Abstract
We demonstrate that mammalian cells can survive for 5 min within high-pressure CO2. Cell survival was investigated by exposing a range of mammalian cell types to supercritical CO2 (scCO2) (35°C, 74 bar; 1 bar = 100 kPa) for increasing exposure and depressurization times. The myoblastic C2C12 cell line, 3T3 fibroblasts, chondrocytes, and hepatocytes all displayed appreciable but varying metabolic activity with exposure times up to 1 min. With depressurization times of 4 min, cell population metabolic activity was ≥70% of the control population. Based on survival data, we developed a single-step scCO2 technique for the rapid production of biodegradable poly(dl-lactic acid) scaffolds containing mammalian cells. By using optimum cell-survival conditions, scCO2 was used to process poly(dl-lactic acid) containing a cell suspension, and, upon pressure release, a polymer sponge containing viable mammalian cells was formed. Cell functionality was demonstrated by retention of an osteogenic response to bone morphogenetic protein-2 in C2C12 cells. A gene microarray analysis showed no statistically significant changes in gene expression across 4,418 genes by a single-class t test. A significance analysis of microarrays revealed only eight genes that were down-regulated based on a δ value of 1.0125 and a false detection rate of 0.
Keywords: biodegradable polymer, bone, scaffolds
Standard methods of combining mammalian cells and synthetic polymers for biotechnological applications (1–3) must minimize disruption to the cell component from fluctuations in solvent composition, temperature, pressure, and shear forces (4, 5). However, processing synthetic polymers by conventional routes requires organic solvents, elevated temperatures or pressures, and sometimes mechanical agitation to produce the required 3D form (6, 7). For this reason, an inefficient two-step process is required to generate the prefabricated polymer structure, with the cell component seeded as a second step (8).
One method for generating macroporous, biodegradable scaffolds is by using high-pressure or supercritical CO2 (scCO2) processing. This technique is solvent-free and has been used for the fabrication of tissue engineering and drug delivery devices because of a number of processing advantages (9, 10). When CO2 is raised above the critical pressure and temperature (31.2°C, 73.8 bar; 1 bar = 100 kPa), it forms a phase with the characteristics of both a liquid and a gas with selective solvent power and high diffusivity (11). It is then able to penetrate into the amorphous regions of polymers, such as poly(dl-lactic acid) (PDLLA), liquefying them at near ambient temperatures. Release of the pressure results in the nucleation of gas bubbles within the polymer, which become permanent upon solidification, creating a reticulated porous structure (9).‖ As a result, scaffold fabrication is possible without introducing high temperatures or organic solvents. This fact has made it possible for sensitive biological molecules, such as protein drugs, to be incorporated into polymeric-controlled release matrices during fabrication (12, 13). This method has already been tailored and used with bone morphogenetic protein-2 (BMP-2), stimulating the formation of bone tissue in vivo (14).
We hypothesized that a similar one-step scCO2 processing technique might be used to produce biodegradable foams containing mammalian cells. The rapid plasticization and subsequent foaming of PDLLA by scCO2 could limit the required survival time for mammalian cells within supercritical conditions to <5 min. However, the retention of cell viability and functionality after exposure to high-pressure CO2 and rapid decompression could be problematic, because other supercritical fluid processes have been used for bacterial cell inactivation (15, 16).
A number of mechanisms have been proposed to explain high-pressure CO2 sterilization, including cell membrane rupture caused by the increase in internal pressure combined with rapid pressure release (17, 18) and the extraction of lipids from the cell membrane (19). Equally, scCO2 diffusion into the cell and the resultant changes in the cellular environment, such as a decrease in pH, could be lethal (17). As a result of this sterilizing power, subcritical CO2 and scCO2 has been used to inactivate bacterial cells and spores when injected into poly(lactic acid) for medical sterilization (17) and for sterilization of food stuffs (20). Even short exposure times (150 sec) to CO2 at just above its critical point (38°C, 74 bar) are sufficient to inactivate both Gram-negative and Gram-positive bacteria (21).
Therefore, we had four main objectives: (i) to process mammalian cells in scCO2 and high-pressure N2 to determine the effects of pressure, exposure time, and processing medium on cell survival; (ii) to investigate the effects of scCO2 on important aspects of gene expression; (iii) to measure cell functionality by quantifying the ability of a scCO2-treated cell line to differentiate in response to growth factors; and (iv) to show cell viability, functionality, and location after combining cells and PDLLA into a single construct.
Results
Cell Survival After scCO2 Processing.
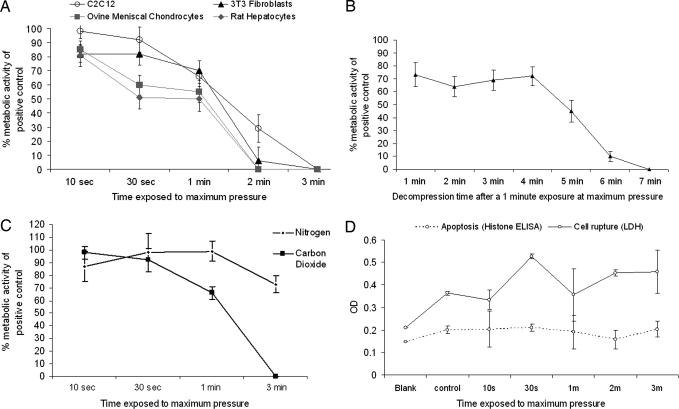
The metabolic activity of cell populations after a 10-sec exposure at maximum pressure and 40-sec depressurization, in comparison with untreated controls, was >80% for all cell types (>90% for C2C12 cell line) (Fig. 1A). Cell survival remained at 50% or greater for all cell types at a 1-min exposure to maximum pressure and a 40-sec depressurization. Between a 1- and 2-min exposure (plus a 40-sec depressurization), there was a steep decline in cell viability, with little or no metabolic activity measured after a 3-min treatment. By reducing the rate of pressure release, cell viability at each exposure time was enhanced (Fig. 1B).
Fig. 1.
Mammalian cell survival and investigation of cell death after exposure to CO2/N2 at 35°C and 74 bar. (A) Cell survival determined by AlamarBlue cell metabolic assay for increasing CO2 exposure times for a variety of cell types. All results are expressed as a percentage of a parallel untreated control with standard deviation. These data indicate that cell survival is both cell type- and time-dependent. (B) Cell survival determined by AlamarBlue cell metabolic assay for increasing CO2 decompression times after a 1-min scCO2 exposure. All results are expressed as a percentage of a parallel untreated control with standard deviation. (C) Comparison of the effects of increasing exposure times to scCO2 and high pressure N2 on C2C12 survival. (D) Levels of cellular histone-DNA fragments (associated with apoptosis) and the cytosolic enzyme, LDH, as a measure of cell membrane rupture after exposure to scCO2 for increasing exposure times.
When C2C12 cells were exposed to N2 at the same pressure and temperature as CO2 in the above studies, survival rates were higher at longer exposure times (Fig. 1C). N2 was chosen as a relatively inert gas to create pressure changes comparable with those achieved with CO2 but without potential chemical association with the aqueous phase. Hence, this study demonstrated that pressure changes in the CO2 phase were not the sole cause of cell death. Exposure to scCO2 did not produce an increase in the cellular histone-DNA fragments associated with apoptosis (Fig. 1D) but did release the cytosolic enzyme lactate dehydrogenase (LDH), indicating mechanical disruption with increasing processing times.
Cell Function and Gene Expression After scCO2 Processing.
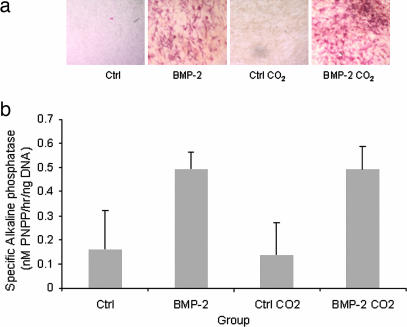
Retention of functionality in the C2C12 cell line was assessed by their ability to undergo osteogenic differentiation measured by using the expression of alkaline phosphatase (AP) as a marker (22, 23). AP activity was detected by using AP staining and a p-nitrophenyl phosphate (p-NPP) colorimetric assay (Fig. 2) (normalized against DNA). AP levels were indistinguishable between the untreated control and the scCO2-exposed cells.
Fig. 2.
Cell functionality after 1-min exposure to scCO2 as assessed by retention of osteogenic response of the C2C12 cell line observed by expression of AP staining (red areas) (a) and a soluble end product (p-NPP) created by AP activity (b). Measured AP activity was normalized against DNA.
Changes in gene expression after exposure to scCO2 were measured in murine C2C12 cells by using the OciChip mouse 30K set A (Ocimum Biosolutions, Hyderabad, India). RNA was harvested from C2C12 cells after scCO2 processing for 1 min (74 bar, 35°C) and labeled and hybridized to the OciChip in comparison with RNA from untreated control cells. Filtering of array data and normalization by using the lowess algorithm were performed within the University of Nottingham microarray database (a version of base Version 1.2.16; Lund University, Lund, Sweden). From the OciChip arrays (9,853 mouse-specific probes), 4,418 genes were detected in three or more arrays and showed a reproducible linear relationship (Fig. 5, which is published as supporting information on the PNAS web site). An initial single-class t test of the data set (against-threshold P = 0.01, with standard Bonferroni correction) did not reveal any significant changes in gene expression from the control samples. The significance analysis of microarrays algorithm found eight significantly down-regulated genes based on a δ value of 1.0125 and a false discovery of 0 (Table 1, which is published as supporting information on the PNAS web site).
Cell Distribution Within PDLLA Scaffolds.
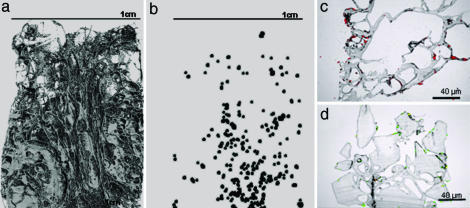
The presence of cells within the scaffolds was demonstrated by a range of imaging techniques. Microcomputed tomography (μCT) images revealed cell distribution throughout the internal surfaces (Fig. 3a and b), which were further verified by sectioning the construct and staining cell nuclei with propidium iodide or LIVE/DEAD (Molecular Probes) (Fig. 3 c and d).
Fig. 3.
The distribution of mammalian cells after incorporation into PDLLA scaffolds by using a one-step scCO2 process. Location of cells throughout the scaffold observed by using μCT images of the polymer foam scaffolds (a) into which fixed cells were incorporated (b) (size and contrast increased for visualization). Cells were also observed throughout the scaffold in 10-μm sections highlighted with propidium iodide (c) and calcein acetoxymethyl ester and ethidium homodimer-1 (LIVE/DEAD) (d) prestained cells.
Cell Survival and Function in PDLLA Scaffoldss.
A viable and spreading cell population within the scaffolds was confirmed by LIVE/DEAD staining (Fig. 4a) and scanning electron microscopy (SEM) imaging (Fig. 4b). Furthermore, staining for AP activity (Fig. 4c) showed red areas on the scaffolds, confirming the expression of AP by the cells, which indicates that, when processed with PDLLA, the cells retain their differentiation capacity.
Fig. 4.
Attachment, viability, and osteogenic differentiation of live mammalian cells in scCO2 foamed PDLLA scaffolds. Live and spreading cells were observed on the scaffolds by using LIVE/DEAD staining of live (green) cells (a) and SEM (b). (c) Functionality of the attached cells was highlighted by osteogenic differentiation of incorporated C2C12 cells as indicated by areas of red-stained AP-active cells (arrow) over the white polymer scaffold material (control scaffold shown in Inset).
Discussion
This study proves that mammalian cells can survive in scCO2 environments with survival rates dependent on cell type, total exposure times, and depressurization rates. For the cells chosen in our study, secondary murine cell lines displayed higher survival rates as a function of exposure time than the primary cells. By reducing the rate of depressurization, cell survival was improved, a result that concurs with the literature on microorganisms for which a more rapid or “explosive” pressure drop can have a more lethal effect (15, 16, 18).
By comparison of the effects of high pressure N2 and CO2, it was found that cell survival was less sensitive to exposure time in N2. Therefore, the loss of cell viability with lengthening exposure to CO2 was caused by additional effects to the rapid pressure changes and high maximum exposure pressure. The detection of LDH release from cells after scCO2 processing and lack of increase in histone-DNA fragments provide more evidence that mechanical trauma is the most important cause of cell damage or death during scCO2 exposure and pressure release. Although our study detects a loss of cell viability on processing, it is apparent that, by minimizing exposure times and slowing depressurization, cell survival rates >70% can be achieved.
A more detailed analysis of cell differentiation and gene expression after exposure to scCO2 revealed that C2C12 cells retained the ability to respond to BMP-2. As measured by AP activity, cells were equally able to differentiate in response to BMP-2 irrespective of their exposure to scCO2. Gene microarray analysis of four samples that were independently exposed to scCO2 confirmed that very few changes in gene expression were induced by the high-pressure CO2 environment and rapid pressure fluctuations. Of eight genes that were significantly down-regulated, survivin, clast4 protein, and glutathione S-transferase could play a role in a biochemical response from the cell to the extreme changes in environment. Survivin is a component of the chromosomal passenger complex that acts as an inhibitor of apoptosis (24). Down-regulation of survivin is the only marker of an apoptotic response we have recorded.
Combining the polymer phase and the cells within scCO2, with a 30-sec exposure time to a maximum pressure of 74 bar and a depressurization time of 40 sec, generated macroporous scaffolds with cells distributed throughout the 3D matrix. Mixing of the cell and polymer components was achieved by combining a fine powder of the polymer and concentrated suspension of the cells. The cells within the scaffold were found to be viable, and C2C12 cells retained the ability to respond to BMP-2. Therefore, a composite structure of biodegradable polymer and mammalian cells could be formed through a process that was completed within 5 min to generate a viable, evenly distributed cell population that was able to differentiate within a porous scaffold.
Conclusion
This study demonstrates that mammalian cells in suspension are able to survive exposure to scCO2 for sufficient time periods to plasticize and foam PDLLA. After exposure to scCO2 and rapid depressurization, cells demonstrated high-population metabolic activity and retention of important aspects of functionality. However, although some cell death did occur, the rate of cell death was higher in CO2 than in N2, and there is evidence of some mechanical disruption of the cell population. The effects of scCO2 on relative gene expression were minimal in the C2C12 cell line, with small down-regulations of survivin, clast4 protein, and glutathione S-transferase providing some evidence of subtle changes at the mRNA level that do not lead to significant apoptosis. Overall, it can be concluded that scCO2 can be used to process cells into polymer composite structures.
Materials and Methods
Equipment and Chemicals.
Food-grade CO2 was supplied by CryoService (Worcester, United Kingdom), N2 was provided by BOC Industrial (Surrey, United Kingdom), and PDLLA (Mw 52,000) was supplied by Purac (Birmingham, United Kingdom). The process was controlled by using a Bronkhorst (Cambridge, United Kingdom) High-Tec BV series flowmeter as a backpressure regulator. The recombinant human BMP-2 was kindly manufactured and supplied by Walter Sebald (Biozentrum der Universität Würzburg, Am Hubland, Würzburg, Germany).
Isolation and Culture of Primary Cells.
Fibrochondrocytes were isolated from the ovine knee joint meniscus by using a method adapted from Collier and Ghosh (25). Tissue was diced into 2-mm3 cubes, and pronase E (VWR International, Leicestershire, United Kingdom) and type II collagenase (Lorne Laboratories, Reading, United Kingdom) were used to enzymatically digest the cells from the tissue. Cells were cultured in DMEM supplemented with 10% FCS, 2% nonessential amino acids, and 2% l-glutamine containing penicillin (100 units/ml), streptomycin (100 μg/ml), and amphotericin B (250 ng/ml) (Sigma). Hepatocytes were isolated from healthy rat liver by using a method modified from Seglen (26). Complete medium for hepatocytes contained Williams medium E (Invitrogen) supplemented with 10% FCS and 20 mM l-glutamine and containing penicillin (100 units/ml), streptomycin (100 μg/ml), and amphotericin B (250 ng/ml) (Sigma).
Secondary Cell Line Culture.
The murine 3T3 and C2C12 cell lines were cultured in DMEM supplemented with 10% FCS, 2% nonessential amino acids, and 2% l-glutamine and containing penicillin (100 units/ml), streptomycin (100 μg/ml), and amphotericin B (250 ng/ml) (Sigma). C2C12 cells were passaged before attaining 70% confluence to avoid spontaneous differentiation to muscle (myoids).
Processing Cells in scCO2 and High-Pressure N2.
Cells were suspended in 200 μl of complete medium and placed into custom-made poly(tetrafluoroethylene) eight-well tissue culture chambers at 2 × 105 cells per well. Cells were processed inside a 60-ml stainless steel pressure vessel at an operating pressure of 74 bar at 35°C. Cells were processed at maximum pressure from 10 sec to 3 min with an additional 80 sec required to pressurize and depressurize the vessel by using sensitive backpressure regulation. This procedure was then repeated with N2 in place of CO2 by using C2C12 cells and exactly the same processing conditions to show any specific effects that CO2 may have on cell survival. After processing, the cells were resuspended in 1 ml of complete DMEM and incubated for 24 h in a 48-well tissue culture plate (Falcon) before further analysis. To test the effects of a less rapid pressure release, 3T3 fibroblasts were subject to increasing depressurization times of up to 7 min after being held at maximum pressure for 1 min.
Metabolic Activity of scCO2 and High-Pressure N2-Processed Cells.
To measure metabolic activity, medium was aspirated and replaced by 1-ml solution of 10% AlamarBlue solution (vol/vol; Serotech) in phenol red-free Hank’s buffered saline solution (Sigma). The cells were incubated for 90 min before 200 μl of solution from each well was transferred to a 96-well plate (Falcon). Fluorescence readings were then obtained at an emission wavelength of λ590 nm by using λ560 nm excitation.
LDH Assay for Damage to Cell Membrane.
C2C12 cells were exposed to scCO2 in DMEM (without phenol red and FCS) for increasing exposure times (as in survival experiments), and the cytosolic LDH released was measured to indicate the extent of damage to the cell membrane. Cells were removed by centrifugation for 2 min (1,000 rpm; 3K15;SciQuip, Freehold, NJ), and 100 μl of the DMEM supernatant was assayed for release of LDH by using an equal volume of the LDH substrate/dye reaction mixture in a 96-well plate (Roche). Absorbance readings were detected at λ490 nm.
ELISA for Apoptosis Detection.
C2C12 cells were exposed to increasing scCO2 exposure times (as in previous experiments) before subsequent culture for 3 h more in fresh medium (so as to observe apoptosis rather than cell mechanical damage). The cells were then assayed for histone-associated DNA fragments indicative of apoptosis through the Cell Death Detection ELISAPLUS (Roche), which was used per the manufacturer’s instructions. Briefly, cells from each processing cycle were lysed by using the provided buffer and centrifuged (200 × g for 10 min), and 20 μl of the lysate supernatant was added to a streptavidin-coated ELISA plate. Eighty microliters of the immunoreagent (containing the enzyme-linked antibody) was added to each well, and the plate was incubated at room temperature (for 2 h). The solution was then removed from each well and replaced with 100 μl of a 2,2′-azino-di-(3-ethyl)benzthiazoline-6-sulfonic acid (ABTS) solution before a 20-min incubation to allow the color to develop in the sample wells. Absorbance was detected at λ490 nm.
Preparation of RNA and Hybridization.
After processing for 1 min at 74 bar and 35°C, murine C2C12 cells were cultured for 3 h more in complete medium. Cells were then rinsed in PBS and homogenized in an RNeasy lysis buffer containing 2-mercaptoethanol (10 μl/ml). Total RNA content of the cell homogenate was assessed by using the RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Slides were OciChip 30K set A arrays, and indirectly labeled probes were generated from 5 μg of total sample RNA with Ambion (Austin, TX) MessageAmp II amplification (per the manufacturer’s protocols). The probes were labeled with Alexa 555 or 647 N-hydroxysuccinimide ester dyes (Invitrogen), and 0.5 μg (≈40 pM of dye label) of each was used for each channel of two-color hybridizations. Hybridizations were performed for 16 h on a TEcan HTS 4800 hybridization station with moderate agitation by using the Ocimum recommended solutions and temperatures.
Microarray Analysis.
Arrays were scanned with a GenePix 4000B laser scanner, and primary data was obtained by using genepix pro 6.0 software (Axon Instruments, Union City, CA). Filtering of array data and normalization by using the lowess algorithm were performed within the University of Nottingham microarray database (a version of base Version 1.2.16; Lund University, Lund, Sweden). Further analysis was performed on this data set by using multiexperiment viewer (mev) software (Institute for Genomic Research, Rockville, MD) and the statistical analysis of microarrays algorithm.
Osteogenic Differentiation of scCO2-Processed C2C12 Cells.
To assess cell function we studied the differentiation of C2C12 cells, which are shown to have multipotential nature dependent on the cell environment (23, 27, 28). The osteoblast lineage can be induced in the C2C12 cell line by BMP-2 (27), an indicator of which is AP activity (22, 23). Before osteogenic culture of C2C12 cells, they were processed the same way as above but then added into six-well plates (Falcon) cultured to 80% confluency (1–2 days) before the addition of 500 ng/ml recombinant human BMP-2 in 2% FCS-supplemented DMEM medium (29). For cells processed into porous scaffolds, the same culture technique was used. After supercritical processing, the cell-loaded scaffolds were cut into four pieces and cultured for 24 h in complete DMEM before the addition of 500 ng/ml recombinant human BMP-2.
AP Activity.
As a measure of the osteogenic status of C2C12 cells, a red color substrate was precipitated on the cells by the action of cellular AP activity. The substrate system was comprised of Naphthol AS-MX buffer solution (Sigma) containing 1 mg/ml fast violet B salt, as described by Oreffo et al. (30). For measurement of AP activity, a p-NPP substrate system was used. Cell cultures were fixed in 75% ethanol for 10 min and homogenized in 600 μl of distilled water containing 0.001% Tween detergent after three cycles of freeze-thawing. Values were compared against known p-NPP standards and matched to DNA content to compensate for changes in cell number. This step was repeated in triplicate over four experiments for osteogenically prompted C2C12 cells.
DNA Assay.
DNA content was estimated by using Hoechst 33258 dye binding, as modified from a method described in ref. 31. In brief, cell cultures were fixed in 75% ethanol and homogenized in 600 μl of distilled water containing 0.001% Tween detergent. Cell homogenate (50 μl) was added to a 96-well plate (Falcon), and 100 μl of 2 μg/ml Hoechst 33258 was added. Fluorescence was detected at an emission wavelength of λ460 nm by using λ360 nm excitation and compared with a series of DNA standards from 0 to 20 μg/ml.
Fabrication of Cell-Loaded PDLLA Scaffolds by Using scCO2.
One hundred milligrams of PDLLA was mixed with 2 × 105 of either osmium tetroxide-stained 3T3 fibroblasts for distribution analysis or with live C2C12 cells suspended in 200 μl DMEM. Cells and PDLLA were mixed together to produce a polymer/cell slurry within the well of a 1-cm-diameter cylindrical poly(tetrafluoroethylene) mould before processing. Polymer and cells were foamed into a scaffold by exposure to scCO2 (35°C, 74 bar) for 30 sec, with an additional 80 sec required to pressurize and depressurize the vessel. After processing, scaffolds were either kept for μCT analysis or cut into four equal pieces and incubated for 24 h in 2 ml of DMEM culture medium in a six-well plate (Falcon) for SEM, viability, and functionality assessment.
μCT Imaging of Cells Within Scaffolds.
C2C12 cells were fixed with 10% formyl saline and then with 1% osmium tetroxide before being processed into PDLLA scaffolds as previously described. The construct was imaged by using a high-resolution μCT system (μCT 40; Scanco Medical, Bassersdorf, Switzerland). The scanner was set to a voltage of 55 kVp, a current of 145 mA. Samples were scanned at 8-μm voxel (3D-pixel) resolution with an integration time of 120 ms to produce 3D reconstructed images. The cell-loaded scaffolds were thresholded at 60 (arbitrary) to view the porous scaffold and then at 190 to view the osmium-stained cells contained within.
Metabolic Activity of Cell-Loaded scCO2-Processed Scaffolds.
Both cell viability and location were assessed with the use of LIVE/DEAD stain (Molecular Probes). The cells and polymers were incubated in 10 ml of complete DMEM containing 20 μl of ethidium homodimer-1 (to highlight the dead cells red) and 5 μl of calcein acetoxymethyl ester (to highlight the live cells green) for 1 h. The cells were then rinsed (four times) in PBS for 1 h before being observed by using fluorescence microscopy (DM IRB; Leica, Deerfield, IL).
Scaffold Histology.
PDLLA scaffolds containing cells were prepared for histology by fixing in 70% methanol for 30 min, drying overnight, and subsequent infiltration with 65°C Paraplast wax (Sigma). Ten-micrometer sections were cut by using a microtome and observed by using a fluorescence microscope for either propidium iodide-stained nuclei to identify cell location or for LIVE/DEAD-stained cells to show cell activity within the scaffolds.
SEM.
Cell-seeded (3T3 fibroblast) scaffolds were fixed in 8% gluteraldehyde for 24 h and prepared for SEM analysis by dehydration and further fixation with 1% osmium tetroxide. The scaffolds were then gold-coated by using a Balzers Union SCD030 sputter coater before being scanned at 20 kV with a Philips (Eindhoven, the Netherlands) 505 scanning electron microscope.
Supplementary Material
Acknowledgments
We thank Martin Dellar, Richard Wilson, and Peter Fields for manufacturing scCO2 equipment; Robert Thomas for the isolation of primary hepatocytes; and the Biotechnology and Biological Sciences Research Council for funding. S.M.H. is a Royal Society Wolfson Research Merit Award holder.
Abbreviations
- AP
alkaline phosphatase
- BMP-2
bone morphogenetic protein-2
- LDH
lactate dehydrogenase
- μCT
microcomputed tomography
- PDLLA
poly(dl-lactic acid)
- p-NPP
p-nitrophenyl phosphate
- scCO2
supercritical CO2
- SEM
scanning electron microscopy.
Footnotes
Conflict of interest statement: Funding for research was received from RegenTec Ltd. and Critical Pharmaceuticals Ltd., which are spin-outs from the University of Nottingham.
This paper was submitted directly (Track II) to the PNAS office.
Parks, K., Sparacio, D., Beckman, E. J. (1996) Abs. Pap. Amer. Chem. Soc. 211, 142-PMSE Part 2.
References
- 1.Langer R., Vacanti J. P. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Emerich D. F., Winn S. R., Christenson L., Palmatier M. A., Gentile F. T., Sandberg P. P. Neurosci. Biobehav. Rev. 1992;16:437–447. doi: 10.1016/s0149-7634(05)80185-x. [DOI] [PubMed] [Google Scholar]
- 3.Ryu J., Oh D. J., Choi C. Y., Kim B. S. Biotechnol. Lett. 2003;25:1363–1367. doi: 10.1023/a:1024992706678. [DOI] [PubMed] [Google Scholar]
- 4.Anson D. S., Austen D. E. G., Brownlee G. G. Nature. 1985;315:683–685. doi: 10.1038/315683a0. [DOI] [PubMed] [Google Scholar]
- 5.Wake M. C., Gupta P. K., Mikos A. G. Cell Transplant. 1996;5:465–473. doi: 10.1177/096368979600500405. [DOI] [PubMed] [Google Scholar]
- 6.Lu L., Peter S. J., Lyman M. D., Leite S. M., Tamada J. A., Vacanti J. P., Langer R., Mikos A. G. Biomaterials. 2000;21:1837–1845. doi: 10.1016/s0142-9612(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 7.Sohier J., Haan R. E., de Groot K., Bezemer J. M. J. Controlled Release. 2003;87:57–68. doi: 10.1016/s0168-3659(02)00350-4. [DOI] [PubMed] [Google Scholar]
- 8.Vunjak-Novakovic G., Freed L. E. Adv. Drug Deliv. Rev. 1998;33:15–30. doi: 10.1016/s0169-409x(98)00017-9. [DOI] [PubMed] [Google Scholar]
- 9.Mooney D. J., Baldwin D. F., Suh N. P., Vacanti L. P., Langer R. Biomaterials. 1996;17:1417–1422. doi: 10.1016/0142-9612(96)87284-x. [DOI] [PubMed] [Google Scholar]
- 10.Yang X. B., Roach H. I., Clarke N. M. P., Howdle S. M., Quirk R., Shakesheff K. M., Oreffo R. O. C. Bone (NY) 2001;29:523–531. doi: 10.1016/s8756-3282(01)00617-2. [DOI] [PubMed] [Google Scholar]
- 11.Woods H. M., Silva M. M. C. G., Nouvel C., Shakesheff K. M., Howdle S. M. J. Mater. Chem. 2004;14:1663–1678. [Google Scholar]
- 12.Howdle S. M., Watson M. S., Whitaker M. J., Popov V. K., Davies M. C., Mandel F. S., Wang J. D., Shakesheff K. M. Chem. Commun. 2001;1:109–110. [Google Scholar]
- 13.Watson M. S., Whitaker M. J., Howdle S. M., Shakesheff K. M. Adv. Mater. 2002;14:1802–1804. [Google Scholar]
- 14.Yang X. B., Whitaker M. J., Sebald W., Clarke N., Howdle S. M., Shakesheff K. M., Oreffo R. O. C. Tissue Eng. 2004;10:1037–1045. doi: 10.1089/ten.2004.10.1037. [DOI] [PubMed] [Google Scholar]
- 15.Fraser D. Nature. 1951;167:33–34. doi: 10.1038/167033b0. [DOI] [PubMed] [Google Scholar]
- 16.Foster J. W., Cowan R. M., Maag T.A. J. Bacteriol. 1962;83:330–334. doi: 10.1128/jb.83.2.330-334.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillow A. K., Dehghani F., Hrkach J. S., Foster N. R., Langer R. Proc. Natl. Acad. Sci. USA. 1999;96:10344–10348. doi: 10.1073/pnas.96.18.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura K., Enomoto A., Fukushima H., Nagai K., Hakoda M. Biosci. Biotechnol. Biochem. 1994;58:1297–1301. [Google Scholar]
- 19.Enomoto A., Nakamura K., Nagai K., Hashimoto T., Hakoda M. Biosci. Biotechnol. Biochem. 1997;61:1133–1137. doi: 10.1271/bbb.61.1133. [DOI] [PubMed] [Google Scholar]
- 20.Haas G. J., Prescott H. E., Dudley E., Dir R., Hintlian C., Keane L. J. Food Safety. 1989;9:253–265. [Google Scholar]
- 21.Spilimbergo S., Elvassore N., Bertucco A. J. Supercrit. Fluids. 2002;22:55–63. [Google Scholar]
- 22.Han B., Tang B., Nimni M. E. J. Orthop. Res. 2003;21:648–654. doi: 10.1016/S0736-0266(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 23.Okubo Y., Bessho K., Fujimura K., Iizuka T., Miyatake S. Biochem. Biophys. Res. Commun. 1999;262:739–743. doi: 10.1006/bbrc.1999.1281. [DOI] [PubMed] [Google Scholar]
- 24.Wheatley S. P., McNeish A. Int. Rev. Cytol. 2005;247:35–88. doi: 10.1016/S0074-7696(05)47002-3. [DOI] [PubMed] [Google Scholar]
- 25.Collier S., Ghosh P. Osteoarthritis Cartilage. 1995;3:127–138. doi: 10.1016/s1063-4584(05)80045-7. [DOI] [PubMed] [Google Scholar]
- 26.Seglen P. O. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 27.Katagiri T., Yamaguchi A., Komaki M., Abe E., Takahashi N., Ikeda T., Rosen V., Wozney J. M., Fujisawa-Sehara A., Suda T. J. Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichida F., Nishimura R., Hata K., Matsubara T., Ikeda F., Hisada K., Yatani H., Cao X., Komori T., Yamaguchi A., Yoneda T. J. Biol. Chem. 2004;279:34015–34022. doi: 10.1074/jbc.M403621200. [DOI] [PubMed] [Google Scholar]
- 29.Tare R. S., Oreffo R. O., Clarke N. M., Roach H. I. J. Bone Miner. Res. 2002;17:2009–2020. doi: 10.1359/jbmr.2002.17.11.2009. [DOI] [PubMed] [Google Scholar]
- 30.Oreffo R. O., Bennett A., Carr A. J., Triffitt J. T. Scand. J. Rheumatol. 1998;27:415–424. doi: 10.1080/030097498442235. [DOI] [PubMed] [Google Scholar]
- 31.Cesarone C. F., Bolognesi C., Santi L. Anal. Biochem. 1979;100:188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.