Abstract
Meiosis is an indispensable process of sexual reproduction. However, detailed information on the regulatory mechanisms that initiate meiosis is not available. Progestins are important steroids regulating final maturation in male and female vertebrates. In male teleosts, it is known that progestin induces spermiation and sperm maturation. However, a role for progestin in early spermatogenesis or meiosis has not yet been described. In this study, we examined the functions of progestin on the initiation of meiosis in male Japanese eel. A natural progestin in teleost fish 17α,20β-dihydroxy-4-pregnen-3-one (DHP) and its receptors were present in the testis at an early stage of spermatogenesis. By using an eel testicular culture system, DHP was shown to induce DNA replication in spermatogonia. Although 11-ketotestosterone, a known initiator of spermatogenesis, also stimulated DNA synthesis in spermatogonia, antibodies against DHP prevented DNA replication when added during the period in which meiosis was initiated. DHP treatment also induced the expression of meiosis-specific markers, such as DmcI and Spo11. Furthermore, Spo11 expression and synaptonemal complexes, specific features of the meiotic prophase, were detected in testicular fragments cultured with DHP in some germ cells that showed morphological characteristics of undifferentiated spermatogonia. We conclude that DHP, a progestin, is an essential factor for the initiation of meiosis.
Keywords: in vitro culture, Japanese eel, spermatogenesis, androgen, Spo11
Meiosis is a special type of cell division that is restricted to germ cells. Meiosis produces haploid cells and forms the basis of sexual reproduction. Many studies on meiosis are directed toward chromosome dynamics (1–3) or to oocyte maturation, which resumes and completes the prophase of the first meiotic division (4, 5). However, the mechanism initiating the first meiotic division is not clear.
The male Japanese eel provides an excellent system for studying the regulation of spermatogenesis, including meiosis, because spermatogonial stem cells are the only type of germ cell present in the testis when eel are in fresh water, and it is the only vertebrate studied to date, in which complete spermatogenesis, including meiotic and postmeiotic stages, can be induced, both in vivo and in vitro (6), by treatment with gonadotropin or 11-ketotestosterone (KT), a major androgen in teleost fish.
Progestins are sex steroid hormones important for reproduction. In mammals, the principal physiological action of progestin is to prepare the reproductive tract for pregnancy and to provide nutritive support for the embryo during gestation (7); however, this is an evolutionarily recent function. In all vertebrates, progestin also plays important roles in gametogenesis. Progestins regulate oocyte maturation (8) by binding to an oocyte plasma membrane receptor, inhibiting oocyte adenylate cyclase, followed by reduced cAMP-dependent protein kinase activity, which induces the activation of maturation promoting factor via Cdc25, eventually triggering the resumption of division I of meiosis (7, 8). In fish spermatogenesis, progestin also plays an important role in spermiation and sperm maturation (9–11). A major progestin in teleost fish, 17α,20β-dihydroxy-4-pregnen-3-one (DHP), induces sperm hydration (9) and acquisition of sperm motility in some species (10, 11). A related progestin, 17α, 20α-dihydroxy-4-pregnen-3-one, is the spermiation-inducing hormone in amphibia (12). Thus, progestin is an indispensable hormone for gametogenesis. However, studies on progestins have been directed mainly on functions in late maturational stages in both sexes.
In several fish species, DHP is found in blood serum at puberty in males (13, 14). Furthermore, we demonstrated that DHP induced spermatogonial DNA synthesis in Japanese huchen (14). These findings suggest that progestin has an important role not only in final maturation but also in early stages of gametogenesis. However, there is no information on the role of DHP in the early stage of spermatogenesis. Here, we show that DHP initiates meiosis in spermatogenesis using eel testis primary tissue and cell culture systems.
Results
Localization of Progesterone Receptor I and II in Testis.
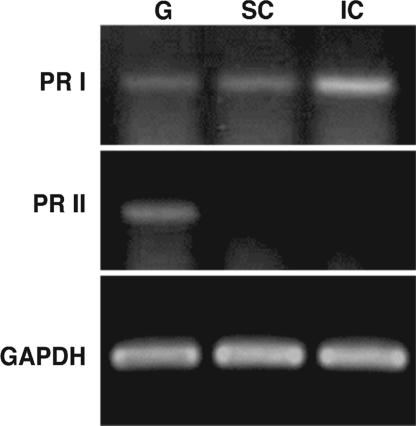
By using RT-PCR, the cellular expression sites of transcripts of progesterone receptor (PR) I and II, both binding DHP, were determined in testis (Fig. 1). PR I was expressed in germ cells, Sertoli cells, and interstitial cells of testis, whereas PR II was detected only in germ cells.
Fig. 1.
Expression of PR I and II in eel testis determined by RT-PCR. RT-PCR was performed by using total RNA extracted from separated germ (G), Sertoli (SC), and interstitial (IC) cells from pooled immature eel testes of 10 eels. For reference, samples were also analyzed for EF-1.
Time Course of Changes in Testicular DHP and 11-KT Levels.
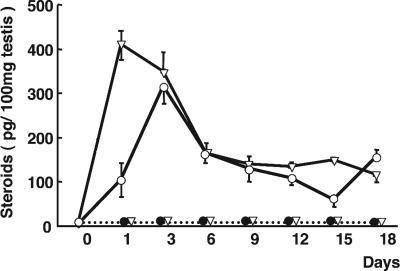
To understand the dynamics of testicular production of DHP and 11-KT, their concentrations were measured in testis by time-resolved fluoroimmunoassay (TR-FIA) (Fig. 2) Before human chorionic gonadotropin (hCG) injection, the testicular levels of DHP and 11-KT were 7 ± 3 and 3 ± 1 pg per 100 mg of tissue, respectively. hCG injection significantly increased the levels of both steroids as early as 1 day after injection. The level of 11-KT peaked on day 1 (410 ± 35 pg per 100 mg of tissue), whereas the peak of DHP levels was observed on day 3 (316 ± 40 pg per 100 mg of tissue), significantly higher than on day 1 after hCG injection. After day 6, the levels of both steroids were maintained between 60 and 170 pg per 100 mg of tissue during the experimental period. Saline injections had no significant effect on the testicular steroid levels throughout the experimental period.
Fig. 2.
Sequential profiles of testicular DHP (○) and 11-KT (▿) during hCG-induced spermatogenesis in eel. Broken lines indicate the saline injection group, DHP (•) and KT (▿). Vertical bars represent the SEM (n = 6 in the initial control and hCG-injected group; n = 5 samples in the saline-injected group).
In Vitro DHP Production in Eel Testis in the Presence of 11-KT.
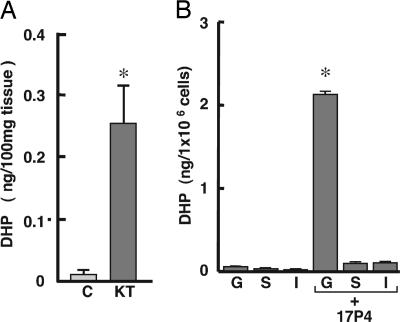
To investigate the effect of 11-KT on DHP production, testicular fragments were cultured with or without 10 ng/ml 11-KT for 6 days, and the concentration of DHP in testis tissue was measured by TR-FIA. The DHP concentration in testis tissue was significantly elevated in the presence of 10 ng/ml 11-KT (Fig. 3A). Furthermore, we identified the site of DHP production by measuring 20β-hydroxysteroid dehydrogenase activity in purified germ, Sertoli, and interstitial cells (Fig. 3B). The precursor of DHP, 17α-hydroxyprogesterone (OHP)4, was converted to DHP only in germ cells.
Fig. 3.
The production of DHP in testis. (A) The effects of 11-KT on DHP production in eel testicular fragments. C, control; mean ± SEM; n = 3 per condition; ∗, P < 0.01). (B) The conversion of 17α-hydroxyprogesterone (17P4) to DHP in separated germ (G), Sertoli (S), and interstitial (I) cells. Results are presented as mean ± SEM; n = 6 per condition; ∗, P < 0.01).
Effect of 11-KT on Eel P450-c17 mRNA Expression in Eel Testes in Vitro.
Northern blot analysis was used to examine the effects of 11-KT on the mRNA expression of eel P450-c17, a key enzyme for the production of sex steroid hormones in the eel testicular organ culture system (Fig. 4). Eel P450-c17 transcripts were prominent in testis before cultivation. Expression of eel P450-c17 did not change during 6 days of culture under control conditions without hormones. However, the signal strength of eel P450-c17 mRNA decreased remarkably in 11-KT-treated testicular fragments. Reprobing the same Northern blot with a labeled EF-1 cDNA showed that similar amounts of EF-1 transcripts were detected in all samples.
Fig. 4.
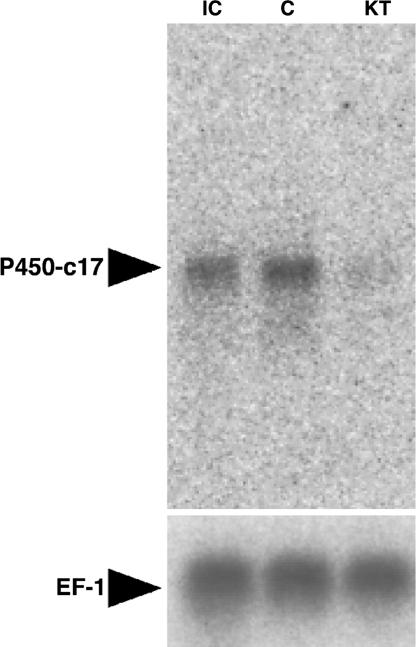
Northern blot analysis of mRNA from cultured testicular fragments for eel P450-c17. Pooled testicular fragments from 10 eels were cultured without (C) or with (KT) 10 ng/ml 11-KT for 6 days. Lane IC shows the initial control before culture. Northern blot of EF-1, which serves as reference, is given below the figure.
The Effect of DHP on DNA Synthesis of Germ Cells.
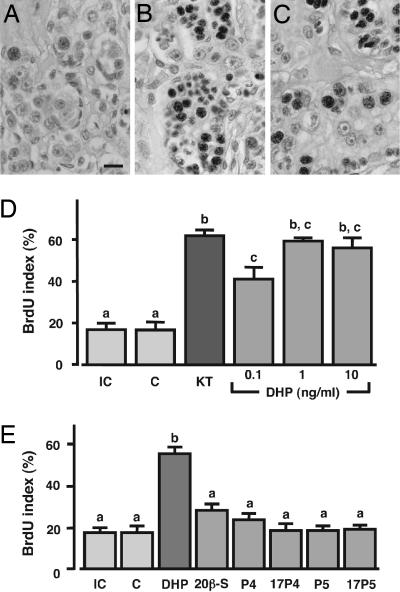
We investigated the effect of DHP during early spermatogenesis in an in vitro organ culture system (Fig. 5A–D). Testicular fragments were cultured with increasing concentrations of DHP (0.1, 1, or 10 ng/ml) or with 10 ng/ml 11-KT as positive control for 6 days. We then monitored DNA synthesis of spermatogonia by exposing the tissue to BrdU. Before cultivation, all germ cells in the testis were undifferentiated spermatogonia (preproliferated spermatogonial stem cells), and the BrdU index was 16.8 ± 3.9%. Adding DHP to the culture medium induced DNA replication with a peak (59.4 ± 2.2%) at 1 ng/ml. Significantly, in controls without any supplement, the BrdU index did not change from its initial value. Addition of 11-KT (positive control) stimulated DNA replication (BrdU index = 62.1 ± 3.3%).
Fig. 5.
Effects of DHP on early spermatogenesis in the eel in vitro. (A–C) Microphotographs show testicular sections from fragments cultured in basal medium without hormone (A), with 10 ng/ml 11-KT (B), and with 1 ng/ml DHP (C). The cells with dark-stained nuclei are BrdU-positive cells. (Scale bar, 50 μm.) (D and E) BrdU labeling index of germ cells in testicular fragments cultured with various concentrations of DHP (D) or 10 ng/ml other progestins (E). The number of BrdU-positive germ cells is expressed as a percentage of the total number of germ cells. IC, initial control; C, negative control without hormone; KT, 10 ng/ml 11-KT; 20β-S, 17α,20β,21-trihydroxy-4-pregnen-3-one; P4, progesterone; 17P4, 17α-hydroxyprogesterone; P5, pregnenolone; 17P5, 17α-hydroxypregnenolone. Results are given as means ± SEM (n = 5). Values with different letters are significantly different (P < 0.05).
Effect of Various Progestins on DNA Synthesis of Germ Cells.
To investigate the effect of other progestins in early spermatogenesis, testicular fragments were cultured with five different progestins (20β-S, P4, 17α-OHP4, P5, and 17α-hydroxypregnenolone (17α-OHP5) for 6 days and BrdU incorporation into germ cells was analyzed (Fig. 5E). A significant increase of DNA replication occurred only when testes were cultured with DHP.
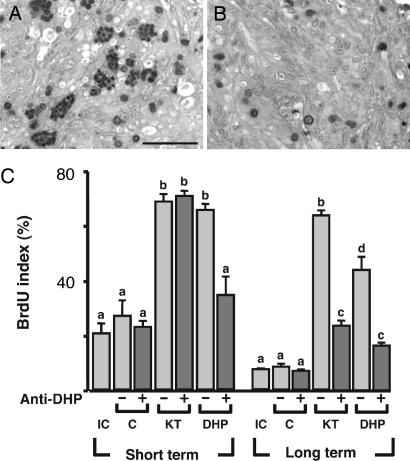
The Effect of Anti-DHP on 11-KT-Induced Spermatogenesis.
To investigate the relationship between 11-KT and DHP, germ cell/somatic cell pellets were cultured in the presence or absence of anti-DHP antibody and/or 11-KT (10 ng/ml) or DHP (1 ng/ml) for 6 (short-term culture) or 15 (long-term culture) days. In the short-term culture, anti-DHP antibodies were present throughout the experimental period (6 days). On the other hand, in the long-term culture, the antibody was present during the last 6 days of the 15-day culture period. After cultivation, spermatogonial DNA synthesis was monitored by the BrdU index (Fig. 6). In short-term culture, 11-KT or DHP treatment alone significantly stimulated the DNA synthesis of spermatogonia compared with the control without hormone. Moreover, DHP-induced, but not 11-KT-induced, DNA synthesis was reduced in the presence of anti-DHP antibodies in the short-term culture. In the long-term culture, on the other hand, the anti-DHP antibodies reduced germ cell DNA synthesis induced by both 11-KT and DHP treatment.
Fig. 6.
Effect of anti-DHP antibody treatment on 11-KT-induced spermatogenesis in a germ cell/somatic cell coculture system. (A and B) Microphotographs show the pellets of germ cells/somatic cells cultured in basal medium alone (A) and 11-KT and anti-DHP antibody together (B) in long-term culture. Dark cells are BrdU-positive cells. (Scale bar, 50 μm.) (C) BrdU index. The number of positively immunoreacted germ cells is expressed as a percentage of the total number of germ cells. IC, initial control; C, negative control without hormone; KT, 10 ng/ml 11-KT; DHP, 1 ng/ml DHP; +, with anti-DHP antibody; −, without anti-DHP antibody. Results are given as means ± SEM calculated for each of three samples. Values with different letters are significantly different (P < 0.05). There are no letters on the graph.
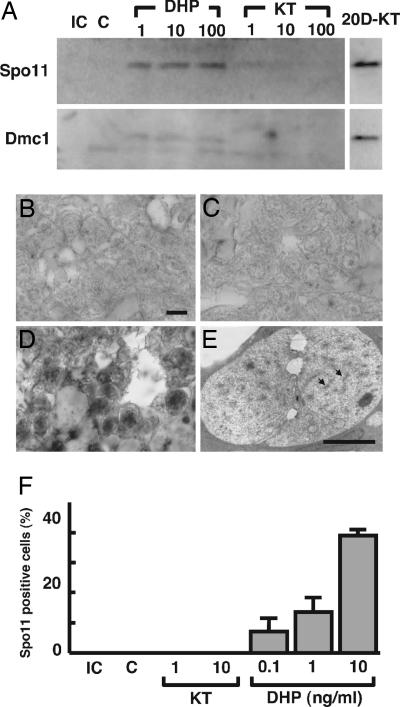
The Effect of DHP on the Expression of Meiosis-Specific Markers.
Western blot analysis was used to examine the effects of DHP on the expression of two meiosis-specific markers, Spo 11 and Dmc 1, in eel testicular organ culture for 6 days (Fig. 7A). Both Spo11 and Dmc1 were detected in DHP-treated testicular fragments but remained undetectable in control and 11-KT-treated testicular fragments. Importantly, after culturing with 10 ng/ml 11-KT for 20 days, when meiosis was progressed and many spermatocyte existed in cultured testicular fragments (15), both markers were detected. After 6 days of DHP treatment, Spo11 was detected only in undifferentiated spermatogonia, i.e., single or paired germ cells in a cyst surrounded by Sertoli cells (Fig. 7 B–D and F). Analyzing such fragments on the ultrastructural level (Fig. 7E) showed that some germ cell nuclei contained synaptonemal complexes characteristic of meiotic cells. However, these meiotic germ cells were bigger than preprolifirated spermatogonia in control testes without hormone.
Fig. 7.
Effect of DHP on induction of meiosis in the Japanese eel in vitro. (A) Expression of meiosis-specific marker proteins Spo11 and Dmc1 in cultured testicular fragments. Testicular fragments were cultured with 0.1, 1, 10, or 100 ng/ml DHP or 1, 10, or 100 ng/ml 11-KT. An initial control sample taken before culture and a control cultured without hormone were run in lanes IC and C, respectively. As a positive control for the expression of meiosis-specific markers, testis tissue fragments cultured with 10 ng/ml KT for 20 days and containing numerous spermatocytes was run in lane 20D-KT. Western blots were repeated five times, using tissue from five males; similar results were obtained in all cases. (B–D) Microphotographs show anti-eelSpo11 immunoreactive material in testicular fragments cultured without hormone (B), with 10 ng/ml 11-KT (C), or with 10 ng/ml DHP (D) for 6 days. (Scale bar, 10 μm.) (E) Electron micrograph of germ cells with synaptonemal complexes (arrows) in testicular fragment cultured with 10 ng/ml DHP for 6 days. (Scale bar, 10 μm.) (F) Percentages of Spo11-positive germ cells. The number of immunoreactive germ cells is expressed as a percentage of the total number of germ cells. IC, initial control; C, control without hormone. Results are given as means ± SEM calculated for each of five samples.
Discussion
To understand the possibility that DHP acts also on early stages of spermatogenesis, we quantified testicular DHP in eel during hCG-induced spermatogenesis and the expression of nuclear PR types in eel testis. Treatment with hCG induced spermatogenesis and a strong increase in testicular DHP levels in vivo. Furthermore, both types of nuclear PRs were expressed in immature testis before initiation of spermatogenesis. These results open the possibility that DHP also acts on the regulation of early spermatogenesis in Japanese eel. Interestingly, DHP has been detected in serum during the proliferation of spermatogonia in some salmonids (13, 14), but the physiological role of this steroid was not clear. Seen in the light of the present data, DHP may be involved in regulating early spermatogenesis in salmonids and other teleosts. Because our RT-PCR studies showed that the two PR types showed partially different cellular sites of expression in the testis, DHP may not have only one function.
We found that DHP stimulated DNA replication of spermatogonia in testicular organ and germ cell/somatic cell coculture, as did 11-KT, a potent androgen in fish. Six other progestins were not effective or had minimal effects, demonstrating an involvement of DHP in the regulation of early spermatogenesis directly on the testicular level. Previous work showed that 11-KT induces full spermatogenesis in eel (15). This androgen promotes spermatogenesis in other fish, e.g., goldfish, African catfish, and Japanese huchen (14, 16, 17). Also, in mice, recent results have suggested a role for androgens in the regulation of spermatogonial numbers (18). Thus, androgen may function in the regulation of early spermatogenesis in vertebrates in general.
What, however, may be the function of DHP in early spermatogenesis, and how does it differ from that of 11-KT? To address these questions, we carried out germ cell/somatic cell coculture, using specific DHP antibodies for 6 (short-term culture) and 15 days (long-term culture). During eel spermatogenesis in vitro, spermatogonial proliferation starts 3 days and meiosis 15 days after commencing the culture with 11-KT. Therefore, the BrdU index of the short-term culture reflects DNA synthesis related to spermatogonial proliferation and that of long-term culture relates to meiosis mainly. In both short- and long-term culture, DHP and 11-KT induced germ cell DNA synthesis. In short-term culture, the DNA synthesis induced by 11-KT stimulation was not prevented by anti-DHP treatment. In long-term culture, however, the DNA synthesis induced by 11-KT stimulation was prevented by anti-DHP treatment. In the long-term culture for 15 days, DHP antibodies were present during the last 6 days only, suggesting that 11-KT can induce two kinds of DNA synthesis in germ cells, one that is not and another, subsequent one that is mediated by DHP. As mentioned above, the germ cells are progressing from spermatogonial proliferation to meiosis on day 15 after initiation of culture with 11-KT (15). These findings suggest that DHP acts on a late stage of spermatogonial proliferation and/or on meiosis.
To understand whether 11-KT is related to DHP production, we investigated the relationship between 11-KT and DHP production by using eel immature testis tissue. We found that 11-KT stimulated DHP production. P450-c17 is a key enzyme for androgen production in Leydig cells (19) showing two catalytic activities (20), C17–20 lyase and 17α-hydroxylase activity. C17–20 lyase activity catalyzes the conversion of 17α-OHP4 to androstendione, a step also important for producing 11-KT in teleost fish (21). Our results indicated that the expression of this enzyme was down-regulated by 11-KT in vitro. Although C17–20 lyase activity was decreased by androgen treatment, the 17α-hydroxylase activity was not in African catfish (22). It is possible that the P450-c17 activity of eel testis is regulated in a similar way. Although further investigation is needed, it is possible that the decrease in C17–20 lyase activity in Leydig cells induced an intratesticular accumulation of 17α-OHP4, the precursor of DHP, possibly leading to DHP production via 20β-hydroxysteroid dehydrogenase activity in germ cells. The delayed peak of testicular DHP compared with that of 11-KT may reflect these changes induced by 11-KT treatment. In future, it is necessary to analyze the mechanisms of steroidogenesis regulated by steroid stimulation.
The activity of 20β-hydroxysteroid dehydrogenase and, hence, DHP production, was restricted to germ cells. It therefore seems possible that DHP acts on early stages of spermatogenesis in a paracrine and/or autocrine manner, considering that PR I was expressed in germ and somatic cells and PR II in germ cells only.
To understand the relationship between DHP and meiosis, we investigated the DHP-induced change of expression of the meiosis-specific markers Dmc1 and Spo11 by using testicular organ culture. Spo11 is involved in the formation of DNA double-strand breaks during the homologous recombination of the meiotic prophase in yeast (23, 24), and Dmc1 is an Escherichia coli RecA-like protein involved only in meiotic recombination in yeast (25, 26). The roles of these proteins in meiosis are conserved throughout eukaryotic species (27, 28). Both markers were induced by DHP in testicular organ culture. Immunocytochemistry showed that the germ cells expressing Spo11 were undifferentiated spermatogonia. Furthermore, synaptonemal complexes, structures specific for the meiotic prophase, were observed in some germ cells of this type in testicular fragments cultured with DHP. Collectively, these data suggest that DHP induced early spermatogonia to enter the meiotic prophase.
Generally, in teleosts, spermatogenesis is perfectly synchronous among all germ cells in a given cyst (29), and the number of mitotic divisions of spermatogonia before entering meiosis is constant in a given species (30) In eel, there are 10 mitotic divisions (31). Therefore, meiotic cells (spermatocytes) form cysts composed of, theoretically, 1,024 cells in Japanese eel. In this report, however, the DHP treatment induced the expression of meiosis markers in undifferentiated spermatogonia, which are single or a pair of cells, surrounded by Sertoli cells after 6 days of culture. It is possible that the DHP caused a precocious termination of spermatogonial mitosis, related to a DHP-induced, advanced entry of spermatogonia into meiosis, in particular at the high dose of DHP.
In conclusion, we demonstrated a new function of progestin by using an eel testis in vitro culture system. Progestin is an essential hormone involved in the regulation of not only final maturation but also of early stages of spermatogenesis, especially the initiation of meiosis in fish. Hence, progestin is an initiator of meiosis in spermatogenesis.
Materials and Methods
Animals.
Cultivated male Japanese eel (Anguilla japonica) were purchased from a commercial eel supplier. For 50 males, a single injection of hCG dissolved in saline was given intramuscularly at a dose of 1,000 IU per fish. Forty control fish were injected with saline alone. Each fish was sampled before injection of either hCG or saline and at 1, 3, 6, 12, and 18 days postinjection. The fish were killed at 0, 1, 3, 6, 9, 12, 15, and 18 days postinjection after anesthetization with 0.1% ethyl aminobenzoate, and testes were sampled. Testicular fragments were used for extraction of poly(A)+ RNA, Western analysis, immunohistochemistry, and measurement of DHP and 11-KT levels in testes.
Testicular Organ Culture Techniques.
Testicular organ culture was performed as described in ref. 15. For culture, testicular explants were maintained in 1 ml of basal medium with or without 0.1, 1, 10, and 100 ng/ml DHP, 10 ng/ml 11-KT, or with 10 ng/ml of one of the following progestins: 17α,20β,21-trihydroxy-4-pregnen-3-one (20β-S), progesterone (P4), 17α-OHP4, pregnenolone (P5), and 17α-hydroxypregnenolone (17α-OHP5). As regards 11-KT, 10 ng/ml is the most effective concentration for inducing spermatogenesis (15). Culture medium was changed 3 days after the start of the culture. Tissue from five eels were used in this experiment for five replicates per condition. After culture for 6 or 20 days, testicular fragments were sampled. Tissue fragments of the 6-day culture were divided into three groups for each treatment. Five fragments of the first group were used for BrdU incorporation studies, three fragments of the second group were used for electron microscopic examination, and 100 mg of the third group was used for Western blot analysis. Testicular fragments of the twenty-day culture were used only for Western blot analysis.
Effect of the Anti-DHP on the Testicular Germ Cells/Somatic Cells Coculture System.
Testicular germ cell/somatic cell coculture techniques were performed as described by Miura et al. (32). In brief, germ cells and somatic cells containing Sertoli cells and interstitial cells isolated from testes of 10 eels were collected by centrifugation, and the cell pellets were incubated for 24 h at 20°C. The culture was performed two times, one time for 6 days (short-term culture), another time for 15 days (long-term culture). In both cultures, the pellets were divided into seven groups, each consisting of five pellets. One group was analyzed immediately to obtain an initial control value; three groups were cultured with 1 ml of basal medium containing 50 μg of anti-DHP antibody (anti-17α, 20β-diOH-P4–3-CMO-BSA, FKA-332; Cosmo Bio, Tokyo; cross reaction with progestins as follows; DHP, 100%; 20β-S, 0.9%; P4, <0.1%; P5, 0.15%; 17α-OHP4, 0.07%) with or without 10 ng/ml 11-KT or 1 ng/ml DHP, and, as a control, three other groups were cultured with 50 μg/ml normal rabbit IgG instead of anti-DHP in the same conditions as the above groups. During the short-term culture, the antibody was present throughout the experiment (0–6 days). In the long-term culture, the antibody was present starting from day 9 until day 15. Three independent experiments were carried out.
Amount of DHP and 11-KT in Testes.
To investigate the testicular content of 11-KT and DHP, 100 mg of testicular fragments of hCG-injected eel were homogenated in 500 μl of eel Ringer's solution and extracted twice with five times volumes of diethyl ether. The 11-KT and DHP were measured by TR-FIA from these extracts according to Yamada et al. (33).
Synthesis of DHP in Testis.
Immature testes of three eels were removed and transferred to glass Petri dishes containing physiological saline solution for eel. Testes were minced with scissors, and 50 mg of testicular fragments were cultured for 6 days with or without 10 ng/ml 11-KT by using the testicular organ culture method described above. After cultivation, testicular fragments were homogenized in 200 μl of physiological saline and DHP extracted from the homogenate by diethyl ether before being measured by TR-FIA, as described above.
Measurement of 20β-Hydroxysteroid Dehydrogenase Activity in Testicular Cells.
Fractions containing spermatogonia, Sertoli, and interstitial cells were prepared from testes of 10 eels as described in ref. 34. Six dishes of 4 × 105 cells per fraction were incubated in eel Ringer's solution with or without 100 ng/ml 17α-OHP4 for 18 h at 20°C before measuring DHP accumulation in the media by TR-FIA, as described above.
Northern Blot Analysis.
poly(A)+ RNA was extracted from testicular fragments cultured with or without 100 ng/ml DHP for 6 days. One microgram of poly(A)+ RNA was electrophoresed on a 1% (wt/vol) agarose denatured gel and blotted onto nylon membranes. The membranes were washed briefly and then baked at 80°C for 1 h. RNA integrity and uniformity of loading and transfer were monitored by hybridization to an eel elongation factor I cDNA. The cDNA fragments of eel P 450-c17 used as probes were labeled by the Random Primer Plus Extension labeling kit (NEN) with [α-32P]dCTP. The membrane containing poly(A)+ RNA was hybridized with 1 × 106 cpm of the radiolabeled probe in hybridization solution at 65°C for 18 h and was then washed with 1× SSC/0.01% SDS solution at 65°C for 1 h. The membrane was exposed to an imaging plate for 24 h and analyzed by using an image analyzer (Fuji).
Antibody.
The production of the rabbit polyclonal antibody against eel Spo11 and Dmc1 was described in ref. 35.
Immunohistochemistry.
By using anti-Spo11, immunohistochemistry was performed as follows. The testicular fragments were fixed in Bouin's solution at 4°C for 18 h, embedded in paraffin wax, and cut into 5-μm serial sections. Sections were deparaffinized in xylene and hydrated in a graded ethanol series. Immunohistochemical analysis was performed by using the HISTOFINE SAB-AP kit (Nichirei, Tokyo). Anti-Spo11 was used at a dilution of 1/1,000. As negative control, immunohistochemical analysis was performed by using 1/1,000 diluted normal rabbit serum (NRS), and NRS did not react any cells (data not shown).
RT-PCR for PRs.
Fractions containing spermatogonia, Sertoli, and interstitial cells were prepared as described above. Total RNA was extracted from 1 × 107 cells per fraction by using Sepasol-RNA I super (Nacalai Tesque, Kyoto). After DNase I treatment, 5 μg of RNA was transcribed by Superscript II (Invitrogen) by using an oligo (dT) primer. The resulting cDNA was amplified by PCR with ePR I-specific primers (sense primer, 5′-CAACTTGGATATGGCGAACAGC-3′; antisense primer, 5′-TGGCACCAAAACGGAGAATCCG-3′) and ePR II-specific primers (sense primer, 5′-CGACTTCGAACAGCTCGTATGG-3′; antisense primer, 5′-CTTTGAGATTCTGGAGACGAG-3′), which were designed based on published eel PR I and II sequences (36, 37). The PCR cycling parameters were as follows: 25 cycles of 94°C for 30 sec, 58°C for 30 sec, and 72°C for 30 sec. GAPDH transcripts were used as the internal standard; specific primers were designed from the nucleotide sequence of the cDNA fragment of Japanese eel GAPDH in the ref. 38. The primers were 5′-GCCATCAACGACCCGTTCATCG-3′(sense) and 5′-GTGCAGGACGCGTTGCTGAC-3′ (antisense). The PCR cycling parameters were as follows: 25 cycles of 94°C for 30 sec, 62°C for 30 sec, and 72°C for 30 sec.
The PCR products were resolved by electrophoresis on a 1.5% agarose gel and stained with ethidium bromide.
BrdU Index.
To detect DNA replication, germ cells were labeled with BrdU according to the manufacturer's instructions (Amersham Pharmacia Bioscience). The number of immunolabeled germ cells was counted and is expressed as a percentage of the total number of germ cells.
Electron Microscopic Observation.
For electron microscopy, testicular fragments were fixed overnight after 6 days of in vitro culture in 1% paraformaldehyde and 1% glutaraldehyde in 0.1 M cacodylate buffer at pH 7.4, postfixed in 1% osmium tetroxide in 0.1 M cacodylate buffer for 1 h, and embedded in epoxy resin according to standard procedures. Ultrathin sections were stained with uranyl acetate and lead citrate for electron microscopic observation.
Electrophoresis and Western Blot Analysis.
Cultured testicular fragments were homogenized in 20 mM Tris·HCl (pH 8.0) containing 1 mM EDTA, 1 mM PMSF and 3 μg/ml leupeptin and centrifuged at 10,000 × g for 60 min at 4°C. The supernatant was mixed with an equal volume of sample buffer [0.125 M Tris·HCl/4% (wt/vol) SDS/20% (vol/vol) glycerol/0.05% (wt/vol) bromophenol blue] with 10% 2-mercaptoethanol. All samples were boiled for 10 min after being mixed with sample buffer. The protein concentration was determined by using the 2-D Quant Kit (Amersham Pharmacia Biosciences).
SDS/PAGE was carried out by using a Tris-glycine buffer system. For Western blot analysis, proteins (5 μg) were separated by SDS/PAGE and transferred to a polyvinylidine difluoride membrane (Millipore). The membrane was immersed overnight at 4°C in a solution containing anti-eSpo11 or anti-eDmc1 at a dilution of 1:1,000 in TBS containing 10% skimmed milk. After washing, the membrane was incubated with alkaline phosphatase (AP)-conjugated goat anti-rabbit IgG (Vector Laboratories) diluted to 1:2,000 in TBS for 1 h. After washing, AP activity was visualized by using CDP-Star Detection Reagent (Amersham Pharmacia Biosciences) and analyzed by using a LAS-1000 mini (Fuji).
Statistics.
Results are expressed as means ± SEM. Data analysis was carried out by the Sceirer, Ray, and Hare extension of the Kruskal–Wallis test (a two-way ANOVA design for ranked data), followed by post hoc Bonferroni adjustment.
Acknowledgments
We thank Dr. R. W. Schulz for reviewing this paper and for his valuable comments on the manuscript. This work was supported by a Grant-in-Aid from the Ministry of Agriculture, Forestry, and Fisheries of Japan and from the Ministry of Education, Culture, Sports, Sciences, and Technology of the Japanese Government.
Abbreviations
- DHP
17α,20β-dhihydroxy-4-pregnen-3-one
- hCG
human chorionic gonadotropin
- KT
ketotestosterone
- PR
progesterone receptor
- TR-FIA
time-resolved fluoroimmunoassay
- 17α-OHP4
17α-hydroxyprogesterone.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Greton J., Hawley R. Nat. Rev. Genet. 2005;6:477–487. doi: 10.1038/nrg1614. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe Y. Trends Genet. 2005;21:405–412. doi: 10.1016/j.tig.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Shinohara A., Shinohara M. Cytogenet. Genome Res. 2004;107:201–207. doi: 10.1159/000080598. [DOI] [PubMed] [Google Scholar]
- 4.Masui Y. Differentiation (Berlin) 2001;69:1–17. doi: 10.1046/j.1432-0436.2001.690101.x. [DOI] [PubMed] [Google Scholar]
- 5.Thomas P., Zhu Y., Pace M. Steroids. 2002;67:511–517. doi: 10.1016/s0039-128x(01)00180-5. [DOI] [PubMed] [Google Scholar]
- 6.Miura T., Miura C. Zool. Sci. 2001;18:1055–1063. [Google Scholar]
- 7.Thomas P. B. In: Encyclopedia of Reproduction. Knobil E., Neil J. D., editors. San Diego: Academic; 1998. pp. 23–30. [Google Scholar]
- 8.Nagahama Y. Steroids. 1997;62:190–196. doi: 10.1016/s0039-128x(96)00180-8. [DOI] [PubMed] [Google Scholar]
- 9.Ueda H., Kanbegawa A., Nagahama Y. Gen. Comp. Endocrinol. 1985;59:24–30. doi: 10.1016/0016-6480(85)90415-0. [DOI] [PubMed] [Google Scholar]
- 10.Miura T., Yamauchi K., Takahashi H., Nagahama Y. Biomed. Res. 1991;12:241–248. [Google Scholar]
- 11.Miura T., Yamauchi K., Takahashi H., Nagahama Y. J. Exp. Zool. 1992;261:359–363. doi: 10.1002/jez.1402610316. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi T., Sakai N., Adachi S., Asahina K., Iwasawa H., Nagahama Y. Endocrinology. 1993;133:321–327. doi: 10.1210/endo.133.1.8319579. [DOI] [PubMed] [Google Scholar]
- 13.Baynes S. M., Scott A. P. Gen. Comp. Endocrinol. 1985;57:150–160. doi: 10.1016/0016-6480(85)90211-4. [DOI] [PubMed] [Google Scholar]
- 14.Amer M. A., Miura T., Miura C., Yamauchi K. Biol. Reprod. 2001;65:1057–1066. doi: 10.1095/biolreprod65.4.1057. [DOI] [PubMed] [Google Scholar]
- 15.Miura T., Yamauchi K., Takahashi H., Nagahama Y. Proc. Natl. Acad. Sci. USA. 1991;88:5774–5778. doi: 10.1073/pnas.88.13.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi M., Aida K., Stacey N. E. Zool. Sci. 1991;8:389–393. [Google Scholar]
- 17.Cavaco J. E. B., Vilrokx C., Trudeau V. L., Schulz R. W., Goos H. J. T. Am. J. Physiol. 1998;44:R1793–R1802. doi: 10.1152/ajpregu.1998.275.6.R1793. [DOI] [PubMed] [Google Scholar]
- 18.Gendt K. D., Swinnen J. V., Saunders P. T. K., Schoonjans L., Dewerchin M., Devos A., Tan K., Atanassova N., Claessens F., Lécureuil C., et al. Proc. Natl. Acad. Sci. USA. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dufau M. L., Miyagawa Y., Takada S., Khanum A., Miyagawa H., Buczko E. Steroids. 1997;62:128–132. doi: 10.1016/s0039-128x(96)00171-7. [DOI] [PubMed] [Google Scholar]
- 20.Nakajin S., Hall P. F. J. Biol. Chem. 1981;256:3871–3876. [PubMed] [Google Scholar]
- 21.Kobayashi T., Nakamura M., Kajiura-Kobayashi H., Young G., Nagahama Y. Cell Tissue Res. 1998;292:573–577. doi: 10.1007/s004410051086. [DOI] [PubMed] [Google Scholar]
- 22.Cavaco J. E. B., van Blijswijk B., Leatherland J. F., Goos H. J. T., Schulz R. W. Cell Tissue Res. 1999;1999:291–299. doi: 10.1007/s004410051357. [DOI] [PubMed] [Google Scholar]
- 23.Keeney S., Giroux C. N., Kleckner N. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 24.Smith K. H., Nicolas A. Curr. Opin. Genet. Dev. 1998;8:200–211. doi: 10.1016/s0959-437x(98)80142-1. [DOI] [PubMed] [Google Scholar]
- 25.Bishop D. K., Park D., Xu L., Kleckner N. Cell. 1992;69:439–456. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]
- 26.Bishop D. K. Cell. 1994;79:1081–1092. doi: 10.1016/0092-8674(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 27.Sauvageau S., Ploquin M., Masson J.-Y. 2004;26:43–56. doi: 10.1002/bies.20150. [DOI] [PubMed] [Google Scholar]
- 28.Keeney S. Curr. Top. Dev. Biol. 2001;52:1–53. doi: 10.1016/s0070-2153(01)52008-6. [DOI] [PubMed] [Google Scholar]
- 29.Grier H. J. Am. Zool. 1981;21:345–357. [Google Scholar]
- 30.Ando N., Miura T., Nader M. R., Miura C., Yamauchi K. Fisheries Sci. 2000;66:299–303. [Google Scholar]
- 31.Miura T., Yamauchi K., Nagahama Y., Takahashi H. Zool. Sci. 1991;8:63–73. [Google Scholar]
- 32.Miura T., Miura C., Konda Y., Yamauchi K. Development (Cambridge, U.K.) 2002;129:2689–2697. doi: 10.1242/dev.129.11.2689. [DOI] [PubMed] [Google Scholar]
- 33.Yamada H., Satoh R.-I., Yamashita T., Kambegawa A., Iwata M. Gen. Comp. Endocrinol. 1997;106:181–188. doi: 10.1006/gcen.1996.6861. [DOI] [PubMed] [Google Scholar]
- 34.Miura C., Miura T., Yamashita M., Yamauchi K., Nagahama Y. Dev. Growth Differ. 1996;38:257–262. doi: 10.1046/j.1440-169X.1996.t01-2-00004.x. [DOI] [PubMed] [Google Scholar]
- 35.Ozaki Y., Miura C., Miura T. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006;143:306–314. doi: 10.1016/j.cbpb.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Todo T., Ikeuchi T., Kobayashi T., Kajiura-Kobayashi H., Suzuki K., Yoshikuni M., Yamauchi K., Nagahama Y. FEBS Lett. 2000;465:12–17. doi: 10.1016/s0014-5793(99)01714-7. [DOI] [PubMed] [Google Scholar]
- 37.Ikeuchi T., Todo T., Kobayashi T., Nagahama Y. FEBS Lett. 2002;510:77–82. doi: 10.1016/s0014-5793(01)03220-3. [DOI] [PubMed] [Google Scholar]
- 38.Takei Y., Inoue K., Ando K., Ihara T., Katafuchi T., Kasiwagi M., Hirose S. Am. J. Physiol. 2001;280:R1727–R1735. doi: 10.1152/ajpregu.2001.280.6.R1727. [DOI] [PubMed] [Google Scholar]