Abstract
Whether nascent glutamatergic synapses acquire their AMPA receptors constitutively or via a regulated pathway triggered by pre-existing NMDA receptor activation is still an open issue. Here, we provide evidence that some glutamatergic synapses develop without expressing NMDA receptors. Using immunocytochemistry, we showed that synapses between developing rat climbing fibres and Purkinje cells expressed GluR2-containing AMPA receptors as soon as they were formed (i.e. on embryonic day 19) but never carried detectable NMDA receptors. This was confirmed by electrophysiological recordings. Excitatory synaptic currents were recorded in Purkinje cells as early as P0. However, no NMDA receptor-mediated component was found in either spontaneous or evoked synaptic responses. In addition, we ruled out a possible role of extrasynaptic NMDA receptors by showing that AMPA receptor clustering at nascent climbing fibre synapses was not modified by chronic in utero NMDA receptor blockade.
Fast transmission at mature glutamatergic synapses is mostly due to the activation of two subtypes of ionotropic glutamate receptors which differ in their biophysical and pharmacological properties: the AMPA and the NMDA receptors (see Dingledine et al. 1999 for review). The AMPA receptor family is made up of four subunits termed GluR1 to GluR4. NMDA receptors include at least one NR1 subunit with NR2 subunits (NR2A to NR2D). NMDA receptors are blocked by magnesium ions at resting but not at depolarized potentials. Since their opening requires both glutamate release (i.e. presynaptic activity) and cell depolarization (i.e. postsynaptic activity), they may be considered as coincidence detectors.
Current hypothesis suggest that the NMDA receptor plays a pivotal role in the activity-dependent conversion of immature glutamatergic synapses into functional ones. Numerous glutamatergic synapses recorded early in development are functionally ‘silent’ synapses which exhibit pure NMDA responses readily observable at depolarized potentials but undetectable at resting potential due to the voltage-dependent block of NMDA receptors by magnesium (Isaac et al. 1995; Durand et al. 1996; Wu et al. 1996). This finding plus the anatomical demonstration of the existence of pure NMDA synapses (Nusser et al. 1998; Petralia et al. 1999; Liao et al. 1999, 2001) has led to the proposal that nascent glutamatergic synapses contain NMDA receptors only and become functional by acquiring AMPA receptors through an activity-dependent process requiring NMDA-receptor activation. Some data, however, have challenged this view (Friedman et al. 2000; Cottrell et al. 2000; Groc et al. 2002). Alternative hypothesis favouring presynaptic mechanisms (Choi et al. 2000; Gasparini et al. 2000; Renger et al. 2001; Maggi et al. 2003) have been proposed to explain the occurrence of pure NMDA responses early in development. On the other hand, a recent study suggests that silent synapses in the neonatal hippocampus are not nascent synapses but result from an activity-dependent removal of AMPA receptors at pre-existing synaptic contacts (Xiao et al. 2004). Therefore, the fundamental question as to whether nascent glutamatergic synapses contain NMDA receptors only and acquire AMPA receptors secondarily through NMDA receptor activation is still a matter of debate.
Neurotransmission between climbing fibres and Purkinje cells is glutamatergic. Adult climbing fibre synapses completely lack the NMDA receptor (Perkel et al. 1990; Llano et al. 1991). However, developing Purkinje cells have been shown to express functional NMDA receptors until P8 (Rosenmund et al. 1992; Momiyama et al. 1996). Determining whether or not these NMDA receptors expressed by developing Purkinje cells are inserted into synapses and whether or not their activation is required for synaptic maturation may help elucidate the role of NMDA receptors in synaptogenesis.
In the present study, we have investigated the expression of AMPA and NMDA receptors at developing climbing fibre synapses using both immunolabelling and electrophysiological recordings. In addition, we have examined the effects of chronic NMDA receptor blockade on climbing fibre synaptogenesis.
Methods
Animals
All procedures were in agreement with the European Communities Council directive (86/609/EEC). Pregnant female Wistar rats were checked daily for delivery. Day of birth was considered as postnatal day zero (P0). Embryos were taken from dated pregnant females purchased from Charles River Laboratory. The day of finding sperm-positive vaginal smear was considered as embryonic day zero (E0). Adult animals (180–200 g) were also used in addition to embryos and developing rat pups.
Chronic NMDA receptor blockade was performed on four pregnant females by i.p. injections of MK-801 (Sigma France). Starting from E17, each animal received two daily injections of MK-801 (0.5 mg kg−1) at 08.00 h and 18.00 h On day E20, embryos were removed by hysterotomy under halothane anaesthesia and immediately killed by decapitation. Embryos from pregnant females that received i.p. injections of saline solution (n = 4) from E17 to E20 were used as controls.
Antigen retrieval
We used antigen retrieval by microwave irradiation in order to increase detection of synaptically located antigens (Fritschy et al. 1998). Embryos (E19–E20), rat pups (P0, P4, P7, P12) and adult rats (180–200 g) were killed by decapitation under halothane anaesthesia. Cerebella were removed and immediately frozen in cold isopentane (−50°C). Coronal cerebellar sections (5–14 μm thick) were obtained on a cryostat, thaw-mounted on gelatinized glass slides and fixed by immersion in phosphate buffer containing 0.5% paraformaldehyde under microwave irradiation (45 s, 800 W; Fritschy et al. 1998; see also Lachamp et al. 2003). Microwave-irradiated sections were used for both confocal and electron microscope analysis.
Immunofluorescent labelling and confocal image acquisition
The following primary antibodies were used: mouse anti-GluR2 (Chemicon, Temecula, CA, USA; 4 μg ml−1), mouse anti-NR1 (Pharmingen, San Diego, CA, USA; 1/1000), rabbit antisynaptophysin (Zymed, San Francisco, CA, USA; 1/50), rabbit anti-VgluT2 (Synaptic System, Göttingen, Germany; 1/1000) and rabbit anticalbindin (Chemicon, 1/1000). Secondary antibodies were Alexa fluor 488-conjugated goat antimouse (Molecular Probes, Eugene, OR, USA; 1/200) and Alexa fluor 546-conjugated goat antirabbit (Molecular Probes, 1/200). All antibodies were diluted in phosphate-buffered saline (PBS, 0.1 m, pH 7.4). Blocking steps were performed with 5% normal goat serum in PBS.
Confocal image acquisition was performed on a Leica TCS SP2 laser scanning microscope (Leica microsystems, Heidelberg, Germany) using the 488 nm band of an Ar laser for excitation of Alexa Fluor 488 (spectral detection: 500–535 nm) and the 543 nm band of an He–Ne laser for excitation of Alexa Fluor 546 (spectral detection: 565–620 nm). High magnification images were acquired using a 63× oil immersion objective (numerical aperture: 1.32). Voxel size was adjusted to 58 nm in x and y and to 162 nm in z. Image editing was performed using Adobe Photoshop.
Image analysis and quantification
The degree of association between receptor immunostaining (GluR2 or NR1) and axon terminal labelling (synaptophysin immunoreactivity) was evaluated on two-channel high magnification confocal images (area sampled: 3500–7000 μm2, depending on the animal). Analysis was performed using the public domain NIH Image program (developed at the US National Institutes of Health and available online at http://rsb.info.nih.gov/nih-image/) and custom-made macros. Axon terminals were first extracted from the synaptophysin channel by image segmentation using a visually adjusted threshold. Two sets of pixels were then selected from the second channel (receptor immunoreactivity). The first set corresponded to the top 0.5% pixels (i.e. the 0.5% pixels exhibiting the highest fluorescence levels in the second channel). The second set was made up of an identical number of pixels randomly chosen. The distance to the nearest axon terminal was then computed for each pixel in each set and histograms representing the distributions of distances were constructed. This was done using a class size (180 nm) that roughly corresponded to the resolution limit. Results obtained from at least three different animals under identical experimental conditions (same age and same area) were pooled. The distributions of distances from the 0.5% top and the random sets were compared using the Kolmogorov-Smirnov test.
Effect of NMDA receptor blockade was evaluated by counting receptor clusters on cerebellar sections from seven MK-801-treated E20 embryos (including the 4 used for detection of apopotosis, see below) and seven age-matched control animals (including the 4 used for detection of apopotosis, see below). Counting was done on high magnification images (one channel single confocal sections, area sampled: 3500 μm2 in each animal). Immunoreactive puncta were extracted by threshold segmentation and counted using the NIH Image software. Results were expressed as number of clusters per square micrometre and analysed using the two-tailed Mann-Whitney test.
Detection of apopotosis
Increase in apoptosis in the ventromedial hypothalamus was used as a positive control for NMDA receptor blockade in utero (see Ikonomidou et al. 1999). Analysis was carried out on sections from four MK-801-treated E20 embryos and four age-matched controls. Apoptosis was detected by TUNEL staining using the Apoptag red in situ apoptosis detection kit (Chemicon, Temacula, CA, USA) according to the manufacturer's instructions. Briefly, cryostat sections were fixed for 10 min in 1% paraformaldehyde in phosphate buffer (PB), postfixed for 5 min in cold (−20°C) ethanol–acetic acid, transferred to equilibration buffer after rinsing and then incubated 1 h at 37°C in the presence of terminal transferase and digoxigenin-labelled nucleotides. Digoxigenin was visualized using a Cy3-conjugated antidigoxigenin antibody. Apoptotic cells were counted on maximum intensity projections of 2.7 μm confocal stacks (area investigated: 0.56 mm2 per animal). Results were analysed using the two-tailed Mann-Whitney test.
Immunostaining for electron microscopy
Microwave-irradiated sections were processed using the mouse anti-GluR2 antibody and a biotinylated goat anti-mouse antibody (Jackson Immunoresearch, West Grove, PA, USA; 1/200) as primary and secondary antibodies, respectively. Sections were then incubated with avidin–biotin–peroxidase complex (ABC kit, Vector Laboratories, Burlingame, CA, USA). Peroxidase labelling was revealed using 3,3′-diaminobenzidine tetrahydrochloride (DAB, 0.025% in PB) and hydrogen peroxide (0.01%). Sections were fixed with 1% osmium tetroxide in PB (45 min), stained with 1% uranyl acetate in distilled water (45 min), dehydrated in ethanol and propylene oxide and flat embedded in Durcupan. Ultra-thin sections were examined and photographed on a Phillips CM10 electron microscope. Negatives were digitalized at 2400 p.p.i. using an Epson Perfection scanner.
Slice preparation and electrophysiological recordings
Preparation of cerebellar slices from young Wistar rats (P0–P7) was adapted from Tell & Bradley (1994). Coronal slices were placed in a recording chamber and continuously superfused with warm (32°C) physiological solution containing (mm): 130 NaCl, 3 KCl, 2.5 CaCl2, 26 NaHCO3, 1.25 KH2PO4, 10 glucose, 0.5 ascorbate, 2 pyruvate, 3 myo-inositol. The solution was aerated with 95% O2–5% CO2 to maintain a pH of 7.4. Patch electrodes tip resistance 2–3 MΩ were filled with a solution containing (mm): 120 caesium methanesulphonate, 10 NaCl, 1 MgCl2, 1 CaCl2, 10 EGTA, 2 ATP, 0.3 GTP, 10 glucose and 10 Hepes (pH 7.3). Bipolar stimulation of climbing fibres (1–10 V, 100 μs, 0.05 Hz) was applied through a tungsten electrode. Whole-cell recordings were performed using an Axopatch 200B (Axon Instruments, Union City, CA, USA) in the presence of a mixture of bicucculine (10 μm) and picrotoxin (50 μm) to prevent putative GABA synaptic responses. All chemicals were obtained from Sigma-Aldrich.
Results
Detection of synaptic AMPA and NMDA receptors using antigen retrieval
In microwave-processed adult cerebellar sections, GluR2 immunoreactivity was present in both the molecular and the granular layer (Fig. 1A). This distribution pattern is in agreement with the fact that GluR2 is the main subunit expressed by Purkinje and granule cells (Sato et al. 1993) and with the demonstrated presence of GluR2 at parallel and climbing fibre synapses (Petralia et al. 1998; Zhao et al. 1998). When viewed at high magnification, GluR2 immunofluorescence appeared to be composed of numerous puncta with apparent diameters less than 1 μm in size. In the molecular layer, GluR2 immunoreactive puncta were apposed to calbindin immunoreactive Purkinje cell dendrites (Fig. 1E). In the granular layer, immunoreactive puncta were grouped in aggregates, 5–10 μm in size, presumably corresponding to synapses between mossy fibres and granule cell dendrites in glomeruli (Fig. 1D). No or only faint immunostaining was observed in Purkinje cell bodies (Fig. 1A). Thus, the size and the distribution of GluR2 immmunoreactive puncta were such as would be expected from labelling occurring at synapses. Furthermore, in double-labelling experiments, immunoreactive puncta were invariably found in close apposition with presynaptic markers. In the granular layer, GluR2 immunoreactive puncta surrounded large patches immunoreactive for both synaptophysin (not shown) and type II vesicular glutamate transporter (VGluT2; Fig. 1D). In the molecular layer also, GluR2 immunoreactive puncta were apposed to synaptophysin immunoreactive patches (not shown). In addition, puncta localized on Purkinje cell main dendrites were invariably associated with VGluT2 (a marker of climbing fibre terminals versus parallel fibre terminals; Fig. 1E). Electron microscopic examination of microwave-processed cerebellar sections confirmed the synaptic localization of GluR2 immunoreactivity after antigen retrieval. As shown in Fig. 1C, peroxidase reaction product deposits within the granular layer were exclusively found at synapses between mossy fibres and granule cell dendrites. Furthermore, immunostaining was present within the synaptic cleft in agreement with the known extracellular location of the epitope recognized by the anti-GluR2 antibody.
Figure 1. Immunodetection of AMPA and NMDA receptors in the adult rat cerebellum after antigen retrieval.
A and B, distributions of GluR2 (A) and NR1 (B) immunofluorescence on coronal cerebellar sections (maximum intensity projections of 1.5 μm thick confocal stacks). Calbindin immunoreactivity (in red) identifies Purkinje cell bodies and dendrites. In A, GluR2 immunoreactivity (green) is distributed over Purkinje cell dendrites in the molecular layer (ML) and regrouped in aggregates reminiscent of synaptic glomeruli in the granular layer (GL). In B, punctate NR1 immunoreactivity (green) is found in synaptic glomeruli in the granular layer. C, electron micrograph of a synaptic glomerulus in the granular layer. GluR2 labelling (immunoperoxidase staining, indicated by arrowheads) is present at synapses between the mossy fibre terminal (mf) and granule cells dendrites. C′, enlargements of boxed areas in C. D, confocal image of a synaptic glomerulus in the granular layer (single optical section). The mossy fibre terminal (identified by VGluT2 immunoreactivity, in red) is decorated with GluR2 immunoreactive puncta (in green, some indicated by arrowheads). E, distribution of GluR2 immunofluorescence (green) in the molecular layer (single confocal section). VGluT2 immunoreactvity (in red) identifies climbing fibre terminals. Lowers panels are enlargements of the boxed area. Arrows and arrowheads indicate GluR2 puncta at presumptive climbing fibre synapses (apposition to VGluT2 immunoreactivity) and parallel fibre synapses, respectively.
NR1 labelling obtained in the rat cerebellum after antigen retrieval by microwaves was very similar to that described in the mouse cerebellum after pepsin pretreatment (Yamada et al. 2001). Unambiguous NR1 immunoreactivity was found in the granular layer and consisted of puncta nearly identical to those observed using the anti-GluR2 antibody (Fig. 1B). These NR1 immunoreactive puncta were grouped in aggregates, as were GluR2 immunoreactive puncta in the same layer, and double-labelling experiments showed that they were closely apposed to presumptive mossy fibre terminals identified by either synaptophysin or VgluT2 immunoreactivity (not shown). They were therefore likely to correspond to the NMDA receptor accumulations previously demonstrated in both glomerular synapses and glomerular attachment plaques (Petralia et al. 2002). Fluorescence levels found in the molecular layer and Purkinje cell bodies were close to background. However, we cannot exclude that part of this fluorescence was due to the labelling of diffuse NR1 proteins. Extrasynaptic receptors have been demonstrated on molecular layer interneurones (which lack synaptic NMDA receptors, see Clark & Cull-Candy, 2002) and on granule cells axons and terminals (Casado et al. 2000). Furthermore, antigen retrieval is known to have opposite effects on antigens located within and outside postsynaptic densities. While enhancing detection of synaptic receptors, it strongly reduces labelling of various nonsynaptic proteins including extrasynaptic receptors (Fritschy et al. 1998; Lachamp et al. 2003; see also Yamada et al. 2001).
Developing climbing fibre--Purkinje cell synapses express GluR2-containing AMPA receptors
Having confirmed that antigen retrieval allow immunodetection of synaptic receptors, we next tried to determine the onset of AMPA receptor clustering at climbing fibre synapses. In the rat cerebellar cortex, the presence of synapses is attested as early as E19 (West & del Cerro, 1976; Morara et al. 2001). These early synapses correspond to contacts between developing climbing fibre terminals and Purkinje cell temporary dendrites (Chedotal & Sotelo, 1993; Morara et al. 2001). They disappear upon regression of the temporary dendrite, between P2 and P4. Meanwhile, climbing fibres migrate over the Purkinje cell surface and establish new synaptic contacts on somatic protrusions, between P4 and P7, and on main dendrites from P8 onwards. We found that GluR2 immunoreactive puncta were present in the cerebellar primordium as early as E19, i.e. at the very onset of climbing fibre synaptogenesis (Fig. 2A). At these early stages, the density of puncta was very low and immunoreactivity was restricted to areas containing Purkinje cell bodies and dendrites. Most GluR2 immunoreactive puncta (but not all, see enlargements in Fig. 2A) were apposed to synaptophysin immunoreactive patches and were therefore likely to correspond to accumulation of AMPA receptors at differentiating synaptic sites between climbing fibres and Purkinje cells. The densities of GluR2 immunoreactive puncta increased between E19 and E20 (Fig. 2B). At this stage, virtually all GluR2 puncta were closely apposed to presynaptic terminals identified by synaptophysin or VGluT2 immunoreactivity. The location of the immunoreactive puncta on Purkinje cells was found to change with ongoing development and translocation of climbing fibre inputs on cell surface. They were on temporary apical dendrites at P0, on perikarya at P4–P7 and on apical poles and developing stem dendrites at P12 (Fig. 2C). In addition to puncta, diffuse immunoreaction presumably corresponding to intracellular GluR2 subunit pools was observed in developing Purkinje cells from E19 to P12. This diffuse intracellular immunoreaction was especially prominent around P4–P7 (Fig. 2C).
Figure 2. Immunodetection of AMPA receptors in the developing cerebellum.
A, distribution of GluR2 immunofluorescence (in green) in the Purkinje cell plate of an E19 embryo (single confocal section). Lowers panels are enlargements of the boxed area. Most immunoreactive puncta (some indicated by arrowheads in the lower panels) are apposed to presumptive axon terminals (identified by synaptophysin immunoreactivity, in red). The double arrow in the lower panels indicates a GluR2 immunoreactive puncta apparently not apposed to synaptophysin labelling. B, distribution of GluR2 immmunofluorescence (in green) in the Purkinje cell plate of an E20 embryo (single confocal sections). Virtually all GluR2-containing AMPA receptor clusters are apposed to the presynaptic markers synaptophysin (left panel, red) and VGluT2 (right panel, red). C, developmental translocation of GluR2-containing AMPA receptor clusters (green) on the cell bodies and dendrites of Purkinje cells identified by calbindin immunolabelling (in red). Note the increased intracellular expression of GluR2 at P4–P7.
The presence of GluR2 at nascent climbing fibre synapses on Purkinje cells before P2 was unexpected since the synaptic contacts found at these early developmental stages are both transient and morphologically very immature (Armengol & Sotelo, 1991; Chedotal & Sotelo, 1993; Morara et al. 2001). Thus, recordings were performed on cerebellar slices of P0 rats to determine whether functional synapses already exist on Purkinje cells at birth. Purkinje cells were selected according to their location, their relatively high capacitance (36 ± 5 pF) and the presence of a fast transient inward current when stepped to 0 mV (Fig. 3A). Eight cells that met these criteria displayed low frequency (0.5–2 Hz) spontaneous excitatory postsynaptic currents (Fig. 3B and C). These spontaneous EPSCs recorded at P0 had fast rise time (0.31 ± 0.3 ms, n = 8) and decay (2.06 ± 1.2 ms, n = 8) and mean amplitudes ranging from 20 to 50 pA. Bath application of 10 μm CNQX suppressed all spontaneous EPSCs, thereby indicating that they were due to AMPA receptor activation (Fig. 3B).
Figure 3. Spontaneous synaptic activity in Purkinje cell at P0.
A, fast inward current recorded in a Purkinje cell at a holding potential of −70 mV when stepped to 0 mV. B, the spontaneous activity recorded in a Purkinje cell at a holding potential of −70 mV (top traces; 96 EPSCs recorded over a 180 s control period) is completely abolished by bath application of CNQX (bottom traces; no EPSCs recorded over a 300-s period of CNQX application). C, 1 and 2, examples of two spontaneous EPSCs observed in A at a larger time scale. 3, averaged trace for the same cell.
Developing climbing fibres–Purkinje cell synapses lack NMDA receptors
Developing Purkinje cells express functional NMDA receptors until P8 (Rosenmund et al. 1992; Momiyama et al. 1996; Misra et al. 2000b). To determine whether these receptors are inserted into nascent climbing fibre synapses, we both analysed the distribution of NR1 immunoreactivity from E19 to P7 and recorded spontaneous and evoked synaptic currents in developing Purkinje cells.
Throughout the period investigated, low but noticeable NR1 immunoreactivity was present in the cerebellar cortex (Fig. 4). Purkinje cells exhibited an intracellular reaction which was especially prominent around P4 (Fig. 4C and D). Puncta exhibiting strong to moderate NR1 immunoreactivity, presumably corresponding to NMDA receptor clusters, were sometimes observed in the molecular layer of embryonic and postnatal cerebellar sections (Fig. 4A and D). However, contrary to GluR2 immmunoreactive puncta (Fig. 4B and E), they were infrequent and virtually never associated with synaptophysin immunoreactive patches. This was confirmed by quantitative analysis (Fig. 5). In the molecular layer, GluR2 immunolabelling was significantly associated with axon terminals whereas NR1 immunolabelling exhibited a more random distribution. The absence of synaptic NR1 labelling in the molecular layer was unlikely to be due to a flaw in the sensitivity of the method used. In sections from P4–P7 animals, NMDA receptors were absent from synapses in the molecular layer (Fig. 4D) but readily detected in association with axon terminals in the granular layer (Fig. 4F; see also Fig. 5).
Figure 4. Compared distributions of AMPA and NMDA receptors in the developing cerebellum.
A and B, comparison of distributions of NR1 (A, green) and GluR2 (B, green) in the Purkinje cell plate of a E20 embryo. Right panels are enlargements of boxed areas. In A, NR1 immunoreactive bright puncta are very few (one indicated by arrowhead in enlargements) and virtually none is apposed to synaptophysin immunoreactivity (in red). In B, virtually all GluR2 immunoreactive bright puncta (some indicated by arrowheads in enlargements) are in close apposition to synaptophysin immunoreactive axon terminals (in red). C, distribution of NR1 immunoreactivity in the cerebellar cortex of a P4 rat pulp (single confocal section). Purkinje cells are identified by calbindin immunolabelling (in red). NR1 immunoreactivity (in green) is found in Purkinje cell bodies (some indicated by stars) and in the developing molecular (ML) and internal granular (IGL) layers. The external granular layer (EGL) exhibits background levels of NR1 immunolabelling. D and E, comparison of distributions of NR1 (D, green) and GluR2 (E, green) in the molecular layer of a P4 rat pup. In D, a few small NR1 immunoreactive puncta are found in the developing molecular layer but none is apposed to terminals. In E, numerous GluR2 immunoreactive puncta (some indicated by arrowheads) are apposed to synaptophysin immunoreactive terminals (red). Stars indicate Purkinje cell bodies. F, distribution of NR1 immunolabelling (green) in the granular layer at P4 (same section as in D). Numerous NR1 immunoreactive puncta (some indicated by arrowheads) are colocalized with axon terminals (in red). All images except C are maximum intensity projections of 0.6 μm thick confocal stacks.
Figure 5. Relationship between receptor immunoreactivity and axon terminal labelling.
Distance-distribution histograms constructed as described in Methods. GluR2 pixels in the molecular layer and NR1 pixels in the granular layer are associated with axon terminals as shown by the fact that their distributions are skewed to the left (significantly different from random: P < 0.05 in both cases, Kolmogorov-Smirnov test). In contrast, the even distribution of NR1 in the molecular layer (not different from random) indicates that there is no association with axon terminals.
Electrophysiological experiments failed to demonstrate any NMDA component in synaptic responses recorded in developing Purkinje cells (Fig. 6). Recordings were performed using a perfusion medium containing no magnesium and a holding potential of −70 mV; d-serine (10 μm) was added to the perfusion medium in order to increase any NMDA response. Controls were also carried out using an holding potential of +40 mV (e.g. Fig. 6A2) to check for a possible blockade of NMDA receptor by remaining magnesium ions. Nevertheless, spontaneous EPSCs recorded in Purkinje cells from P0 to P7 (n = 26; 8 at P0, 4 at P2, 2 at P6, 6 at P5 and 6 at P7) had fast rise and decay times that did not significantly changed over the period studied (0.34 ± 0.08 ms for rise times; 1.91 ± 0.53 ms for decays). However membrane capacitance nearly doubled from P0 to P7 (from 36 ± 5 to 74 ± 9 pF; P < 0.005). Furthermore, whatever the holding potential, normalized averaged spontaneous EPSCs recorded before and during application of APV (50 μm) had similar rise times and decays (0.34 ± 0.08 ms for rise times and 1.91 ± 0.53 ms for decays before APV; 0.37 ± 0.10 ms for rise times and 1.99 ± 0.63 ms for decays after APV; n = 26), thereby confirming the absence of an NMDA receptor-mediated component (Fig. 6A). We next investigated a possible contribution of NMDA receptors to the response evoked by climbing fibre activation (Fig. 6B). EPSCs elicited by stimulation in Purkinje cells at P4 (n = 5) had features of immature climbing fibre responses (Crepel et al. 1976; Llano et al. 1991). They were large (200–1000 pA) and consisted of two to three discrete components with different thresholds (and in some cases different latencies, see for instance Fig. 6B1) that presumably reflected multi-innervations. Bath application of APV (50 μm, Fig. 6B2) failed to induce significant change in either the amplitudes or the rise times and decays of the climbing fibre responses (rise times: 0.91 ± 0.11 ms before APV versus 0.86 ± 0.11 ms after APV; decays: 13.3 ± 3.1 ms before APV versus 13.0 ± 3.5 ms after APV; n = 5).
Figure 6. Absence of an NMDA component in the synaptic responses of Purkinje cells.
A, normalized superimposed averaged traces of spontaneous EPSC before and after application of APV in a Mg2+-free saline in a P0 Purkinje cell at two different holding potentials (1 and 2) and in a P2 (3) and a P5 (4) Purkinje cells. Note that APV never alters the decay rate. B, superimposed evoked synaptic responses in two P4 Purkinje cells before and after APV application at two different holding potentials (1 and 2). Stimulation (arrows) was applied on climbing fibres. Note the biphasic response in 1 presumably reflecting multi-innervation of the Purkinje cell at this developmental stage.
Clustering of GluR2-containing AMPA receptors at developing climbing fibre–Purkinje cell synapses does not require activation of NMDA receptors
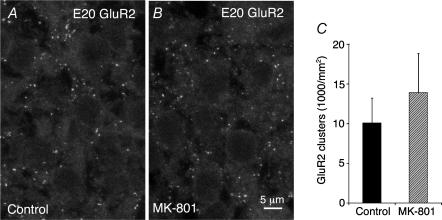
Having demonstrated that NMDA receptors expressed by developing Purkinje cells remain extra-synaptic, we next considered whether they play a role in AMPA receptor clustering. We performed chronic NMDA receptor blockade in pregnant female rat using MK-801, a non-competitive antagonist that undergoes placental transfer (Ikonomidou et al. 1999; Adams et al. 2004). NMDA receptor blockade was started at E17 (i.e. 2 days before the onset of cerebellar synaptogenesis) and maintained until E20. A positive control for NMDA receptor blockade was obtained by analysing apoptosis in the hypothalamus. In agreement with Ikonomidou et al. (1999), we found that the densities of apoptotic cells was increased in sections of MK-801-treated embryos as compared to controls (251 ± 42 versus 129 ± 31 apoptotic cells per mm2, P < 0.05). NMDA receptor blockade failed to prevent AMPA receptor clustering. Numerous GluR2 immunoreactive puncta were apparent in the Purkinje cell layers of both control and MK-801-treated embryos (Fig. 7A and B). Virtually all these GluR2 puncta were closely apposed to synaptophysin immunoreactive terminals (not shown). The lack of effect of NMDA receptor blockade on AMPA receptor clustering was confirmed by quantitative analysis (Fig. 7C), which failed to demonstrate any significant difference between GluR2 puncta densities measured in control and MK-801-treated animals (10.1 ± 3.1 and 13.9 ± 4.9 thousands of clusters per mm2, respectively).
Figure 7. NMDA blockade does not impair AMPA receptor clustering in the developing cerebellum.
A and B, GluR2 immunofluorescence in the Purkinje cell plates of a control (A) and an MK-801-treated (B) E20 embryo. Maximum intensity projections of 2.8 μm thick confocal stacks. Numerous immunoreactive puncta are present in both cases. C, densities of AMPA receptor clusters in the Purkinje cell plates of control (n = 7) and MK-801-treated (n = 7) E20 embryos. Increase after MK-801 is not significant.
Discussion
Our findings support the view that the events involved in climbing fibre synapse formation differ from those which give rise to hippocampal and neocortical synapses. Climbing fibre synapses never expressed a detectable amount of NMDA receptors. In addition, we found that climbing fibre synapses accumulated AMPA receptors early after they were formed via a mechanism that did not depend on NMDA receptor activation.
The first climbing fibre synapses appears in the Purkinje cell plate at E19. This early synaptic investment has led to the suggestion that climbing fibres may influence the embryonic differentiation of their targets (Chedotal & Sotelo, 1993; Morara et al. 2001). There is still a controversy as to whether these early synapses are functional. They are morphologically immature (Armengol & Sotelo, 1991; Chedotal & Sotelo, 1993; Morara et al. 2001) and previous electrophysiological studies have failed to detect climbing fibre responses before P2 (Mariani & Changeux, 1981; Crepel et al. 1981). Here, we show that climbing fibre synapses were competent to elicit postsynaptic responses as early as P0. Furthermore, we found that AMPA receptor clusters were associated with VGluT-expressing presumptive terminals in the Purkinje cell plate as early as E20. Since VGluT expression is a key determinant of synaptic vesicle loading and cycling (Fremeau et al. 2004; Wojcik et al. 2004), this finding suggests that some climbing fibres established functional synapses before birth. These early climbing fibre synapses are unlikely to play a role in dendritic arbor growth and stabilization (Cline, 2001). First, they form on temporary dendrites that disappear upon development. In addition, recent in vitro experiments indicate that afferent activity is of minor importance for the development of Purkinje cell dendritic tree (Adcock et al. 2004). Alternatively, this early synaptic activity may serve to establish a blueprint of the olivocerebellar map by stabilizing climbing fibre terminals via retrograde signals. Electron microscope observations indicate that the retraction of Purkinje cell temporary dendrites occurs without any evidence of axon terminal degeneration or regression (Morara et al. 2001).
The lack of detectable NMDA receptors in developing climbing fibre synapses is intriguing since Purkinje cells express functional NMDA receptors during the first postnatal week (Rosenmund et al. 1992; Momiyama et al. 1996; Misra et al. 2000b). The possibility that Purkinje cells lack the specific mechanisms that drive NMDA receptors to synapses, such as those involving EphB2 (Henderson et al. 2001) or α-neurexin (Kattenstroth et al. 2004), appears unlikely. Transfection experiments with a recombinant Sindbis virus encoding the NR2B subunit indicate that these mechanisms operate in Purkinje cells, at least in mature ones (Kakegawa et al. 2003). Alternatively, NMDA receptors expressed by developing Purkinje cells may lack the molecular determinants required for synaptic targeting. Synaptic delivery and anchoring of NMDA receptors depends on interactions between C-termini and intracellular proteins (Prybylowski & Wenthold, 2004). Furthermore, the identity of the NR2 subunits is critical. NMDA receptors containing wild-type NR2B or NR2A subunits are targeted to synapses, whereas those made of NR2A or NR2B subunits that lack the C-terminal PDZ-interacting domain are still delivered to the cell surface but excluded from synapses (Steigerwald et al. 2000; Prybylowski et al. 2002; Barria & Malinow, 2002). Developing Purkinje cells express NR1 and NR2D subunits (Akazawa et al. 1994; Misra et al. 2000b). The C-terminus of NR2D differs from that of NR2A and NR2B in term of PDZ-interacting domain. In addition, there is growing evidence suggesting that NR2D-containing NMDA receptors are restricted to extrasynaptic sites in cerebellar and spinal neurones (Momiyama, 2000; Misra et al. 2000a; Brickley et al. 2003; Mi et al. 2004). Thus, the presence of NR2D (and the absence of other NR2 subunits) may explain the exclusion of NMDA receptors from synapses in developing Purkinje cells.
Correlated activity is thought to play a pivotal role in the construction of neural circuits during development (Katz & Shatz, 1996; Zhang & Poo, 2001). Synaptic connections between neurones that fire together are reinforced and stabilized while other connections are weakened or even removed. NMDA receptors are thought to provide the molecular basis for this activity-dependent mechanisms of synapse selection (Constantine-Paton & Cline, 1998). Since their opening requires both glutamate release and cell depolarization, NMDA receptors may behave as Hebbian coincidence detectors that sense synchronized pre- and postsynaptic activity. Furthermore, it has been proposed that NMDA receptor activation induces synapse strengthening and stabilization by driving AMPA receptors into the postsynaptic membrane. Experiments performed to address this question have provided conflicting data. Some studies suggest that AMPA receptor acquisition during development or potentiation requires both electrical activity and NMDA receptor activation (Zhu et al. 2000). Others show that neither genetic ablation of NR1 (Li et al. 1994) nor chronic pharmacological blockade of NMDA receptors (Luthi et al. 2001; Colonnese et al. 2003; Vincent et al. 2004) prevents synapse development or AMPA receptor acquisition. Consistent with the latter, we found that NMDA receptor blockade by MK-801 did not impair AMPA receptor delivery to nascent climbing fibre synapses. It may be argued that chronic NMDA receptor blockade triggered compensatory NMDA-independent mechanisms of AMPA receptor acquisition (Zhu & Malinow, 2002). However, NMDA receptors in Purkinje cells are unlikely to behave as coincidence detectors. Contrary to what occurs in most other neurones, they were not concentrated at synapses. Furthermore, we found no NMDA component in the spontaneous or evoked excitatory postsynaptic currents, presumably because of limited spillover and/or very low NMDA receptor densities in the immediate vicinity of release sites. Thus, due to their location outside synapses, NMDA receptors in developing Purkinje cells may be involved in sensing ambient levels of glutamate but are unlikely to be activated by focal release of glutamate correlated with postsynaptic activity. Alternatively, there is evidence suggesting that presynaptic NMDA receptors in cerebellar interneurones contain the NR2D subunit (Glitsch & Marty, 1999). Likewise, NMDA receptors expressed by developing Purkinje cells may be routed to axons and serve as presynaptic receptors controlling GABA release in deep cerebellar nuclei.
The present data suggest that NMDA receptors play only a minor role if any in the formation of climbing fibre synapses. Thus, they appear at odds with current views on synapse development which place emphasis on NMDA receptors as detectors of correlated activity. The fact that different neuronal populations express different NMDA receptor subtypes targeted to different subcellular compartments may explain this discrepancy. For instance, NR2B and NR2A are the main subunits in developing telencephalic neurones (i.e. in models used in most influential studies on synapse development) whereas NR2D, which produce extrasynaptic receptors predominates in developing Purkinje cells and most brainstem structures (Akazawa et al. 1994). Conceivably, the mechanisms involved in synapse differentiation may differ between neurones, depending on whether they mainly express synaptic or extrasynaptic NMDA receptors.
Acknowledgments
We thank Dr A. Marty and Dr C. Levenes for helpful comments on the manuscript.
References
- Adams SM, de Rivero Vacari JC, Corriveau RA. Pronounced cell death in the absence of NMDA receptors in the developing somatosensory thalamus. J Neurosci. 2004;24:9441–9450. doi: 10.1523/JNEUROSCI.3290-04.2004. 10.1523/JNEUROSCI.3290-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adcock KH, Metzger F, Kapfhammer JP. Purkinje cell dendritic tree development in the absence of excitatory neurotransmission and of brain-derived neurotrophic factor in organotypic slice cultures. Neuroscience. 2004;127:137–145. doi: 10.1016/j.neuroscience.2004.04.032. 10.1016/j.neuroscience.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol. 1994;347:150–160. doi: 10.1002/cne.903470112. 10.1002/cne.903470112. [DOI] [PubMed] [Google Scholar]
- Armengol JA, Sotelo C. Early dendritic development of Purkinje cells in the rat cerebellum. A light and electron microscopic study using axonal tracing in ‘in vitro’ slices. Dev Brain Res. 1991;64:95–114. doi: 10.1016/0165-3806(91)90213-3. 10.1016/0165-3806(91)90213-3. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Misra C, Mok MH, Mishina M, Cull-Candy SG. NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J Neurosci. 2003;23:4958–4966. doi: 10.1523/JNEUROSCI.23-12-04958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado M, Dieudonne S, Ascher P. Presynaptic N-methyl-D-aspartate receptors at the parallel fiber-Purkinje cell synapse. Proc Natl Acad Sci U S A. 2000;97:11593–11597. doi: 10.1073/pnas.200354297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedotal A, Sotelo C. The ‘creeper stage’ in cerebellar climbing fiber synaptogenesis precedes the ‘pericellular nest’-ultrastructural evidence with parvalbumin immunocytochemistry. Dev Brain Res. 1993;76:207–220. doi: 10.1016/0165-3806(93)90209-s. [DOI] [PubMed] [Google Scholar]
- Choi SW, Klingauf J, Tsien RW. Postfusional regulation of cleft glutamate concentration during LTP at ‘silent synapses’. Nat Neurosci. 2000;3:330–336. doi: 10.1038/73895. 10.1038/73895. [DOI] [PubMed] [Google Scholar]
- Clark BA, Cull-Candy SG. Activity-dependent recruitment of extrasynaptic NMDA receptor activation at an AMPA receptor-only synapse. J Neurosci. 2002;22:4428–4436. doi: 10.1523/JNEUROSCI.22-11-04428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT. Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol. 2001;11:118–126. doi: 10.1016/s0959-4388(00)00182-3. [DOI] [PubMed] [Google Scholar]
- Colonnese MT, Shi J, Constantine-Paton M. Chronic NMDA receptor blockade from birth delays the maturation of NMDA currents, but does not affect AMPA/kainate currents. J Neurophysiol. 2003;89:57–68. doi: 10.1152/jn.00049.2002. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT. LTP and activity-dependent synaptogenesis: the more alike they are, the more different they become. Curr Opin Neurobiol. 1998;8:139–148. doi: 10.1016/s0959-4388(98)80017-2. [DOI] [PubMed] [Google Scholar]
- Cottrell JR, Dube GR, Egles C, Liu GS. Distribution, density, and clustering of functional glutamate receptors before and after synaptogenesis in hippocampal neurons. J Neurophysiol. 2000;84:1573–1587. doi: 10.1152/jn.2000.84.3.1573. [DOI] [PubMed] [Google Scholar]
- Crepel F, Delhaye-Bouchaud N, Dupont JL. fate of the multiple innervation of cerebellar purkinje cells by climbing fibers in immature control, X-irradiated and hypothyroid rats. Brain Res. 1981;227:59–71. doi: 10.1016/0165-3806(81)90094-8. [DOI] [PubMed] [Google Scholar]
- Crepel F, Mariani J, Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976;7:567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304:1815–1819. doi: 10.1126/science.1097468. 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: Time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. 10.1016/S0896-6273(00)00009-X. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABAA receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol. 1998;390:194–210. 10.1002/(SICI)1096-9861(19980112)390:2<194::AID-CNE3>3.0.CO;2-X. [PubMed] [Google Scholar]
- Gasparini S, Saviane C, Voronin LL, Cherubini E. Silent synapses in the developing hippocampus: Lack of functional AMPA receptors or low probability of glutamate release? Proc Natl Acad Sci U S A. 2000;97:9741–9746. doi: 10.1073/pnas.170032297. 10.1073/pnas.170032297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch M, Marty A. Presynaptic effects of NMDA in cerebellar Purkinje cells and interneurons. J Neurosci. 1999;19:511–519. doi: 10.1523/JNEUROSCI.19-02-00511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Gustafsson B, Hanse E. Spontaneous unitary synaptic activity in CA1 pyramidal neurons during early postnatal development: constant contribution of AMPA and NMDA receptors. J Neurosci. 2002;22:5552–5562. doi: 10.1523/JNEUROSCI.22-13-05552.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JT, Georgiou J, Jia Z, Robertson J, Elowe S, Roder JC, Pawson T. The receptor tyrosine kinase EphB2 regulates NMDA-dependent synaptic function. Neuron. 2001;32:1041–1056. doi: 10.1016/s0896-6273(01)00553-0. 10.1016/S0896-6273(01)00553-0. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Kakegawa W, Tsuzuki K, Iino M, Ozawa S. Functional NMDA receptor channels generated by NMDAR2B gene transfer in rat cerebellar Purkinje cells. Eur J Neurosci. 2003;17:887–891. doi: 10.1046/j.1460-9568.2003.02498.x. 10.1046/j.1460-9568.2003.02498.x. [DOI] [PubMed] [Google Scholar]
- Kattenstroth G, Tantalaki E, Sudhof TC, Gottmann K, Missler M. Postsynaptic N-methyl-D-aspartate receptor function requires alpha-neurexins. Proc Natl Acad Sci U S A. 2004;101:2607–2612. doi: 10.1073/pnas.0308626100. 10.1073/pnas.0308626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Lachamp P, Balland B, Tell F, Crest M, Kessler JP. Synaptic localization of the glutamate receptor subunit GluR2 in the rat nucleus tractus solitarii. Eur J Neurosci. 2003;17:892–896. doi: 10.1046/j.1460-9568.2003.02494.x. 10.1046/j.1460-9568.2003.02494.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994;76:427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Liao D, Scannevin RH, Huganir R. Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J Neurosci. 2001;21:6008–6017. doi: 10.1523/JNEUROSCI.21-16-06008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Zhang X, O'Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci. 1999;2:37–43. doi: 10.1038/4540. 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- Llano I, Marty A, Armstrong CM, Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, Schwyzer L, Mateos JM, Gahwiler BH, McKinney RA. NMDA receptor activation limits the number of synaptic connections during hippocampal development. Nat Neurosci. 2001;4:1102–1107. doi: 10.1038/nn744. 10.1038/nn744. [DOI] [PubMed] [Google Scholar]
- Maggi L, Le Magueresse C, Changeux JP, Cherubini E. Nicotine activates immature ‘silent’ connections in the developing hippocampus. Proc Natl Acad Sci U S A. 2003;100:2059–2064. doi: 10.1073/pnas.0437947100. 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Changeux JP. Ontogenesis of olivocerebellar relationships. I. Studies by intracellular recordings of the multiple innervation of Purkinje cells by climbing fibers in the developing rat cerebellum. J Neurosci. 1981;1:696–702. doi: 10.1523/JNEUROSCI.01-07-00696.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi R, Sia GM, Rosen K, Tang X, Moghekar A, Black JL, McEnery M, Huganir RL, O'Brien RJ. AMPA receptor-dependent clustering of synaptic NMDA receptors is mediated by stargazin and NR2A/B in spinal neurons and hippocampal interneurons. Neuron. 2004;44:335–349. doi: 10.1016/j.neuron.2004.09.029. 10.1016/j.neuron.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Misra C, Brickley SG, Farrant M, Cull-Candy SG. Identification of subunits contributing to synaptic and extrasynaptic NMDA receptors in Golgi cells of the rat cerebellum. J Physiol. 2000a;524:147–162. doi: 10.1111/j.1469-7793.2000.00147.x. 10.1111/j.1469-7793.2000.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra C, Brickley SG, Wyllie DJ, Cull-Candy SG. Slow deactivation kinetics of NMDA receptors containing NR1 and NR2D subunits in rat cerebellar Purkinje cells. J Physiol. 2000b;525:299–305. doi: 10.1111/j.1469-7793.2000.t01-1-00299.x. 10.1111/j.1469-7793.2000.t01-1-00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama A. Distinct synaptic and extrasynaptic NMDA receptors identified in dorsal horn neurones of the adult rat spinal cord. J Physiol. 2000;523:621–628. doi: 10.1111/j.1469-7793.2000.t01-1-00621.x. 10.1111/j.1469-7793.2000.t01-1-00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama A, Feldmeyer D, Cull-Candy SG. Identification of a native low-conductance NMDA channel with reduced sensitivity to Mg2+ in rat central neurones. J Physiol. 1996;494:479–492. doi: 10.1113/jphysiol.1996.sp021507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morara S, van der Want JJ, de Weerd H, Provini L, Rosina A. Ultrastructural analysis of climbing fiber-Purkinje cell synaptogenesis in the rat cerebellum. Neuroscience. 2001;108:655–671. doi: 10.1016/s0306-4522(01)00433-x. 10.1016/S0306-4522(01)00433-X. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Lujan R, Laube G, Roberts JDB, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. 10.1016/S0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- Perkel DJ, Hestrin S, Sah P, Nicoll RA. Excitatory synaptic currents in Purkinje cells. Proc R Soc Lond B Biol Sci. 1990;241:116–121. doi: 10.1098/rspb.1990.0074. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal-development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. NMDA receptors and PSD-95 are found in attachment plaques in cerebellar granular layer glomeruli. Eur J Neurosci. 2002;15:583–587. doi: 10.1046/j.1460-9568.2002.01896.x. 10.1046/j.1460-9568.2002.01896.x. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Zhao HM, Wang YX, Wenthold RJ. Variations in the tangential distribution of postsynaptic glutamate receptors in Purkinje cell parallel and climbing fiber synapses during development. Neuropharmacology. 1998;37:1321–1334. doi: 10.1016/s0028-3908(98)00118-x. 10.1016/S0028-3908(98)00118-X. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, Fu Z, Losi G, Hawkins LM, Luo J, Chang K, Wenthold RJ, Vicini S. Relationship between availability of NMDA receptor subunits and their expression at the synapse. J Neurosci. 2002;22:8902–8910. doi: 10.1523/JNEUROSCI.22-20-08902.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski K, Wenthold RJ. N-Methyl-D-aspartate receptors: subunit assembly and trafficking to the synapse. J Biol Chem. 2004;279:9673–9676. doi: 10.1074/jbc.R300029200. 10.1074/jbc.R300029200. [DOI] [PubMed] [Google Scholar]
- Renger JJ, Egles C, Liu G. A developmental switch in neurotransmitter flux enhances synaptic efficacy by affecting AMPA receptor activation. Neuron. 2001;29:469–484. doi: 10.1016/s0896-6273(01)00219-7. 10.1016/S0896-6273(01)00219-7. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Legendre P, Westbrook GL. Expression of NMDA channels on cerebellar Purkinje cells acutely dissociated from newborn rats. J Neurophysiol. 1992;68:1901–1905. doi: 10.1152/jn.1992.68.5.1901. [DOI] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Tohyama M. The differential expression patterns of messenger RNAs encoding non-N-methyl-D-aspartate glutamate receptor subunits (GluR1–4) in the rat brain. Neuroscience. 1993;52:515–539. doi: 10.1016/0306-4522(93)90403-3. 10.1016/0306-4522(93)90403-3. [DOI] [PubMed] [Google Scholar]
- Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Kohr G. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci. 2000;20:4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tell F, Bradley RM. Whole-cell analysis of ionic currents underlying the firing pattern of neurons in the gustatory zone of the nucleus tractus solitarii. J Neurophysiol. 1994;71:479–492. doi: 10.1152/jn.1994.71.2.479. [DOI] [PubMed] [Google Scholar]
- Vincent A, Kessler JP, Baude A, Dipasquale E, Tell F. N-methyl-D-aspartate receptor activation exerts a dual control on postnatal development of nucleus tractus solitarii neurons in vivo. Neuroscience. 2004;126:185–194. doi: 10.1016/j.neuroscience.2004.03.012. 10.1016/j.neuroscience.2004.03.012. [DOI] [PubMed] [Google Scholar]
- West MJ, del Cerro M. Early formation of synapses in the molecular layer of the fetal rat cerebellum. J Comp Neurol. 1976;165:137–153. doi: 10.1002/cne.901650203. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GY, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- Xiao MY, Wasling P, Hanse E, Gustafsson B. Creation of AMPA-silent synapses in the neonatal hippocampus. Nat Neurosci. 2004;7:236–243. doi: 10.1038/nn1196. 10.1038/nn1196. [DOI] [PubMed] [Google Scholar]
- Yamada K, Fukaya M, Shimizu H, Sakimura K, Watanabe M. NMDA receptor subunits GluRepsilon1, GluRepsilon3 and GluRzeta1 are enriched at the mossy fibre-granule cell synapse in the adult mouse cerebellum. Eur J Neurosci. 2001;13:2025–2036. doi: 10.1046/j.0953-816x.2001.01580.x. 10.1046/j.0953-816x.2001.01580.x. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;4:1207–1214. doi: 10.1038/nn753. 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zhao HM, Wenthold RJ, Petralia RS. Glutamate-receptor targeting to synaptic populations on Purkinje-cells is developmentally-regulated. J Neurosci. 1998;18:5517–5528. doi: 10.1523/JNEUROSCI.18-14-05517.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Malinow R. Acute versus chronic NMDA receptor blockade and synaptic AMPA receptor delivery. Nat Neurosci. 2002;5:513–514. doi: 10.1038/nn0602-850. [DOI] [PubMed] [Google Scholar]