Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) plays a crucial role in regulating fluid secretion by the airways, intestines, sweat glands and other epithelial tissues. It is well established that the CFTR is a cAMP-activated, nucleotide-dependent anion channel, but additional functions are often attributed to it, including regulation of the epithelial sodium channel (ENaC). The absence of CFTR-dependent ENaC inhibition and the resulting sodium hyperabsorption were postulated to be a major electrolyte transport abnormality in cystic fibrosis (CF)-affected epithelia. Several ex vivo studies, including those that used the Xenopus oocyte expression system, have reported ENaC inhibition by activated CFTR, but contradictory results have also been obtained. Because CFTR–ENaC interactions have important implications in the pathogenesis of CF, the present investigation was undertaken by our three independent laboratories to resolve whether CFTR regulates ENaC in oocytes and to clarify potential sources of previously reported dissimilar observations. Using different experimental protocols and a wide range of channel expression levels, we found no evidence that activated CFTR regulates ENaC when oocyte membrane potential was carefully clamped. We determined that an apparent CFTR-dependent ENaC inhibition could be observed when resistance in series with the oocyte membrane was not low enough or the feedback voltage gain was not high enough. We suggest that the inhibitory effect of CFTR on ENaC reported in some earlier oocyte studies could be attributed to problems arising from high levels of channel expression and suboptimal recording conditions, that is, large series resistance and/or insufficient feedback voltage gain.
The primary function of the cystic fibrosis transmembrane conductance regulator (CFTR) is to mediate cAMP-activated anion (Cl−) conductance across the apical membrane of epithelial cells (Anderson et al. 1991; Nagel et al. 1992; Riordan, 1993; Gadsby et al. 1995; Quinton, 1999; Sheppard & Welsh, 1999; Dawson et al. 1999; Gadsby & Nairn, 1999; Nagel, 1999; Akabas, 2000). Consistent with its Cl− channel function, disease-causing mutations in the CFTR gene result in impaired transepithelial Cl− conductance, a hallmark of cystic fibrosis (CF) (Stutts & Boucher, 1999; Pilewski & Frizzell, 1999; Quinton, 1999). However, additional functions have been attributed to the CFTR, including regulation of the epithelial Na+ channel (ENaC) in airways and sweat glands (Stutts et al. 1995, 1997; Reddy et al. 1999; Reddy & Quinton, 2003), regulation of the outwardly rectifying Cl− channel (Schwiebert et al. 1995, 1999), calcium-activated Cl− channel (Kunzelmann et al. 1997; Tarran et al. 2002) and ROMK2 potassium channel (McNicholas et al. 1997), vesicle trafficking (Bradbury et al. 1992), regulation of bicarbonate transport (Ko et al. 2002; Park et al. 2002) and the expression of inflammatory mediators (Donaldson & Boucher, 2003). These additional functions of the CFTR remain the subject of intense research and debate, while some earlier claims, such as CFTR-mediated ATP release (Reisin et al. 1994) or acidification of intracellular organelles (Barasch et al. 1991), have been questioned by later studies (Reddy et al. 1996; Bradbury, 1999).
Abnormal Na+ transport by CF-affected airway epithelia has been suggested by many in vivo and in vitro observations in humans and mice, showing increased amiloride-sensitive transepithelial potentials in CF (Knowles et al. 1981, 1983; Boucher et al. 1986; Grubb et al. 1994; Mall et al. 1998; reviewed by Stutts & Boucher, 1999). The simplest interpretation of these early observations was that the rate of Na+ absorption was increased in CF, thereby explaining the dehydration of the airway surface liquid layer and the impaired clearance of pathogens. Na+ hyperabsorption was subsequently attributed to the absence of CFTR in the plasma membrane and to the lack of CFTR-dependent tonic inhibition of ENaC (Stutts et al. 1995, 1997). According to this hypothesis, loss of regulatory functions of CFTR is central to the development of CF pathology in the lungs. However, it is well established for human reabsorptive sweat ducts, where both the CFTR and the ENaC reside in the same apical membrane, that absence of the CFTR in CF-affected ducts does not elevate Na+ conductance (Bijman & Fromter, 1986), but under certain conditions may even significantly reduce it (Reddy et al. 1999; Reddy & Quinton, 2003). A direct relationship between ENaC and CFTR conductances in sweat ducts may not necessitate regulatory protein–protein interaction. As pointed out previously by Nagel et al. (2001b) and Horisberger (2003), due to an imposed Na+ concentration gradient in those experiments, at least part of the Na+ conductance reduction in CF-affected sweat ducts (Reddy et al. 1999) can arise from voltage-dependence of ENaC conductance, as predicted by the Goldmann–Hodgkin–Katz equation (Hodgkin & Katz, 1949; Hille, 1992). Because CFTR activation induces a large voltage shift, Na+ current is then measured at a voltage where ENaC conductance is elevated (Nagel et al. 2001b). These observations in sweat glands are consistent with several studies in mouse lungs. First, Barbry & Lazdunski (1996) reviewed several studies on animal models describing an inactivation of CFTR which found no alteration of ion transport capacities in mouse airways. Second, Fang et al. (2002) identified the role played by the CFTR in the distal airspaces of the lung after stimulation of the cAMP cascade. Importantly, these authors clearly demonstrated that the presence or absence of functional CFTR did not affect basal lung liquid clearance, suggesting that the CFTR has no influence on ENaC activity in that tissue.
ENaC–CFTR interactions have been directly tested in several heterologous expression systems (Stutts et al. 1995, 1997). However, the most compelling demonstration of CFTR-dependent ENaC inhibition has come from studies on Xenopus oocytes co-expressing both channels. Significant reduction of macroscopic amiloride-sensitive Na+ current by cAMP-stimulated CFTR was reported by several research groups, including one of our laboratories (Mall et al. 1996; Briel et al. 1998; Chabot et al. 1999; Jiang et al. 2000; Ji et al. 2000; Suaud et al. 2002a, b). Assuming that specific protein–protein interactions were involved, the oocyte expression system was further used as a functional assay in an attempt to identify regions on the CFTR or ENaC protein implicated in these interactions, but results obtained by different groups did not provide a consistent model (Schreiber et al. 1999; Jiang et al. 2000; Ji et al. 2000). In more recent studies, when series resistance was minimized (see below), ENaC inhibition by activated CFTR was often very small (< 20%) or statistically insignificant (Suaud et al. 2002a, b; Samaha et al. 2004; Yan et al. 2004). A modified hypothesis suggested that CFTR-mediated changes of intracellular [Cl−] or Cl− flux could inhibit ENaC (König et al. 2001). Published observations, however, are not unambiguous, for example Briel et al. (1998) stated that ENaC is inhibited in a voltage-dependent manner by Cl− influx rather than by the cytosolic Cl− concentration, whereas Konstas et al. (2003) found voltage-independent inhibition. König et al. (2001) attributed inhibition to the elevation of intracellular [Cl−], although this parameter was not measured directly in that study. In addition, a chloride-dependent inactivation mechanism would require tissue-specific regulation to explain the opposing effects observed in airways and sweat glands and is in contrast to the stimulation of 22Na+ uptake by Cl− influx in ENaC/CFTR-co-expressing oocytes (Nagel et al. 2001b).
Some recent studies did not find specific CFTR-dependent ENaC inhibition in MDCK epithelial cells or in Xenopus oocytes (Lahr et al. 2000; Nagel et al. 2001b). In particular, Nagel et al. (2001b) proposed that in Xenopus oocytes, under certain experimental conditions, apparent CFTR-dependent reduction of amiloride-sensitive current may be artefactual, a result of excessively large series resistance leading to considerable voltage-clamp errors. Because the resulting errors grow with increasing membrane conductance, activation of CFTR will reduce the fraction of voltage acting on the membrane. As a result, ENaC current is reduced due to a smaller electrical driving force, which could be misinterpreted as inhibition (Nagel et al. 2001b). This conclusion was supported by Chabot et al. (2002) in a recent erratum.
The aim of the present study was to determine whether cAMP activation of CFTR downregulates ENaC in Xenopus oocytes and to identify potential sources of dissimilar findings reported by different laboratories. The effect of CFTR activation on ENaC was examined in three independent laboratories, each with a different experimental protocol. We paid special attention to minimize voltage-clamp errors. Our three laboratories found no evidence of ENaC inhibition by activated CFTR if oocytes were voltage clamped with minimal series resistance and high feedback gain of the amplifier was used. Part of this study has been presented in preliminary form (Nagel et al. 2001a).
Methods
Electrophysiology
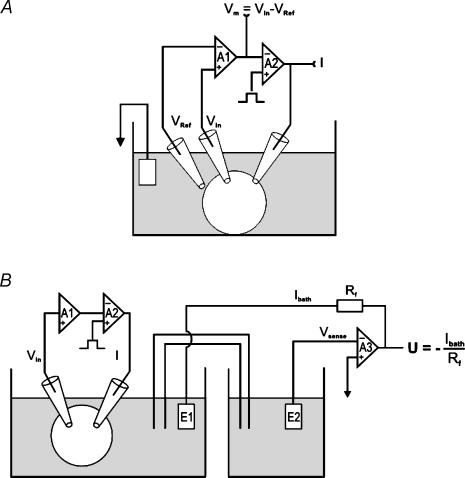
Two-electrode voltage-clamp experiments were performed with Turbo-Tec 05 (NPI Electronic, D-71732 Tamm, Germany), GeneClamp-500 (Axon Instruments, Union City, CA, USA) or TEV-200 voltage clamp (Dagan Corporation, Minneapolis, MN, USA) amplifiers (Chabot et al. 1999; Nagel et al. 2001b). Because membrane resistance (Rm) could be reduced significantly, sometimes even down to ∼1 kΩ in oocytes expressing ENaC and/or CFTR (Nagel et al. 2001b; Nagel, 2004), special care was taken to keep other resistances in series with the membrane and between intra-and extracellular voltage-recording electrodes as low as possible. When Rm becomes comparable to the series resistance (Rs) of the recording circuit, only a fraction of the applied voltage will be experienced by the oocyte membrane, while the rest will drop across the Rs. Neglecting the Rs in such situations may lead to serious misinterpretation of the experimental data (Nagel et al. 2001b; Nagel, 2004). Therefore, the components contributing to Rs and the possibilities to reduce it are considered here in some detail (see also Hodgkin et al. 1952; Taylor et al. 1960; Armstrong & Gilly, 1992; Axon Instruments, 1993). In principle, any resistance in series with the membrane and between the electrodes measuring voltage across the membrane contributes to Rs (access resistance). Major sources to be considered are resistance of the cytoplasm, tissue covering the oocyte (e.g. the vitellin layer), the electrolyte (bath) solution, agar bridges and Ag–AgCl electrodes. By careful design of the experiment, some of these elements may be eliminated, and resistance of others may be reduced. The remaining Rs can be compensated electronically, at least partially if necessary (Moore et al. 1984). Techniques to measure Rs have been described by Binstock et al. (1975). Generally, two electrodes, separate from the current-passing electrode, were used for differential membrane-potential measurements. Figure 1 presents schematic representations of the voltage-clamp arrangements employed here and the corresponding electrical circuits.
Figure 1. Schematic representation of different voltage-clamp configurations.
Voltage-clamp arrangement as used with the Turbo-Tec (A) and the GeneClamp-500 (B) amplifier. See Methods for details.
Voltage-clamp configuration
Membrane potential (Vm) is measured as the difference between an intracellular (Vin) and extracellular (Vref) electrode with a high impedance differential amplifier (Vm=Vin−Vref) in case of the Turbo-Tec 05 or Dagan amplifier. As with the Geneclamp 500 (Axon Instruments) differential measurement of the voltage is not possible, we used the arrangement suggested by Axon Instruments. A virtual ground amplifier (VG-2A) was used to measure current. This amplifier was connected to two bath electrodes, one to pass current and one to sense voltage, virtually without passing current. In all three cases, the extracellular electrode to measure Vm is placed, via an agar bridge, very close to the oocyte. The second bath electrode, used to pass current, is a Ag–AgCl wire.
Bath-fluid resistance was measured as described below and was typically close to 100 Ω when the external voltage-reference electrode (Vref) was kept close to the oocyte (Nagel, 2004). This is in agreement with the calculated access resistance to a sphere of Ø 1 mm in ND96 solution (see below) (Hille, 1992; Baumgartner et al. 1999). However, Rs may increase up to several kiloOhms for some commercially available experimental chambers, which have a separate well for the bath electrode located at some distance from the oocyte (e.g. RC-10, Warner Instruments, Hamden, CT, USA).
Bath-fluid resistance measurements
Bath-fluid resistance of the recording chamber, an important part of the Rs in two-electrode voltage-clamp experiments (Nagel et al. 2001b; Nagel, 2004), could be estimated by the method described by Nagel (2004). Briefly, in the absence of an oocyte, the two glass microelectrodes that are normally used to impale the oocyte are introduced in the bath solution close to the position where an oocyte is normally placed. The external voltage reference electrode (a semi-microelectrode filled with KCl or an agar bridge) was placed close to the intracellular voltage electrode as in an experiment with an oocyte. In the set-up mode of the amplifier (current-clamp mode), the potential difference of the two microelectrodes to the reference was zeroed, and the amplifier was then switched to voltage clamp. The applied voltage was slowly increased until 10 μA of current was passing between the current-injecting electrode and the bath reference ground electrode. The voltage (in mV) required to drive 1 μA of current corresponds numerically to the combined Rs (in kΩ). With typical positions of the electrodes (i.e. the saline or agar bridge connecting to the reference electrode, as close as possible to the oocyte), a resistance of about 100 Ω was found between the voltage electrodes.
Determination of total Rs
To measure the total resistance in series with the membrane, current steps were applied and the resulting voltage drop was measured. Because the Rm is initially short-circuited by membrane capacity, the initial voltage drop is attributed to the resistance in series with the membrane.
Other sources of voltage-clamp errors
Insufficient feedback gain of the voltage-clamp amplifier is another source of voltage-clamp error which depends on the magnitude of membrane conductance. Under stationary voltage-clamp conditions, the difference between the command voltage and measured voltage depends on the Rm, the gain of the feedback amplifier and the resistance of the current electrode plus the output resistance of the amplifier (see e.g. Axon Instruments, 1993):
where Vcmd is the command voltage, α is the feedback gain of the amplifier, Rm is the resistance of the cell membrane, Rin is the resistance of the current-injecting electrode, and Rout the output resistance of the voltage clamp amplifier.
To give an example: with a feed back gain of 1000, Rm= 5000 Ω, Rin= 0.5 MΩ plus output resistance of the voltage clamp amplifier (1 MΩ, e.g. the HS-2Ax10 headstage of Axon), the stationary voltage error is 23%. Increasing the Rm to 10 kΩ decreases the error to 13%. In contrast to errors due to Rs this type of error can be recognized by monitoring the measured Vm and comparing it with the command voltage. It is possible to correct the error by increasing the feedback gain although this is not always feasible because the voltage-clamp circuit has a tendency to oscillate at high gains. The interesting point here is that an elevation of membrane conductance, for example CFTR activation, will increase the voltage error and decrease the driving force on total conductance. Thus, with low feedback gain, activation of the CFTR could result in an apparent decrease of amiloride-sensitive ENaC conductance, which could be misinterpreted as the result of interaction between the two channels.
Oocyte acquisition and injection
Oocyte isolation and injection procedures were described in previous publications from our laboratories (Weinreich et al. 1997, 1999; Chabot et al. 1999). Mature female Xenopus laevis were maintained at 18–20°C with a 12-h light–dark cycle. Oocyte clusters were surgically removed from the ovaries and torn apart with forceps in ND96 medium containing (mm): NaCl 96, KCl 2, Hepes 10, CaCl2 1.8; at pH 7.4. Denuded oocytes were obtained by collagenase digestion (type IA, 370 U ml−1, Sigma) for 2 h at room temperature and rinsed several times in ND96 or ORi solutions (see below). Stage 5–6 oocytes were selected and incubated overnight at 18°C in ND96 or ORi medium with gentamycin (50 μg ml−1). Healthy oocytes were selected and injected with up to 50 nl cRNA (5–200 ng μl−1). The oocytes were incubated for 2–4 days after injection in ND96 or ORi medium supplemented with gentamycin and 10 μmol l−1 amiloride.
Solutions
The ND96 solution contained (mm): NaCl 96, KCl 2, MgCl2 1, Hepes 5, sodium pyruvate 2.5 and CaCl2 1.8; and 40 U ml−1penicillin, 40 μg ml−1 streptomycin and 50 mg l−1 gentamycin; at pH 7.6. The ORi solution contained (mm): NaCl 110, KCl 5, CaCl2 2, MgCl2 1.8 and Mops 5; at pH 7.6.
Experimental protocols
Two different experimental protocols were followed to study the effect of CFTR activation on ENaC co-expressed in oocytes. Protocol 1 (data in Fig. 2): oocytes were kept under open-circuit conditions except for short periods (< 30 s) during which they were voltage clamped, and the voltage-ramp protocol (V from −150 mV to +100 mV in 10 s) was applied to determine the current–voltage (I–V) relationship. A fast perfusion system allowed complete change of the bath solution within less than 10 s. The following solutions were applied sequentially to the oocyte during an experiment to measure ENaC and CFTR current (Protocol 1, Table 1, data in Fig. 2): A1, ND96 + 10 μm amiloride; B, ND96; A2, ND96 + 10 μm amiloride; C1, ND96 + 10 μm amiloride + 10 μm forskolin +100 μm 8-(4-chlorophentylthio)cAMP (cpt cAMP) +100 μm 3-isobutyl-1-methylxanthine (IBMX); D, ND96 + 10 μm forskolin + 100 μm cpt cAMP + 100 μm IBMX; C2, ND96 + 10 μm amiloride +10 μm forskolin + 100 μm cpt cAMP + 100 μm IBMX. The specific control ENaC current was determined as: B −1/2(A1 + A2). The specific ENaC current in cAMP-stimulated oocytes was determined as: D −1/2(C1 + C2). The specific CFTR current was determined as 1/2[(C1 + C2) − (A1 + A2)]. Protocol 2 (data in Fig. 3): oocytes were voltage clamped at −60 mV, and membrane current was recorded continuously. The specific control ENaC current was determined as the average of the amiloride-sensitive (10 μm amiloride) current before stimulation with 1 mm IBMX at the beginning of an experiment and after washout of IBMX at the end of that experiment. The specific ENaC current in the presence of activated CFTR was determined as amiloride-sensitive current after full activation of CFTR with 1 mm IBMX. The specific CFTR current was determined as amiloride-insensitive, IBMX-stimulated current.
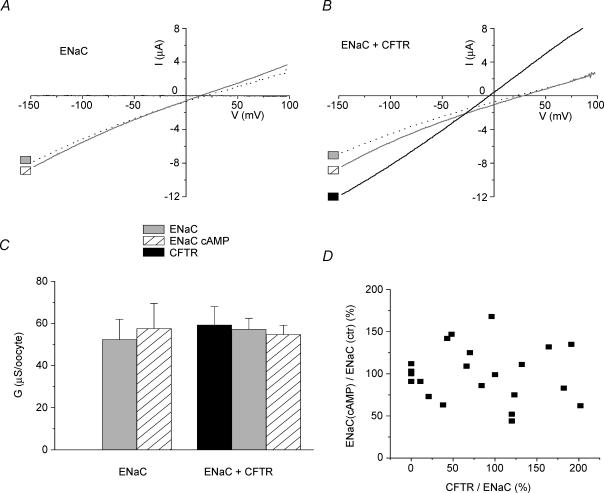
Figure 2. Human ENaC is not regulated by the human CFTR co-expressed in Xenopus oocytes.
A, representative current–voltage (I–V) relationships obtained with an oocyte expressing human α-,β-and γ-ENaC only. Specific amiloride-sensitive (10 μm), ENaC-mediated current is shown in response to a voltage ramp (see Methods). The two lines represent the I–V relationship before (dotted line), and after application of cAMP-elevating cocktail (see Methods, continuous grey line). Note the lack of effect of cAMP elevation on ENaC-mediated current. B, representative I–V relationships obtained with an oocyte co-expressing hENaC and hCFTR. The graph shows specific amiloride-sensitive, ENaC-mediated current before and after the application of a cAMP-elevating cocktail, dotted and continous grey lines, respectively. The continuous black line represents cAMP-stimulated, CFTR-mediated current measured in the presence of amiloride. Note that in the presence of the CFTR, elevation of cAMP had no significant (NS) effect on the slope of ENaC-mediated current, although its reversal potential was slightly, but statistically significantly, increased (change in Vr= 13 mV, P < 0.001). C, summary of results: conductances GCFTR, GENaC and GENaC(cAMP) were calculated from the slopes of the I–V relationships such as those shown in A and B. The difference between the number of oocytes measured in the presence of the CFTR (n = 19) and the number of oocytes measured in the absence of the CFTR (n = 4) is due to the fact that only oocytes exhibiting similar levels of ENaC conductance were presented here (four oocytes), but cAMP insensitivity was also noticed in oocytes exhibiting higher levels of conductance (see D below). D, effect of CFTR activation on ENaC-mediated conductance in oocytes expressing different GCFTR/GENaC ratios. The graph shows relative change of ENaC-mediated conductance GENaC(cAMP)/GENaC in each individual oocyte measured before and after CFTR activation. The figure illustrates that CFTR activation had no effect on the hENaC at GCFTR/GENaC ratios up to 2.
Table 1.
Solutions used in experiments shown in Fig. 2
| Solution | ENaC activated | CFTR activated |
|---|---|---|
| A1 | − | − |
| A2 | − | − |
| B | + | − |
| C1 | − | + |
| C2 | − | + |
| D | + | + |
Effect of solutes on ENaC and CFTR conductance. Activity (+) and lacking activity on inhibition (−) of the CFTR or ENaC conductance, respectively, are indicated.
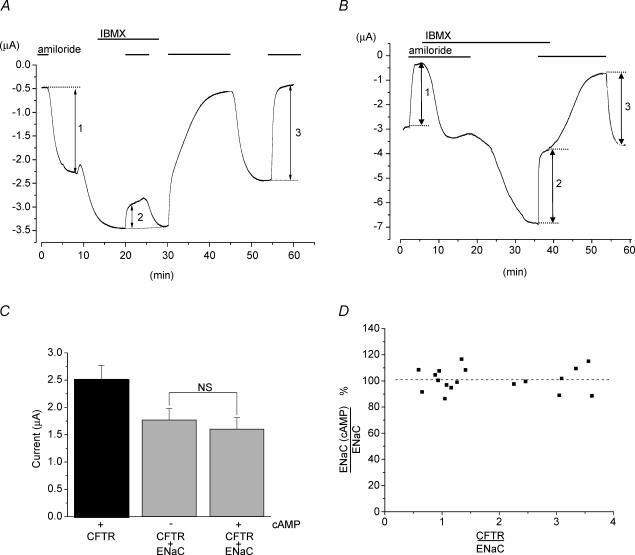
Figure 3. Rat ENaC is not regulated by the human CFTR co-expressed in Xenopus oocytes.
A, example of a current trace recorded at −60 mV from an oocyte co-expressing α-, β-and γ-rENaC and hCFTR recorded with a single bath electrode (see Fig. 1; i.e. relatively large Rs of about 6 kΩ; see below). Horizontal lines indicate the application of 10 μm amiloride to block the ENaC or 1 mm IBMX to stimulate the CFTR. Vertical arrows indicate the amplitudes of ENaC-mediated, amiloride-sensitive Na+ current (IENaC) observed before, during and after IBMX stimulation, arrows 1, 2 and 3, respectively. Note that CFTR stimulation, seen as increased inward current during IBMX application, resulted in apparent inhibition of amiloride-sensitive current (compare vertical arrow 2 with 1 or 3). The bath-fluid resistance of the experimental chamber (RC-10, Warner Instruments Co) filled with ND96 solution was ∼4.5 kΩ, the combined resistance of the reference bath electrode and the agar bridge was ∼1.5 kΩ. B, an example of an experiment similar to A, but performed with the virtual ground amplifier connected with two electrodes to the bath to reduce Rs (cf. Fig. 1). No reduction of ENaC current by the CFTR was observed under these conditions; compare the inhibition of ENaC by amiloride in the presence of activated CFTR (arrow 2) with that before (arrow 1) and after CFTR deactivation (arrow 3). C, summary of ENaC-mediated and CFTR-mediated currents measured with low Rs as in B, filled bar, ICFTR; grey bars, IENaC without (left) and with (right) stimulation of the CFTR by IBMX. Oocytes were clamped at the holding potential of −60 mV. The data are means ±s.e.m, n = 23. The observed ENaC current amplitudes were not statistically significantly different (NS) before and after CFTR stimulation. D, effect of activated CFTR on IENaC observed in oocytes expressing different ratios of ICFTR/IENaC. Oocytes were voltage-clamped at −60 mV and stimulated with 1 mm IBMX. The slope of the linear regression fitted to the data points was not significantly different from 0 (P= 0.99, n = 18 oocytes from seven different frogs).
Results
CFTR fails to inhibit human and rat ENaC co-expressed in Xenopus oocytes
Figure 2A shows the I–V relationships of amiloride-sensitive current in oocytes expressing the α-, β-and γ-subunits of human ENaC (hENaC). It demonstrates that amiloride-sensitive, hENaC-mediated current was not affected by cAMP stimulation. When the hENaC was co-expressed with the human CFTR (hCFTR), application of cAMP-elevating cocktail to these oocytes activated a large CFTR-mediated current, but had no effect on amiloride-sensitive current (Fig. 2B). Figure 2C summarizes whole-cell ENaC-and CFTR-mediated conductances (GENaC and GCFTR, respectively) calculated from the slope of the I–V relationships, such as those shown in Fig. 2A and B. Mean GENaC was not different in oocytes expressing ENaC alone and those co-expressing CFTR in the absence of cAMP stimulation. Furthermore, in the latter group of oocytes, GENaC was also not affected by cAMP stimulation of the CFTR. Thus, our results provide no evidence of the negative regulation of hENaC by the CFTR. As ENaC inhibition may require higher expression levels of GCFTR relative to GENaC, we have examined oocytes expressing different GCFTR/GENaC ratios (Fig. 2D). This figure shows that even at GCFTR/GENaC ratios of ∼2, stimulation of CFTR had no effect on hENaC activity.
In an independent study, the α-, β-and γ-subunits of rat ENaC (rENaC) were expressed instead of hENaC (Fig. 3). In this study, a different experimental protocol was used (i.e. oocytes co-expressing rENaC and CFTR were voltage clamped at a fixed Vm of −60 mV) and the oocyte current was recorded continuously during the entire experiment. Figure 3A gives an example of a current trace from such an experiment performed without compensating the Rs of the bath fluid and the ground electrode. Elevation of intracellular cAMP by including 1 mm IBMX in the perfusate resulted in significant stimulation of CFTR-mediated current, and, under these conditions, an apparent reduction of amiloride-sensitive, ENaC-mediated current. However, when the Rs was reduced by using the virtual ground bath amplifier with two bath electrodes, no inhibition of amiloride-sensitive current was observed (Fig. 3B). This demonstrates that apparent inhibition of the ENaC by the CFTR may inadvertently occur if the Rs is not properly reduced. Figure 3C summarizes the data from several experiments, such as those in Fig. 3B, showing that CFTR activation had no statistically significant effect on rENaC. Figure 3D examines this effect in oocytes expressing different CFTR/ENaC current ratios and reveals that even at ratios approaching 4, the CFTR did not inhibit rENaC. Thus, our results with hENaC and rENaC confirm the previous report by Nagel et al. (2001b) that the CFTR does not inhibit the ENaC in oocytes, if oocyte Vm is properly controlled.
In a further series of voltage-clamp experiments with rENaC/h CFTR-co-expressing oocytes, we examined the effect of the feedback gain (voltage gain) on apparent ENaC conductance and its apparent ‘regulation’ by activated CFTR. In these experiments, the actual membrane voltage was also measured, but the observed voltage deviations from the target value at the different gains (see Methods: ‘Other sources of voltage-clamp errors’) were not taken into account when calculating ‘apparent conductances’, as is usually done by all commercial software. In addition, we determined real conductances from the actually observed current and voltage values. Table 2 shows both apparent and real ENaC conductances before and after CFTR activation, determined at three different voltage gains. As expected, lower voltage gain leads to a decreased apparent ENaC conductance when the CFTR is activated, and this directly results from voltage-clamp errors (see Methods: ‘Other sources of voltage clamp errors’).
Table 2.
Apparent and real ENaC conductances (in μS) in rENaC/hCFTR-co-expressing oocytes measured with three different voltage gains
| No CFTR activation | Full CFTR activation | |||
|---|---|---|---|---|
| Voltage gain | Gapp | Greal | Gapp | Greal |
| 1 k | 55 | 62 | 25 | 67 |
| 4 k | 62 | 65 | 52 | 68 |
| 10 k | 61 | 62 | 59 | 68 |
In this example 2.8 ng rENaC-cRNA and 0.8 ng hCFTR-cRNA were injected and oocyte conductance was measured after 50 h of incubation; representative of four other experiments with low cRNA amounts. CFTR was activated by 0.5 mm IBMX + 10 μm forskolin. Apparent conductance (Gapp) was determined as the current slope between −20 mV and +20 mV and real conductance (Greal) was determined as the current slope between the actually observed voltages, with −20 mV and +20 mV as target values in the voltage clamp protocol. Both Gapp and Greal are given in μS. Configuration as in Fig. 1B with two bath electrodes and an estimated Rs of less than 200 Ω. Although in this example Greal is slightly larger during CFTR activation, ENaC seems significantly inhibited by CFTR activation when compared to Gapp, obtained at a voltage gain of 1 k.
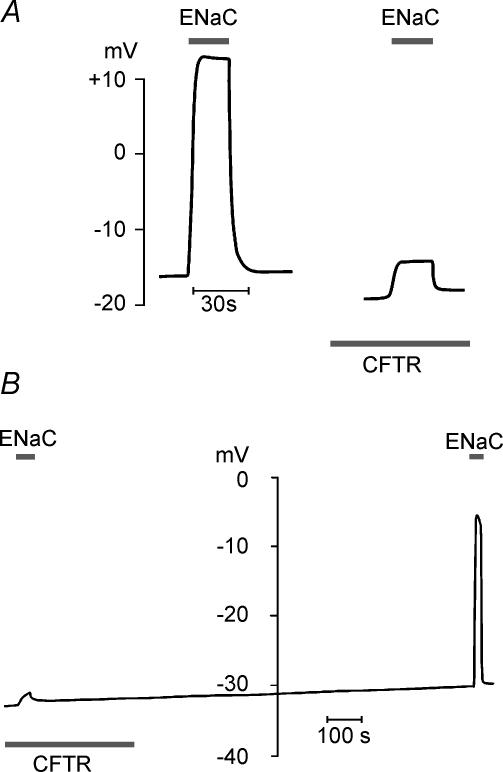
To demonstrate that CFTR activation modulates the amiloride-sensitive component of the Vm, we measured Vm under current-clamp conditions (with I = 0) before and after CFTR stimulation. Amiloride was briefly removed (to activate ENaC conductance), and corresponding shifts of Vm were measured. Figure 4A shows the voltage shift induced by amiloride removal in hCFTR/rENaC-co-expressing oocytes before and after CFTR activation. Clearly, the amiloride-induced voltage shift was much smaller, once the CFTR was activated. The effect of CFTR activation on the amiloride-sensitive voltage shift was fully reversible, as demonstrated in Fig. 4B, where amiloride removal-induced voltage shift was examined first with activated CFTR and then after CFTR inactivation. The mean amiloride-sensitive voltage shift for rENaC/hCFTR-co-expressing oocytes was 35 ± 7 mV with CFTR inactive and dropped to 10 ± 5 mV after CFTR activation (n = 8). This effect was not specific for CFTR-mediated conductance, because non-specific increase of membrane conductance introduced, for example by simply rupturing the oocyte membrane, also decreased the ENaC-related, amiloride-sensitive voltage shift (data not shown). It is important to note that for each oocyte tested in these current-clamp experiments, we also confirmed that ENaC conductance was not influenced by CFTR activation under voltage-clamp conditions with high voltage gain and low Rs. Although this might seem paradoxical at first glance, modulation of the amiloride-induced voltage shift by other conductances is in fact expected and will be explained in the Discussion.
Figure 4. Amiloride-induced voltage shift is modulated by membrane conductance changes.
A, when the hCFTR is inactive, rENaC activation by removal of amiloride (indicated by the bar labelled ENaC) depolarizes an oocyte in this example from −16 mV to +13 mV. Activation of the CFTR (by 0.5 mm IBMX + 10 μm forskolin, indicated by the bar labelled CFTR) yields slight hyperpolarization (to −19 mV), and subsequent activation of ENaC depolarizes the oocyte to only −14 mV. The trace shown is representative of seven similar experiments in which voltage-clamp measurements showed that ENaC conductance is not affected by CFTR activation. B, continuous voltage recording from another hCFTR/rENaC-co-expressing oocyte, where CFTR and ENaC were activated in a reversed order compared to A (i.e. CFTR was activated first, at the beginning of the experiment, and then inactivated). The data show increase of ENaC-related, amiloride-induced voltage shift after inactivating CFTR (washout of IBMX/forskolin), demonstrating reversibility of the effect.
Discussion
The hypothesis that the CFTR inhibits the ENaC has its roots in early studies before the involved channels, the CFTR and the ENaC, were identified at the molecular level. In vivo and in vitro transepithelial potential measurements on normal and CF-affected airway epithelia detected increased amiloride sensitivity of CF-affected tissues (Knowles et al. 1981, 1983). This was attributed to increased rates of Na absorption (hyperabsorption) by CF-affected epithelia and seemed to explain elegantly the abnormally dehydrated mucus in CF-affected airways (Boucher et al. 1986). After cloning the CFTR and ENaC (Riordan et al. 1989; Canessa et al. 1993; Lingueglia et al. 1993), it was expected that one of the functions of the CFTR was to inhibit the ENaC (Stutts et al. 1995). Indeed, several laboratories subsequently reported direct inhibition of the ENaC by the CFTR in several experimental systems, including voltage-clamped oocytes of Xenopus laevis (Mall et al. 1996; Letz & Korbmacher, 1997; Jiang et al. 2000; Suaud et al. 2002a, b; Konstas et al. 2003). Our present results demonstrate that the CFTR does not inhibit ENaC in oocytes and are thus in direct contrast to previous reports, which used the same expression system. This could not be attributed to low expression ratios of the CFTR compared to the ENaC (Kunzelmann, 2003), because we have examined the effect at different CFTR/ENaC conductance ratios (up to 4, absolute conductance ranges were 10–100 μS for the ENaC and 10–300 μS for the CFTR)and under widely varying conditions. Furthermore, we found no inhibitory effect with both hENaC and rENaC (Fig. 2D and 3D; and Nagel et al. 2001b). It was also suggested that functional ENaC–CFTR interactions may differ between murine and human ENaC, as well as, that they could be influenced by naturally occurring polymorphism of α-hENaC (Yan et al. 2004). They found less than 35% inhibition of murine ENaC by activated CFTR (their Fig. 1A), only a modest 20% inhibition for wildtype α-hENaC and no change for T663A α-hENaC, where threonine 663 (wildtype) was replaced by alanine (their Fig. 2). Because in these recent experiments Yan et al. (2004) also used a virtual ground, as in our experiments, voltage-clamp errors could be avoided if experiments were performed at high voltage gain and with low series resistance. Thus, the absence or negligible inhibition is expected and agrees with our data. Indeed, in our present study we used the same variant T663 for which Yan et al. (2004) found a modest inhibition (20%, their Fig. 2), whereas we found no inhibition when Rs was fully compensated and high gain of the amplifier was used.
As our three laboratories did not observe ENaC inhibition by CFTR activation, the obvious question arises: how to reconcile our findings with those reported by other investigators? After careful examination of all the different experimental conditions, we come to the conclusion that the only reasonable explanation for such divergent results is the way the two-electrode voltage-clamp techniques were deployed. For example, high Rs or too low feedback voltage gain could both limit the ENaC conductance measured. Because an Rs problem can arise easily and inadvertently, and indeed it happened to one of us (Chabot et al. 1999, 2002), we made an effort to closely examine the problem and to find a simple method to estimate the actual Rs of the recording setup. As recently demonstrated by one of us (Nagel et al. 2001b), the Rs in the measuring circuit may simulate ENaC inhibition if the Rm drops due to activation of large membrane conductance (Nagel et al. 2001b; Nagel, 2004; see also Fig. 3A). This hypothesis is further strengthened by closely examining experimental data published by other laboratories. For example, König et al. (2001) reported ENaC inhibition by the CFTR and intracellular Cl−. However, under their experimental conditions, the ENaC was not only inhibited by activation of a completely unrelated chloride channel, ClC-0, but also by permeabilization of the membrane with amphotericin. Thus, all manoeuvres that increased membrane conductance – expression of the CFTR or ClC-0 or amphotericin-induced membrane permeabilization – resulted in apparent ENaC inhibition. To us, these data suggest that the Rs probably limits the measurable conductance and, in this way, simulates ENaC ‘inhibition’. In addition, apparent ENaC inhibition could result when too low gain in the voltage feedback loop is used, once additional conductance is activated (see Table 2). Other groups recently studied ENaC–CFTR interactions in oocytes that were voltage clamped with a presumably low Rs. However, their actual data show that cAMP stimulation of wild-type CFTR had a very small (< 20%) or statistically non-significant effect on the ENaC (Suaud et al. 2002a, b; Yan et al. 2004). Such results are expected if oocytes were clamped with minimal Rs and, thus, are consistent with our interpretation.
The assumption that the apparent interaction between the CFTR and ENaC is due to voltage-clamp errors explains a variety of observations reported in the literature. First, it explains why the CFTR seems to interact with almost all other electrogenic transport systems – channels as well as transporters. Second, it explains that the degree of inhibition depends on the expression level, i.e. the CFTR-mediated conductance. Third, it also explains the results of mutation experiments if one takes into account that the conductances induced by mutated CFTR channels are much lower (Mall et al. 1996; Briel et al. 1998; Schreiber et al. 1999) and fourth, it explains why the reduction of Cl− concentration, and therefore membrane conductance, reduces the apparent interaction between the CFTR and ENaC.
It might be also interesting to review the early findings of elevated amiloride-induced voltage shifts in CF-affected tissues which ultimately led to the notion of increased sodium absorption in CF. In fact, we also observed similar effects in CFTR/ENaC-co-expressing oocytes. As Fig. 4 shows, the amiloride-induced voltage shift is smaller once the CFTR is activated. It is often assumed that such reduction of voltage shift hints of CFTR-dependent inhibition of amiloride-sensitive sodium current. However, this is not necessarily the case and alternative explanations are possible. In the following example, we will consider amiloride-induced voltage shifts in oocytes expressing the CFTR and ENaC. Vm can be well described by the Goldmann–Hodgkin–Katz equation:
| (1) |
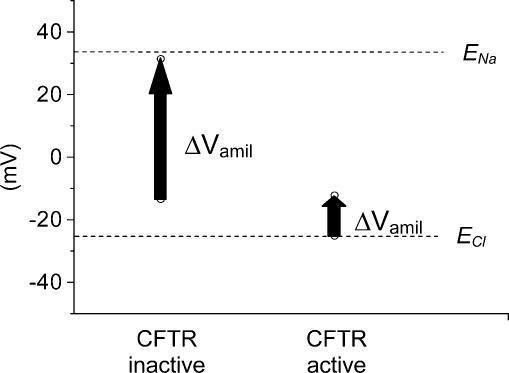
where [Cl−]i= 45 mm, [Na+]i= 30 mm and [K+]i= 120 mm, and [Cl−]o= 121 mm, [Na+]o= 110 mm and [K+]o= 5 mm. As an illustration, let us assume that PK= 0.01 ×PNa and that in the absence of CFTR stimulation, the residual PCl= 0.01 ×PNa, while after CFTR activation, PCl= 3 ×PNa. Also, let us assume that in the presence of amiloride, residual PNa= 0.01 ×PNa and then calculate Vm for different experimental situations. With the CFTR inactive and amiloride present, Vm= −13.5 mV, while upon amiloride removal, it will increase to +31.4 mV. After CFTR stimulation and in the presence of amiloride, Vm will be −25.2 mV, whereas with active CFTR and ENaC, the Vm will be −12.2 mV.
Thus, the amiloride-induced voltage shift when the CFTR is inactive will be ∼45 mV, while it will be much smaller after CFTR activation: 13 mV (see Fig. 5). Indeed, this confirms qualitatively what we observed in voltage measurements on CFTR/ENaC-co-expressing oocytes (Fig. 4). The experimentally observed values are slightly different because the actual conductances and intracellular ion concentrations may be somewhat different from those used in our simple example. Not surprisingly, activation of a chloride conductance, which is not mediated by the CFTR, may also lead to a reduced amiloride-induced voltage shift in transepithelial voltage measurements, without the need to invoke ‘regulatory interactions’ as is done often (e.g. Schreiber et al. 2003). Of course, this argument does not apply to careful conductance estimates derived from application of current injections. Conductance measurements by current injections can, under certain conditions, accurately reflect amiloride-sensitive sodium conductance. Such conductance measurements will, however, only yield reliable results if residual conductance is not overwhelming and if Rs is not too large. It is also important to stress that our study is limited to only one expression system, amphibian oocytes. It may well be that CFTR–ENaC regulatory interactions cannot be reproduced in oocytes because some factor(s), which are required for such interactions, are missing in these cells. Thus, it will be critical to extend our study to other cellular systems, while ensuring optimal recording conditions.
Figure 5. Amiloride-induced voltage shifts in oocytes expressing ENaC and CFTR.
The diagram shows predicted membrane voltage shifts induced by amiloride removal (ΔVamil, vertical arrows) for oocytes under current-clamp condition with CFTR inactive or after CFTR activation. ΔVamil was calculated as described in the Discussion. ENa and ECl (dashed lines) represent Nernst potentials for Na+ and Cl−, respectively.
In summary, the results from our three independent laboratories univocally demonstrated the absence of ENaC inhibition by the CFTR in Xenopus oocytes, when Rs of the recording circuitry was low (∼100 Ω). We suggest that the inhibitory effects previously reported in the literature could be attributed to either unfavourably large Rs or insufficient voltage gain or both, resulting in apparent reduction of ENaC conductance. Lessons from the oocyte expression system argue for careful re-examination of other in vitro experimental systems in which CFTR–ENaC regulatory interactions are studied, especially in whole-cell patch-clamp experiments, where it is known that access resistance has to be monitored carefully (Armstrong & Gilly, 1992).
Acknowledgments
G.N. thanks Professor B. Rossier for the rENaC plasmids, Professor J. Riordan for the hCFTR plasmid, Professor E. Bamberg for generous support and constant encouragement, and Doris Ollig, Saskia Schröder-Lang, and Eva-Verena Bongartz for technical assistance. The suppport of the Canadian Cystic Fibrosis Foundation (to R.G.) is gratefully acknowledged. The authors thank Ovid Da Silva, Editor, Research Support Office, Research Centre, CHUM, for editing this manuscript.
References
- Akabas MH. Cystic fibrosis transmembrane conductance regulator. Structure and function of an epithelial chloride channel. J Biol Chem. 2000;275:3729–3732. doi: 10.1074/jbc.275.6.3729. 10.1074/jbc.275.6.3729. [DOI] [PubMed] [Google Scholar]
- Anderson MP, Berger HA, Rich DP, Gregory RJ, Smith AE, Welsh MJ. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991;67:775–784. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Gilly WF. Access resistance and space clamp problems associated with whole-cell patch clamping. Methods Enzymol. 1992;207:100–122. doi: 10.1016/0076-6879(92)07007-b. [DOI] [PubMed] [Google Scholar]
- Axon Instruments. The Axon Guide. Foster City CA: Axon Instruments Inc; 1993. pp. 1–282. [Google Scholar]
- Barasch J, Kiss B, Prince A, Saiman L, Gruenert D, al Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991;352:70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- Barbry P, Lazdunski M. Structure and regulation of the amiloride-sensitive epithelial sodium channel. Ion Channels. 1996;4:115–167. doi: 10.1007/978-1-4899-1775-1_4. [DOI] [PubMed] [Google Scholar]
- Baumgartner W, Islas L, Sigworth FJ. Two-microelectrode voltage clamp of Xenopus oocytes: voltage errors and compensation for local current flow. Biophys J. 1999;77:1980–1991. doi: 10.1016/S0006-3495(99)77039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijman J, Fromter E. Direct demonstration of high transepithelial chloride-conductance in normal human sweat duct which is absent in cystic fibrosis. Pflugers Arch. 1986;407:S123–S127. doi: 10.1007/BF00584941. [DOI] [PubMed] [Google Scholar]
- Binstock L, Adelman WJ, Senft P, Jr, Lecar H. Determination of the resistance in series with the membranes of giant axons. J Membr Biol. 1975;21:25–47. doi: 10.1007/BF01941060. [DOI] [PubMed] [Google Scholar]
- Boucher RC, Stutts MJ, Knowles MR, Cantley L, Gatzy JT. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest. 1986;78:1245–1252. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury NA. Intracellular CFTR: localization and function. Physiol Rev. 1999;79:S175–S191. doi: 10.1152/physrev.1999.79.1.S175. [DOI] [PubMed] [Google Scholar]
- Bradbury NA, Jilling T, Berta G, Sorscher EJ, Bridges RJ, Kirk KL. Regulation of plasma membrane recycling by CFTR. Science. 1992;256:530–532. doi: 10.1126/science.1373908. [DOI] [PubMed] [Google Scholar]
- Briel M, Greger R, Kunzelmann K. Cl− transport by cystic fibrosis transmembrane conductance regulator (CFTR) contributes to the inhibition of epithelial Na+ channels (ENaCs) in Xenopus oocytes co-expressing CFTR and ENaC. J Physiol. 1998;508:825–836. doi: 10.1111/j.1469-7793.1998.825bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa CM, Horisberger JD, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Chabot H, Vives MF, Dagenais A, Grygorczyk C, Berthiaume Y, Grygorczyk R. Downregulation of epithelial sodium channel (ENaC) by CFTR co-expressed in Xenopus oocytes is independent of Cl− conductance. J Membr Biol. 1999;169:175–188. doi: 10.1007/s002329900529. [DOI] [PubMed] [Google Scholar]
- Chabot H, Vives MF, Dagenais A, Grygorczyk C, Berthiaume Y, Grygorczyk R. Downregulation of epithelial sodium channel (ENaC) by CFTR co-expressed in Xenopus oocytes is independent of Cl− conductance – Erratum. J Membr Biol. 2002;186:185. doi: 10.1007/s002329900529. 10.1007/s00232-001-0158-2. [DOI] [PubMed] [Google Scholar]
- Dawson DC, Smith SS, Mansoura MK. CFTR: mechanism of anion conduction. Physiol Rev. 1999;79:S47–S75. doi: 10.1152/physrev.1999.79.1.S47. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Boucher RC. Update on pathogenesis of cystic fibrosis lung disease. Curr Opin Pulm Med. 2003;9:486–491. doi: 10.1097/00063198-200311000-00007. 10.1097/00063198-200311000-00007. [DOI] [PubMed] [Google Scholar]
- Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS, Matthay MA. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol. 2002;119:199–208. doi: 10.1085/jgp.119.2.199. 10.1085/jgp.119.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby DC, Nagel G, Hwang TC. The CFTR chloride channel of mammalian heart. Annu Rev Physiol. 1995;57:387–416. doi: 10.1146/annurev.ph.57.030195.002131. 10.1146/annurev.ph.57.030195.002131. [DOI] [PubMed] [Google Scholar]
- Gadsby DC, Nairn AC. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol Rev. 1999;79:S77–S107. doi: 10.1152/physrev.1999.79.1.S77. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Vick RN, Boucher RC. Hyperabsorption of Na+ and raised Ca2+-mediated Cl− secretion in nasal epithelia of CF mice. Am J Physiol. 1994;266:C1478–C1483. doi: 10.1152/ajpcell.1994.266.5.C1478. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates Inc.; 1992. [Google Scholar]
- Hodgkin AL, Huxley AF, Katz B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952;116:424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol. 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger JD. ENaC–CFTR interactions: the role of electrical coupling of ion fluxes explored in an epithelial cell model. Pflugers Arch. 2003;445:522–528. doi: 10.1007/s00424-002-0956-0. [DOI] [PubMed] [Google Scholar]
- Ji HL, Chalfant ML, Jovov B, Lockhart JP, Parker SB, Fuller CM, Stanton BA, Benos DJ. The cytosolic termini of the beta-and gamma-ENaC subunits are involved in the functional interactions between CFTR and ENaC. J Biol Chem. 2000;275:27947–27956. doi: 10.1074/jbc.M002848200. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Li J, Dubroff R, Ahn YJ, Foskett JK, Engelhardt J, Kleyman TR. Epithelial sodium channels regulate cystic fibrosis transmembrane conductance regulator chloride channels in Xenopus oocytes. J Biol Chem. 2000;275:13266–13274. doi: 10.1074/jbc.275.18.13266. 10.1074/jbc.275.18.13266. [DOI] [PubMed] [Google Scholar]
- Knowles MR, Gatzy J, Boucher RC. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981;305:1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- Knowles MR, Stutts MJ, Spock A, Fischer N, Gatzy JT, Boucher RC. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983;221:1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J. 2002;21:5662–5672. doi: 10.1093/emboj/cdf580. 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König J, Schreiber R, Voelcker T, Mall M, Kunzelmann K. The cystic fibrosis transmembrane conductance regulator (CFTR) inhibits ENaC through an increase in the intracellular Cl− concentration. EMBO Rep. 2001;2:1047–1051. doi: 10.1093/embo-reports/kve232. 10.1093/embo-reports/kve232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstas AA, Koch JP, Korbmacher C. cAMP-dependent activation of CFTR inhibits the epithelial sodium channel (ENaC) without affecting its surface expression. Pflugers Arch. 2003;445:513–521. doi: 10.1007/s00424-002-0957-z. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K. ENaC is inhibited by an increase in the intracellular Cl−concentration mediated through activation of Cl− channels. Pflugers Arch. 2003;445:504–512. doi: 10.1007/s00424-002-0958-y. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Mall M, Briel M, Hipper A, Nitschke R, Ricken S, Greger R. The cystic fibrosis transmembrane conductance regulator attenuates the endogenous Ca2+ activated Cl− conductance of Xenopus oocytes. Pflugers Arch. 1997;435:178–181. doi: 10.1007/s004240050498. 10.1007/s004240050498. [DOI] [PubMed] [Google Scholar]
- Lahr TF, Record RD, Hoover DK, Hughes CL, Blazer-Yost BL. Characterization of the ion transport responses to ADH in the MDCK-C7 cell line. Pflugers Arch. 2000;439:610–617. doi: 10.1007/s004249900222. 10.1007/s004240050984. [DOI] [PubMed] [Google Scholar]
- Letz B, Korbmacher C. cAMP stimulates CFTR-like Cl− channels and inhibits amiloride-sensitive Na+ channels in mouse CCD cells. Am J Physiol. 1997;272:C657–C666. doi: 10.1152/ajpcell.1997.272.2.C657. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett. 1993;318:95–99. doi: 10.1016/0014-5793(93)81336-x. 10.1016/0014-5793(93)81336-X. [DOI] [PubMed] [Google Scholar]
- McNicholas CM, Nason MW, Jr, Guggino WB, Schwiebert EM, Hebert SC, Giebisch G, Egan ME. A functional CFTR-NBF1 is required for ROMK2–CFTR interaction. Am J Physiol. 1997;273:F843–F848. doi: 10.1152/ajprenal.1997.273.5.F843. [DOI] [PubMed] [Google Scholar]
- Mall M, Bleich M, Greger R, Schreiber R, Kunzelmann K. The amiloride-inhibitable Na+ conductance is reduced by the cystic fibrosis transmembrane conductance regulator in normal but not in cystic fibrosis airways. J Clin Invest. 1998;102:15–21. doi: 10.1172/JCI2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall M, Hipper A, Greger R, Kunzelmann K. Wild type but not deltaF508 CFTR inhibits Na+ conductance when co-expressed in Xenopus oocytes. FEBS Lett. 1996;381:47–52. doi: 10.1016/0014-5793(96)00079-8. 10.1016/0014-5793(96)00079-8. [DOI] [PubMed] [Google Scholar]
- Moore JW, Hines M, Harris EM. Compensation for resistance in series with excitable membranes. Biophys J. 1984;46:507–514. doi: 10.1016/S0006-3495(84)84048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G. Differential function of the two nucleotide binding domains on cystic fibrosis transmembrane conductance regulator. Biochim Biophys Acta. 1999;1461:263–274. doi: 10.1016/s0005-2736(99)00162-5. [DOI] [PubMed] [Google Scholar]
- Nagel G. CFTR investigated with the two-electrode voltage-clamp technique: the importance of knowing the series resistance. J Cystic Fibros. 2004;3:109–111. doi: 10.1016/j.jcf.2004.05.043. 10.1016/j.jcf.2004.05.043. [DOI] [PubMed] [Google Scholar]
- Nagel G, Hwang TC, Nastiuk KL, Nairn AC, Gadsby DC. The protein kinase A-regulated cardiac Cl− channel resembles the cystic fibrosis transmembrane conductance regulator. Nature. 1992;360:81–84. doi: 10.1038/360081a0. 10.1038/360081a0. [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Grygorczyk R, Barbry P. Sodium uptake and voltage-clamp experiments in Xenopus oocytes provide no evidence for specific regulation of ENaC by CFTR. Pediatr Pulmonol. 2001a;22(Suppl.):209. [Google Scholar]
- Nagel G, Szellas T, Riordan JR, Friedrich T, Hartung K. Non-specific activation of the epithelial sodium channel by the CFTR chloride channel. EMBO Rep. 2001b;2:249–254. doi: 10.1093/embo-reports/kve045. 10.1093/embo-reports/kve045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Ko SB, Choi JY, Muallem G, Thomas PJ, Pushkin A, Lee MS, Kim JY, Lee MG, Muallem S, Kurtz I. The cystic fibrosis transmembrane conductance regulator interacts with and regulates the activity of the HCO3− salvage transporter human Na+-HCO3− cotransport isoform 3. J Biol Chem. 2002;277:50503–50509. doi: 10.1074/jbc.M201862200. 10.1074/jbc.M201862200. [DOI] [PubMed] [Google Scholar]
- Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev. 1999;79:S215–S255. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev. 1999;79:S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Light MJ, Quinton PM. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl− channel function. Nature. 1999;402:301–304. doi: 10.1038/46297. 10.1038/46297. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM. Functional interaction of CFTR and ENaC in sweat glands. Pflugers Arch. 2003;445:499–503. doi: 10.1007/s00424-002-0959-x. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM, Haws C, Wine JJ, Grygorczyk R, Tabcharani JA, Hanrahan JW, Gunderson KL, Kopito RR. Failure of the cystic fibrosis transmembrane conductance regulator to conduct ATP. Science. 1996;271:1876–1879. doi: 10.1126/science.271.5257.1876. [DOI] [PubMed] [Google Scholar]
- Reisin IL, Prat AG, Abraham EH, Amara JF, Gregory RJ, Ausiello DA, Cantiello HF. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J Biol Chem. 1994;269:20584–20591. [PubMed] [Google Scholar]
- Riordan JR. The cystic fibrosis transmembrane conductance regulator. Annu Rev Physiol. 1993;55:609–630. doi: 10.1146/annurev.ph.55.030193.003141. 10.1146/annurev.ph.55.030193.003141. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Samaha FF, Rubenstein RC, Yan W, Ramkumar M, Levy DI, Ahn YJ, Sheng S, Kleyman TR. Functional polymorphism in the carboxyl terminus of the alpha-subunit of the human epithelial sodium channel. J Biol Chem. 2004;279:23900–23907. doi: 10.1074/jbc.M401941200. 10.1074/jbc.M401941200. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Hopf A, Mall M, Greger R, Kunzelmann K. The first-nucleotide binding domain of the cystic-fibrosis transmembrane conductance regulator is important for inhibition of the epithelial Na+ channel. Proc Natl Acad Sci U S A. 1999;96:5310–5315. doi: 10.1073/pnas.96.9.5310. 10.1073/pnas.96.9.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, König J, Sun J, Markovich D, Kunzelmann K. Effects of purinergic stimulation, CFTR and osmotic stress on amiloride-sensitive Na+ transport in epithelia and Xenopus oocytes. J Membr Biol. 2003;192:101–110. doi: 10.1007/s00232-002-1067-8. 10.1007/s00232-002-1067-8. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Benos DJ, Egan ME, Stutts MJ, Guggino WB. CFTR is a conductance regulator as well as a chloride channel. Physiol Rev. 1999;79:S145–S166. doi: 10.1152/physrev.1999.79.1.S145. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, Guggino WB. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Boucher . Cystic fibrosis gene and functions of CFTR. Implications of dysfunctional ion transport for pulmonary pathogenesis. In: Yankaskas JR, Knowles MR, editors. Cystic Fibrosis in Adults. Philadelphia: Lippincott-Raven Publishers; 1999. pp. 3–25. [Google Scholar]
- Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J Biol Chem. 1997;272:14037–14040. doi: 10.1074/jbc.272.22.14037. 10.1074/jbc.272.22.14037. [DOI] [PubMed] [Google Scholar]
- Suaud L, Carattino M, Kleyman TR, Rubenstein RC. Genistein improves regulatory interactions between G551D-CFTR and the epithelial sodium channel in Xenopus oocytes. J Biol Chem. 2002a;277:50341–50347. doi: 10.1074/jbc.M209641200. 10.1074/jbc.M209641200. [DOI] [PubMed] [Google Scholar]
- Suaud L, Li J, Jiang Q, Rubenstein RC, Kleyman TR. Genistein restores functional interactions between Delta F508-CFTR and ENaC in Xenopus oocytes. J Biol Chem. 2002b;277:8928–8933. doi: 10.1074/jbc.M111482200. 10.1074/jbc.M111482200. [DOI] [PubMed] [Google Scholar]
- Tarran R, Loewen ME, Paradiso AM, Olsen JC, Gray MA, Argent BE, Boucher RC, Gabriel SE. Regulation of murine airway surface liquid volume by CFTR and Ca2+-activated Cl− conductances. J Gen Physiol. 2002;120:407–418. doi: 10.1085/jgp.20028599. 10.1085/jgp.20028599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RE, Moore JW, Cole KS. Analysis of certain errors in squid axon voltage clamp measurements. Biophys J. 1960;1:161–202. doi: 10.1016/s0006-3495(60)86882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich F, Riordan JR, Nagel G. Dual effects of ADP and adenylylimidodiphosphate on CFTR channel kinetics show binding to two different nucleotide binding sites. J Gen Physiol. 1999;114:55–70. doi: 10.1085/jgp.114.1.55. 10.1085/jgp.114.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich F, Wood PG, Riordan JR, Nagel G. Direct action of genistein on CFTR. Pflugers Arch. 1997;434:484–491. doi: 10.1007/s004240050424. 10.1007/s004240050424. [DOI] [PubMed] [Google Scholar]
- Yan W, Samaha FF, Ramkumar M, Kleyman TR, Rubenstein RC. Cystic fibrosis transmembrane conductance regulator differentially regulates human and mouse epithelial sodium channels in Xenopus oocytes. J Biol Chem. 2004;279:23183–23192. doi: 10.1074/jbc.M402373200. 10.1074/jbc.M402373200. [DOI] [PubMed] [Google Scholar]