0% (Rfree = 19.1%) for all data (38 − 1.55 Å), with bond length and angle rmsds of 0.018 Å and 1.61°, respectively. The program lsqman (41) was used to superimpose structures and determine rmsds for Cα atoms. The coordinates and structure factors have been submitted to the Protein Data Bank with PDB ID codes 2FTA (AZAMI), 2FT6 [Cu(II) AZAMI-F], 2FT7 [Cu(I) AZAMI-F, pH 6] and 2FT8 [Cu(I) AZAMI-F, pH 8].
Supplementary Material
Acknowledgments
We thank the staff of the Daresbury Synchrotron Radiation Source for assistance with data collection. This work was supported by Biotechnology and Biological Sciences Research Council Grant BB/C504519/1 (to C.D.), a Universities UK Overseas Research Students Award (to S.Y.), the Deutsche Forschungsgemeinschaft (B.M.M.), and the University of Newcastle upon Tyne. M.J.B. is supported by a Royal Society (United Kingdom) University Research Fellowship.
Abbreviations
- AMI
amicyanin
- AZ
azurin
- Em
reduction potential
- ESE
electron self-exchange
- ET
electron transfer
- rmsd
rms deviation
- T1
type 1
- vis
visible.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 2FTA, 2FT6, 2FT7, and 2FT8).
References
- 1.Lu Y., Berry S. M., Pfister T. D. Chem. Rev. 2001;101:3047–3080. doi: 10.1021/cr0000574. [DOI] [PubMed] [Google Scholar]
- 2.Hellinga H. W. J. Am. Chem. Soc. 1998;120:10055–10066. [Google Scholar]
- 3.Schnepf R., Hörth P., Bill E., Wieghardt K., Hildebrandt P., Haehnel W. J. Am. Chem. Soc. 2001;123:2186–2195. doi: 10.1021/ja001880k. [DOI] [PubMed] [Google Scholar]
- 4.Daugherty R. G., Wasowicz T., Gibney B. R., DeRose V. J. Inorg. Chem. 2002;41:2623–2632. doi: 10.1021/ic010555a. [DOI] [PubMed] [Google Scholar]
- 5.Gray H. B., Malmström B. G., Williams R. J. P. J. Biol. Inorg. Chem. 2000;5:551–559. doi: 10.1007/s007750000146. [DOI] [PubMed] [Google Scholar]
- 6.DeLano W. pmol. San Carlos, CA: DeLano Scientific; 2002. [Google Scholar]
- 7.Adman E. T. Adv. Protein Chem. 1991;42:144–197. doi: 10.1016/s0065-3233(08)60536-7. [DOI] [PubMed] [Google Scholar]
- 8.Dennison C. Coord. Chem. Rev. 2005;249:3025–3054. [Google Scholar]
- 9.Buning C., Canters G. W., Comba P., Dennison C., Jeuken L., Melter M., Sanders-Loehr J. J. Am. Chem. Soc. 2000;122:204–211. [Google Scholar]
- 10.Remenyi R., Jeuken L. J. C., Comba P., Canters G. W. J. Biol. Inorg. Chem. 2001;6:23–26. doi: 10.1007/s007750000178. [DOI] [PubMed] [Google Scholar]
- 11.Yanagisawa S., Dennison C. J. Am. Chem. Soc. 2004;126:15711–15719. doi: 10.1021/ja047295r. [DOI] [PubMed] [Google Scholar]
- 12.Dennison C. Dalton Trans. 2005:3436–3442. doi: 10.1039/b507440c. [DOI] [PubMed] [Google Scholar]
- 13.Baker E. N. J. Mol. Biol. 1988;203:1071–1095. doi: 10.1016/0022-2836(88)90129-5. [DOI] [PubMed] [Google Scholar]
- 14.Shepard W. E. B., Anderson B. F., Lewandoski D. A., Norris G. E., Baker E. N. J. Am. Chem. Soc. 1990;112:7817–7819. [Google Scholar]
- 15.Nar H., Messerschmidt A., Huber R., van de Kamp M., Canters G. W. J. Mol. Biol. 1991;221:765–772. doi: 10.1016/0022-2836(91)80173-r. [DOI] [PubMed] [Google Scholar]
- 16.Crane B. R., Di Bilio A. J., Winkler J. R., Gray H. B. J. Am. Chem. Soc. 2001;123:11623–11631. doi: 10.1021/ja0115870. [DOI] [PubMed] [Google Scholar]
- 17.Solomon E. I., Szilagyi R. K., De Beer George S., Basumallick L. Chem. Rev. 2004;104:419–458. doi: 10.1021/cr0206317. [DOI] [PubMed] [Google Scholar]
- 18.Sweeney J. A., Harmon P. A., Asher S. A., Hutnik C. M., Szabo A. G. J. Am. Chem. Soc. 1991;113:7531–7537. [Google Scholar]
- 19.Kalverda A. P., Salgado J., Dennison C., Canters G. W. Biochemistry. 1996;35:3085–3092. doi: 10.1021/bi9518508. [DOI] [PubMed] [Google Scholar]
- 20.Jeuken L. J. C., van Vliet P., Verbeet M. P., Camba R., McEvoy J. P., Armstrong F. A., Canters G. W. J. Am. Chem. Soc. 2000;122:12186–12194. [Google Scholar]
- 21.Battistuzzi G., Borsari M., Canters G. W., de Waal E., Leonardi A., Ranieri A., Sola M. Biochemistry. 2002;41:14293–14298. doi: 10.1021/bi026564s. [DOI] [PubMed] [Google Scholar]
- 22.Lommen A., Canters G. W. J. Biol. Chem. 1990;265:2768–2774. [PubMed] [Google Scholar]
- 23.Groeneveld C. M., Canters G. W. Eur. J. Biochem. 1985;153:559–564. doi: 10.1111/j.1432-1033.1985.tb09337.x. [DOI] [PubMed] [Google Scholar]
- 24.Bertini I., Fernández C. O., Karlsson B. G., Leckner J., Luchinat C., Malmström B. G., Nersissian A. M., Pierattelli R., Shipp E., Valentine J. S., Vila A. J. J. Am. Chem. Soc. 2000;122:3701–3707. [Google Scholar]
- 25.Armstrong F. A., Hill H. A. O., Oliver B. N., Whitford D. J. Am. Chem. Soc. 1985;107:1473–1476. [Google Scholar]
- 26.Guss J. M., Harrowell P. R., Murata M., Norris V. A., Freeman H. C. J. Mol. Biol. 1986;192:361–387. doi: 10.1016/0022-2836(86)90371-2. [DOI] [PubMed] [Google Scholar]
- 27.Dennison C., Kohzuma T., McFarlane W., Suzuki S., Sykes A. G. Chem. Commun. 1994:581–582. [Google Scholar]
- 28.Vakoufari E., Wilson K. S., Petratos K. FEBS Lett. 1994;347:203–206. doi: 10.1016/0014-5793(94)00544-3. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Z., Cunane L. M., Chen Z. W., Durley R. C. E., Mathews F. S., Davidson V. L. Biochemistry. 1998;37:17128–17136. doi: 10.1021/bi9817919. [DOI] [PubMed] [Google Scholar]
- 30.Cunane L. M., Chen Z. W., Durley R. C. E., Mathews F. S. Acta Crystallogr. D. 1996;52:676–686. doi: 10.1107/S0907444996001072. [DOI] [PubMed] [Google Scholar]
- 31.Musiani F., Carloni P., Ciurli S. J. Phys. Chem. B. 2004;108:7495–7499. [Google Scholar]
- 32.Li H., Webb S. P., Ivanic J., Jensen J. H. J. Am. Chem. Soc. 2004;126:8010–8019. doi: 10.1021/ja049345y. [DOI] [PubMed] [Google Scholar]
- 33.Olsson M. H. M., Hong G., Warshel A. J. Am. Chem. Soc. 2003;125:5025–5039. doi: 10.1021/ja0212157. [DOI] [PubMed] [Google Scholar]
- 34.Winkler J. R., Wittung-Stafshede P., Leckner J., Malmström B. G., Gray H. B. Proc. Natl. Acad. Sci. USA. 1997;94:4246–4249. doi: 10.1073/pnas.94.9.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryde U., Olsson M. H. M. Int. J. Quantum Chem. 2001;81:335–347. [Google Scholar]
- 36.van de Kamp M., Floris R., Hali F. C., Canters G. W. J. Am. Chem. Soc. 1990;112:907–908. [Google Scholar]
- 37.Vijgenboom E., Busch J. E., Canters G. W. Microbiology. 1997;143:2853–2863. doi: 10.1099/00221287-143-9-2853. [DOI] [PubMed] [Google Scholar]
- 38.Battistuzzi G., Borsari M., Canters G. W., di Rocco G., de Waal E., Arendsen Y., Leonardi A., Ranieri A., Sola M. Biochemistry. 2005;44:9944–9949. doi: 10.1021/bi050261r. [DOI] [PubMed] [Google Scholar]
- 39.Machczynski M. C., Gray H. B., Richards J. H. J. Inorg. Biochem. 2002;88:375–380. doi: 10.1016/s0162-0134(02)00364-1. [DOI] [PubMed] [Google Scholar]
- 40.Sato K., Kohzuma T., Dennison C. J. Am. Chem. Soc. 2003;125:2101–2112. doi: 10.1021/ja021005u. [DOI] [PubMed] [Google Scholar]
- 41.Kleywegt G. J., Zou J. Y., Kjeldgaard M., Jones T. A. In: International Tables for Crystallography, Vol. F. Crystallography of Biological Macromolecules. Rossmann M. G., Arnold E., editors. Dordrecht, The Netherlands: Kluwer; 2001. pp. 353–356. and 366–367. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.