Abstract
Functional neuroimaging relies on the robust coupling between neuronal activity, metabolism and cerebral blood flow (CBF), but the physiological basis of the neuroimaging signals is still poorly understood. We examined the mechanisms of activity-dependent changes in tissue oxygenation in relation to variations in CBF responses and postsynaptic activity in rat cerebellar cortex. To increase synaptic activity we stimulated the monosynaptic, glutamatergic climbing fibres that excite Purkinje cells via AMPA receptors. We used local field potentials to indicate synaptic activity, and recorded tissue oxygen partial pressure (Ptiss,O2) by polarographic microelectrodes, and CBF using laser-Doppler flowmetry. The disappearance rate of oxygen in the tissue increased linearly with synaptic activity. This indicated that, without a threshold, oxygen consumption increased as a linear function of synaptic activity. The reduction in Ptiss,O2 preceded the rise in CBF. The time integral (area) of the negative Ptiss,O2 response increased non-linearly showing saturation at high levels of synaptic activity, concomitant with a steep rise in CBF. This was accompanied by a positive change in Ptiss,O2. Neuronal nitric oxide synthase inhibition enhanced the initial negative Ptiss,O2 response (‘dip’), while attenuating the evoked CBF increase and positive Ptiss,O2 response equally. This indicates that increases in CBF counteract activity-induced reductions in Ptiss,O2, and suggests the presence of a tissue oxygen reserve. The changes in Ptiss,O2 and CBF were strongly attenuated by AMPA receptor blockade. Our findings suggest an inverse relationship between negative Ptiss,O2 and CBF responses, and provide direct in vivo evidence for a tight coupling between activity in postsynaptic AMPA receptors and cerebellar oxygen consumption.
Nerve tissue is among the highest oxygen-consuming tissues in the body. The oxygen is used for oxidation of glucose which is thought to provide almost all the energy needed by neurones to support brain activity (Siesjo, 1978). There is a tight coupling between neuronal activity, blood flow and glucose and oxygen consumption (Clarke & Sokoloff, 1994). The tight and robust neurovascular coupling (Iadecola, 2004) is driven by synaptic mechanisms (Lauritzen, 2005), and ensures that oxygen and glucose are supplied to the brain to match activity (Raichle, 1998). There is a strong interest in exploring the energetic costs of single elements of chemical neurotransmission in order to provide an energy budget for signalling in the grey matter of the brain (Attwell & Laughlin, 2001; Lennie, 2003). This, as well as an exact knowledge of the timing and the quantitative relationship between neuronal activities, blood flow and oxygen consumption, forms the basis for correct interpretation of signals obtained by functional neuroimaging in the working brain (Raichle, 2003; Obata et al. 2004).
The aim of the present work was to investigate oxygen consumption by examining the changes in tissue oxygen partial pressure (Ptiss,O2) during activation in a simple neuronal network in relation to excitatory synaptic activity and changes in blood flow. We chose the excitatory, monosynaptic glutamatergic climbing fibre–Purkinje cell synapse for that purpose. One of the advantages of this system is that synaptic excitation is robust, and that the cerebral blood flow (CBF) response develops slowly during stimulation; that is, over tens of seconds (Mathiesen et al. 1998). In comparison, the CBF response in the somatosensory system develops much faster; that is, of the order of hundreds of milliseconds (Nielsen & Lauritzen, 2001), which may cloud rapid initial decreases in Ptiss,O2. Therefore, the chances of observing changes in Ptiss,O2 independently of blood flow changes were greater in the cerebellar cortex. This allowed us to explore the basis for the activity-induced initial ‘dip’, and the following overshoot in tissue oxygenation. As far as we can ascertain, this is the first study to simultaneously measure tissue oxygenation changes and CBF responses to a wide range of synaptic activation and quantitatively assess their relationships. Specifically, we tested the hypothesis that clamping the CBF response by inhibiting activity-dependent vasodilatation would enhance the oxygen dip without affecting the evoked synaptic activity. At the same time we examined the hypothesis that the tissue oxygenation overshoot that follows the initial early oxygen dip depended on the rise in blood flow. Finally, we tested the hypothesis that blocking postsynaptic activity by glutamatergic AMPA receptor inhibitors would block the early oxygen dip.
Methods
Animal preparation
Experiments were performed in 26 male Wistar rats (250–350 g). The study was in full compliance with the guidelines of the European Council's Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes and was approved by the Danish National Ethics Committee. Anaesthesia was induced with isoflurane (5.0% for induction, 1.5% during surgery). After surgery, anaesthesia was switched to intravenous α-chloralose (bolus, 45 mg kg−1; supplement, 15 mg kg−1 (20 min) −1) for at least 1 h before data acquisition. Extra supplements of α-chloralose were given upon pilo-erection or increased blood pressure (> 10%). The trachea was cannulated for mechanical ventilation (30% O2–70% N2O during surgery; O2-enriched air thereafter) and the left femoral vein and artery for anaesthesia delivery and for continuous monitoring of mean arterial blood pressure (MABP) and periodic arterial blood gas sampling, respectively (MABP, 108 ± 1 mmHg; PCO2, 36.8 ± 0.3 mmHg; PO2, 123.9 ± 2.0 mmHg; pH 7.39 ± 0.004; mean ± s.e.m.; n = 26). Rectal temperature was monitored and maintained at 37 ± 0.5°C. Intraperitoneal catheters were inserted for application of 7-nitroindazole (7-NI). The animals were placed in a head holder with lignocaine (lidocaine) gel (2%) applied to the contact spots of the ear bars. An open cranial window preparation was placed over parts of the cerebellar vermis and medulla oblongata, and the brain was superfused with artificial cerebrospinal fluid (aCSF) as previously described (Mathiesen et al. 1998). At the end of the experiment animals were killed by an intravenous injection of air. The experimental setup is depicted in Fig. 1.
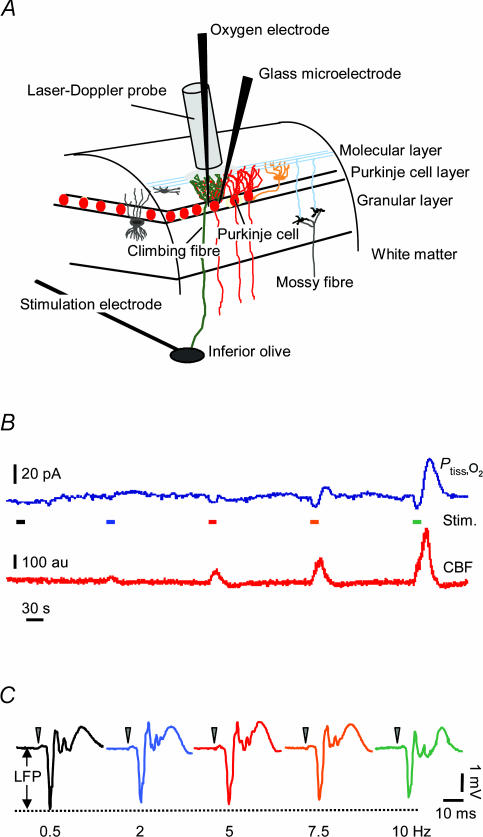
Figure 1. Recordings of Ptiss,O2, neuronal activity and blood flow in rat cerebellar cortex.
A, schematic three-dimensional drawing of experimental set-up, including neurones of interest and placement of stimulation and recording devices. A bi-polar electrode placed stereotaxically in the caudal part of the inferior olive stimulated climbing fibres (green) that give a monosynaptic excitatory input to Purkinje cells (red). Concurrent electrophysiological recordings (LFPs) were performed by a glass microelectrode at the level of the Purkinje cell layer. An oxygen microelectrode located at the same depth, simultaneously recorded changes in tissue oxygen tension (Ptiss,O2). A specific characteristic of this modified Clark-type polarographic electrode is its built-in guard cathode, which promotes the long-term stability of oxygen measurements. Tip diameter ranged from 3 to 5 μm. CBF was recorded by LDF with a probe located in close apposition to the microelectrodes. B, shows original recordings of both Ptiss,O2 (upper trace) and CBF (lower trace) in response to climbing fibre stimulation with increasing stimulation frequency. Coloured bars indicate stimulation periods (15 s) and frequencies (same colour code as in C). C, the corresponding averaged LFPs for each stimulation frequency, where LFP amplitudes tended to decrease with increasing frequencies. The double-headed arrow indicates the measured LFP amplitude for the 0.5-Hz stimulation. Time points of stimulation are marked by grey arrowheads. Examples are from the same animal as in Fig. 2.
Electrophysiological recordings
A single-barrelled glass microelectrode filled with 2 m saline (impedance, 2–3 MΩ; tip diameter, ∼2 μm) was lowered to a depth of 300–500 μm into the vermis of the cerebellar cortex. An Ag–AgCl ground electrode was placed in the neck muscle. Extracellular local field potentials (LFPs) and single unit activity (spikes) of Purkinje cells were recorded at the level of the Purkinje cell layer. Purkinje cells were identified by their ability to fire both simple and complex spikes spontaneously or the production of a complex spike at 5–8 ms after electrical stimulation of the inferior olive. The preamplified (× 10) signal was A/D-converted, amplified, filtered (spikes, 300–6000 Hz bandwidth; LFPs, 0.5–2400 Hz bandwidth) and digitally sampled using the Power 1401 interface (Cambridge Electronic Design, Cambridge, UK) connected to a PC running the Spike 2.5 software (Cambridge Electronic Design). Digital sampling rates were at 25 kHz for spikes and 5 kHz for LFPs.
Climbing fibre stimulation
A coated, bipolar stainless steel electrode (SNEX 200, Rhodes Medical Instruments, Inc., Woodland Hills, CA, USA; contact separation, 0.25 mm) was stereotaxically lowered into the caudal part of the inferior olive as described (Mathiesen et al. 1998). Positioning was optimized by means of the maximal response of LFPs to low-frequency stimulation (0.5 Hz) and by the occurrence of complex spikes in response to stimulation. Each Purkinje cell receives excitatory input from one climbing fibre that makes synaptic contact with the Purkinje cell soma and proximal dendrites. Climbing fibre stimulation induces release of glutamate, which activates Purkinje cells via postsynaptic AMPA receptors (Mathiesen et al. 1998), resulting in activation of Purkinje cells located within parasagittal bands (Dunbar et al. 2004). In optical imaging studies and multiple electrode recordings the width of these bands in the mediolateral direction ranged from 250 to 500 μm (Hanson et al. 2000; Fukuda et al. 2001). Stimulation was given as 0.2-ms square-wave pulses at 0.15 mA (ISO-flex, A.M.P.I., Israel) with increasing frequencies for 15 s. We used stimulation frequencies between 0.5 and 10 Hz; 10 Hz corresponds to the highest firing frequency of the inferior olive–climbing fibre system (Llinas & Volkind, 1973; Llinas et al. 2002) and evoked synaptic currents might be unreliable above this frequency (Silver et al. 1998).
Protocol
Three sets of experiments were performed for this study. First, we examined the relationship between increases in excitatory synaptic activity and local changes in Ptiss,O2 and CBF within the activated region. To increase neuronal activity we stimulated climbing fibres with increasing frequencies of 0.5, 2, 5, 7.5 and 10 Hz (n = 10). We correlated the summed LFP (ΣLFP, see below) to evoked changes in Ptiss,O2 and CBF to obtain a quantitative relationship between these variables.
In the second part of the study, we clamped CBF during activation thereby diminishing the supply of O2 to the activated area. To this end, we applied the neuronal nitric oxide synthase (nNOS, type I NOS) inhibitor, 7-NI (40 mg kg−1i.p., in 5% cremophor; Sigma Chemicals, Vallensbaek, Denmark; n = 8). Climbing fibres were stimulated at 10 Hz and the responses compared before (CTR) and 30 min after drug application (7-NI).
Finally, we wanted to establish whether postsynaptic activity in Purkinje cells could explain the oxygen consumption found during activation. Thus, the third part of this study examined the effect of AMPA receptor blockade on activation-induced Ptiss,O2 changes. We superfused the cerebellum with the AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 0.5 mm in aCSF; n = 8). Responses to stimulation at 10 Hz were compared before (CTR), during CNQX (CNQX), and after a wash out period (Wash out). Additionally, in five of the 7-NI-treated animals from the second part of the study, CNQX (0.5 mm) was applied after 7-NI had been given. In order to obtain complete recovery of responses, CNQX application was kept brief and no attempt was made to achieve a complete attenuation of signals in all animals. Only animals exhibiting a significant negative Ptiss,O2 response (area below 2 s.d. from baseline) were included in the CNQX experiments. Interstimulus interval in all three groups was 3 min.
Cerebellar cortical blood flow measurement
CBF was recorded continuously using laser-Doppler flowmetry (LDF). The LDF probe was at a fixed position ∼0.3 mm above the pial surface in a region devoid of large vessels (wavelengths, 780 nm; fibre separation, 250 μm; PeriMed, Järfälla, Sweden). The probe, measuring CBF changes down to a depth of 1000 μm, was placed as close as possible to the micro-and oxygen electrode. The LDF signal was smoothed with a time constant of 0.2 s (PeriFlux 4001 Master, PeriMed), sampled with 10 Hz, A/D converted and digitally recorded using Spike 2.5 software (Cambridge Electronic Design).
Tissue PO2 measurements
We used a modified Clark-type polarographic oxygen microelectrode (OX-10, Unisense A/S, Aarhus, Denmark) with a guard cathode for Ptiss,O2 measurements (Revsbech, 1989). The advantage of this electrode type is its small tip size (3–5 μm in this study) and its built-in guard cathode which removes all oxygen from the electrolyte reservoir. This enabled us to measure Ptiss,O2 over time (and different treatment conditions) with excellent long-term stability (signal drift, 0–0.5% h−1). The field of sensitivity is a sphere of 2 × tip diameter. The electrodes used in this study were constructed so that the 90% response time was < 1 s and the stirring sensitivity was nearly negligible at < 0.8% (Revsbech, 1989). Oxygen microsensors respond linearly to changes in oxygen concentration. Calibration of each electrode was performed in air-saturated and oxygen-free saline (0.9% at 37°C) before and after each experiment with reproducible oxygen measurements. Mean Ptiss,O2 was 38.4 ± 2.6 mmHg (mean ± s.e.m.; n = 26) and ranged from 12.7 to 64.4 mmHg. The oxygen electrodes were stepwise, vertically inserted in the cerebellar cortex and positioned in the same sagittal line and at the same cortical depth as the glass microelectrodes. The distance between the two electrodes was ∼100–200 μm. Both electrodes and the LDF probe recorded from the same cerebellar folia and were kept in the same position throughout the experiment. No linear drifts in baseline occurred during the experiments. The oxygen electrodes were connected to a high impedance picoammeter (PA 2000, Unisense A/S) sensing the currents of the oxygen electrodes. Signals were A/D converted and recorded at 100 Hz (Power 1401 and Spike 2.5 (Cambridge Electronic Design)). Off-line filtering using a low-pass filter of 0.3 Hz was used to remove noise induced by heartbeat and mechanical ventilation as described by Masamoto et al. (2003a).
Data analysis and statistics
Simultaneously recorded LFP, CBF and Ptiss,O2 signals were used for analysis. Stimulation-induced CBF and Ptiss,O2 responses were calculated as percentage change from baseline (20 s prior to stimulation). The magnitude of the response was estimated as the area under the curve above (CBF, positive Ptiss,O2 responses) or below (negative Ptiss,O2 responses) two standard deviations of baseline. This, in conjunction with the long baseline period, ensured that only statistically significant responses were included for further analysis. Individual animals had constant levels of baseline noise throughout the experiment. The initial slope (disappearance rate of oxygen in the tissue; −k) of the early Ptiss,O2 decrease is useful as an indicator of local oxygen consumption (Leniger-Follert, 1977). −k was calculated from the initial constant decrease phase of the negative Ptiss,O2 response (∼1–3 s after stimulation onset). −k was determined as the tangent of the steeply decreasing Ptiss,O2 curve during this period and is defined as the difference in tissue oxygen partial pressure (in percentage change from baseline) divided by time (in seconds). The initial slope was not calculated for the CNQX-treatment group because negative Ptiss,O2 responses were abolished or too small during CNQX application to obtain reliable initial slope calculations. Calculating oxygen consumption from the initial slope requires the determination of oxygen release from haemoglobin in the area of interest. Due to this, and the well-described spatial heterogeneity of brain Ptiss,O2 and local oxygen consumption (Leniger-Follert, 1977; Erecinska & Silver, 2001; Baumgartl et al. 2002; Masamoto et al. 2003b), no attempt was made to calculate local cerebral metabolic rate of oxygen (CMRO2) in absolute terms from our data. Another practical consequence of this spatial heterogeneity was that the temporal profiles of Ptiss,O2 responses were not averaged for groups of animals. The response magnitudes and the initial slopes of the oxygen signals were calculated for each animal from averaged responses of four to six stimulations per treatment condition or stimulation frequency. Calculations were performed by a custom-made analysis program based on Matlab 6.5 (MathWorks Inc., Natick, MA, USA). Responses in which the oxygen signal did not return to baseline (± 2 s.d.) within 75 s after stimulation onset were excluded.
The LFP is a potential change in the extracellular fluid induced by ion fluxes resulting from synchronized activity of ensembles of nerve cells (Llinas & Nicholson, 1974). LFPs in the cerebellar cortex are characterized by dendritic transmembrane ion fluxes (Nicholson & Llinas, 1971). Climbing fibre stimulation-induced LFPs consist of a negligible presynaptic component and a large excitatory postsynaptic potential which is followed by a smaller and slower positive potential (Eccles et al. 1967). A net influx of positive ions into the dendrites of Purkinje cells at the site of synaptic excitation produces a current sink and causes the negative deflection of the LFP. The following decline in negative potential and even its reversal to a positive wave are attributable to the somata and axons of the Purkinje cells, where a net efflux of positive ions acts as a current source (Eccles et al. 1966). We calculated LFP amplitudes as the difference between the large negative deflection and the baseline before stimulation onset. Additional off-line digital low-pass filtering (0.5–300 Hz) of the LFP signal ensured that no high frequency (spike) signals contaminated the LFP measurements. Mean LFP amplitudes were calculated for each frequency (0.5–10 Hz) or treatment condition (e.g. CTR, 7-NI, CNQX, Wash out) and the summed potential (ΣLFP) was calculated as the product of the LFP amplitude (in mV) and the stimulus rate (in Hz). The ΣLFP was used as an indicator of synaptic activity (Mathiesen et al. 1998).
To be able to compare data between animals, responses (LFP, CBF, Ptiss,O2) were normalized to the response evoked by 10 Hz stimulation. ΣLFP was correlated to the evoked CBF and Ptiss,O2 responses, either by least-squares linear regression analysis or by polynomial regression in cases where non-linearity was evident from the distribution of data. All data are expressed as means ± s.e.m. ANOVA on repeated measures followed by Bonferroni post hoc analysis was performed for statistical comparison of multiple treatment conditions. When appropriate for simple comparisons within groups (‘before-after’), a paired t test was used. Values were considered statistically significant at P < 0.05.
Results
Co-localized synaptic activity, Ptiss,O2 and CBF were simultaneously measured (Fig. 1). We used local field potential recordings, a Clark-type polarographic oxygen microelectrode and a LDF probe to assess the interdependency of synaptic activity, Ptiss,O2 and CBF responses and to clarify the nature of the biphasic Ptiss,O2 signals evoked by neuronal activation.
Experiments were performed in the cerebellar cortex using the well-described purely excitatory monosynaptic climbing fibre–Purkinje cell pathway. Stimulation of climbing fibres results in an all-or-none activation of postsynaptic Purkinje cells (Eccles et al. 1966). Synaptic transmission in the climbing fibre–Purkinje cell pathway is glutamatergic and results in activation of postsynaptic AMPA receptors (Mathiesen et al. 1998; Beitz & Saxon, 2004). Thus, there is a clear association between climbing fibre stimulation and postsynaptic excitatory activity in Purkinje cells (Eccles et al. 1966; Llano et al. 1991; Konnerth et al. 1990; Mathiesen et al. 1998) (Fig. 1C).
Frequency-dependent changes in Ptiss,O2 and CBF
We varied the input function by increasing the frequency of climbing fibre stimulation. Evoked responses of local Ptiss,O2 and CBF were frequency-dependent, as illustrated in Figs 1B, 2 and 3. The CBF responses were monophasic increments, whereas the Ptiss,O2 response pattern ranged from decreases alone at low frequencies to biphasic responses at higher stimulus frequencies (Fig. 2A). The biphasic responses consisted of an initial decrease in Ptiss,O2 (negative Ptiss,O2 response) followed by a slower and longer lasting positive peak (positive Ptiss,O2 response). Comparison of the time courses of the Ptiss,O2 and CBF signals showed that the decrease in Ptiss,O2 began before the increase in CBF was evident (Fig. 2A).
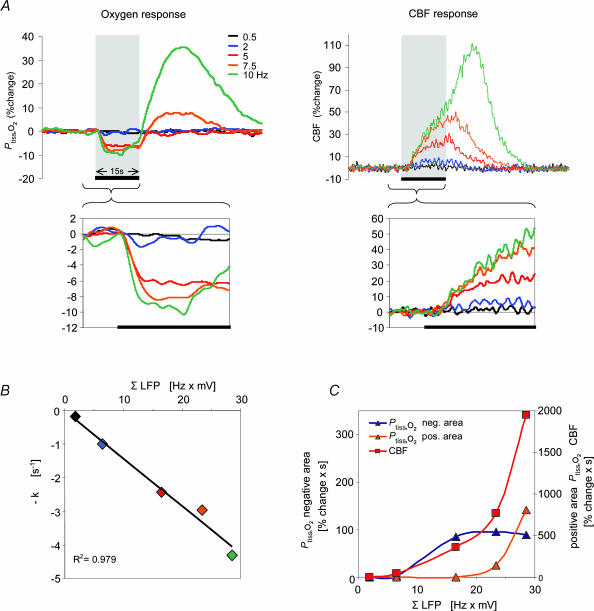
Figure 2. Frequency-dependent changes of Ptiss,O2, CBF and LFP responses: original recording from one animal.
A, superimposed Ptiss,O2 and CBF responses (averages of four responses from one animal) to varying stimulation frequencies from 0.5 to 10 Hz. The Ptiss,O2 response pattern ranged from monophasic decreases (0.5–5 Hz) to biphasic responses (7.5 and 10 Hz) where an initial decrease (negative Ptiss,O2 response) was followed by a tissue oxygenation overshoot (positive Ptiss,O2 response). The CBF response increased monotonically with increasing stimulation frequency. The inset boxes enlarge the responses during the stimulation period. The disappearance rate of oxygen in the tissue (−k) during the initial phase of stimulation, was frequency dependent (left inset), as indicated by the increasing steepness of the initial negative slope. Tissue deoxygenation began before CBF started to rise. The grey bars, and the black lines at the bottom of the inset boxes indicate the 15-s stimulation period. B, shows a correlation plot between ΣLFP during stimulation (averaged LFP amplitude × stimulation frequency) and −k. Colour coding is the same as in A. C, shows correlation plots between ΣLFP and evoked CBF, negative and positive Ptiss,O2 responses.
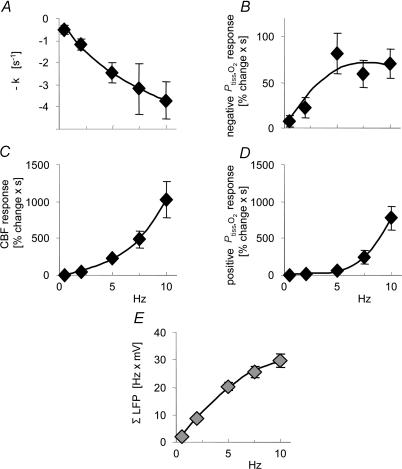
Figure 3. Frequency-dependency of Ptiss,O2 and CBF dynamics.
Stimulation-induced responses are plotted against stimulation frequencies (n = 10 rats). Data are averaged by frequency and illustrate the frequency dependency of the haemodynamic and oxygen responses. A, the disappearance rate of oxygen in the tissue (−k; initial slope of the negative Ptiss,O2 response) increased with increasing stimulation frequency. B, a plot of the mean area of the negative Ptiss,O2 response. The area of the negative Ptiss,O2 response increased up to frequencies of 5 Hz and then levelled off. C and D, the CBF and positive Ptiss,O2 responses showed an ongoing increase without signs of saturation. E, shows ΣLFP plotted against stimulation frequency. ΣLFP (expressing the overall level of synaptic activity) tends to saturate at frequencies above 5 Hz. The x-axis labels for C and D also apply for A and B. All values are given as mean ± s.e.m.
The disappearance rate of oxygen in the tissue (−k), reflecting early local oxygen consumption during activation, increased as a function of stimulation frequency, which is shown by the increasing initial slope of the negative Ptiss,O2 signal during the first seconds of stimulation (Fig. 2A inset, and Fig. 3A). In comparison, the magnitude of the area of the negative Ptiss,O2 response increased only up to stimulation frequencies of 5 Hz, after which it clearly levelled off or even decreased (Fig. 3B). The area of the positive Ptiss,O2 response paralleled the CBF increase at stimulation frequencies above 5 Hz (Fig. 3C and D).
Relationship of ΣLFP to Ptiss,O2 and CBF responses
As a measure of neuronal activation, synaptic activity was calculated for each stimulation train as the average field potential amplitude multiplied by stimulus frequency (ΣLFP). The LFP represents the spatially weighted sum of extracellular currents due to synchronous synaptic activity in a group of neurones in response to one stimulation impulse (Llinas & Nicholson, 1974). The summation of LFP amplitudes over time is therefore a plausible indicator of total synaptic activity evoked by repeated stimulation impulses and has been widely used to describe the inter-relationship of synaptic activity and CBF or metabolism (Mathiesen et al. 1998; Devor et al. 2003; Sheth et al. 2004; Thomsen et al. 2004). LFP amplitudes decreased at higher stimulation frequencies (Fig. 1C) due to reduced glutamate release (Silver et al. 1998; Foster & Regehr, 2004) or postsynaptic AMPA receptor saturation (Harrison & Jahr, 2003). In consequence, ΣLFP started to plateau at frequencies above 5 Hz (Fig. 3E).
The relationship between ΣLFP and the initial slope of the negative Ptiss,O2 response (−k) is shown in Fig. 2B (one animal) and Fig. 4A (all animals, normalized data). Evoked synaptic activity was found to be linearly related to −k and the data were well described by a linear regression passing through the origin (R2= 0.987; P= 3.37 × 10−6). This finding implies that during the early phase of activation, oxygen is consumed at all levels of synaptic activity.
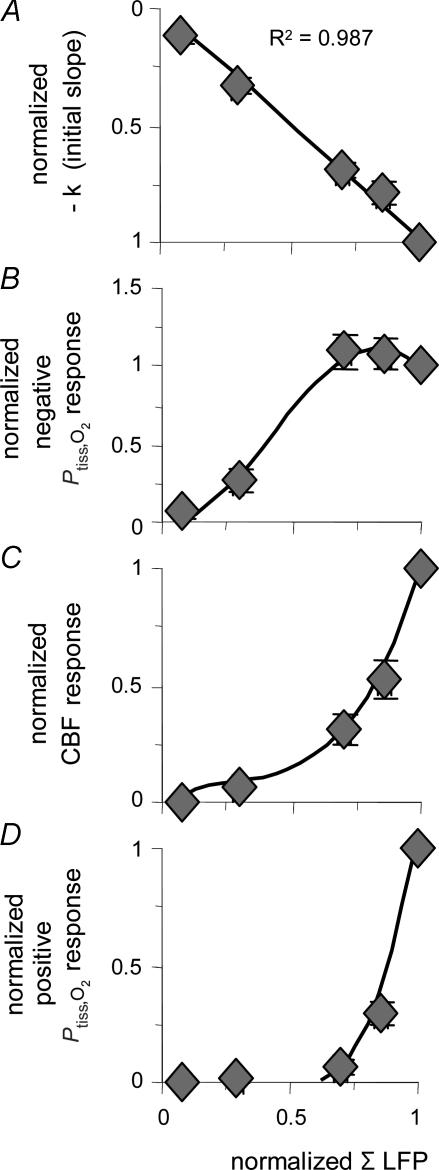
Figure 4. Correlation between accumulated synaptic activity and Ptiss,O2 and CBF responses.
Normalized values of evoked CBF and Ptiss,O2 responses are plotted against normalized values of ΣLFP (n = 10 rats). All responses were normalized to the corresponding 10-Hz response and were averaged and grouped by stimulation frequency. A, the disappearance rate of oxygen in the tissue (−k) plotted against synaptic activity (ΣLFP) shows an inverse linear relationship, well fitted by a linear regression passing through the origin (P < 0.05). This suggests that oxygen consumption increases at all levels of synaptic activity. B, the correlation between ΣLFP and the area of the negative Ptiss,O2 response demonstrated saturation of the negative Ptiss,O2 response at high levels of synaptic activity. The CBF (C) and the positive Ptiss,O2 response (D) both exhibited a non-linear rise over the range of induced synaptic activities without reaching saturation. C and D, indicate that synaptic activity, and thereby CBF, has to increase above a given threshold to evoke a Ptiss,O2 overshoot. Note that the level of synaptic activity where the CBF response curve started to increase more steeply coincides not only with the point where a positive Ptiss,O2 signal became evident but also with the point where the negative Ptiss,O2 response started to level off. The x-axis label for D applies to all panels. Polynomial regression was used to illustrate the distribution of data in B–D. All values are given as mean ± s.e.m.
The area of the negative Ptiss,O2 response was calculated to obtain a measure of the integrated effect of synaptic activity on Ptiss,O2 over time. However, in contrast to −k, the relationship between the area of the negative Ptiss,O2 response and synaptic activity (ΣLFP) was non-linear (Fig. 4B). The negative Ptiss,O2 area increased linearly at low or moderate levels of synaptic activity, but reached a plateau at higher levels. In comparison, neither the area of the positive Ptiss,O2 response nor the CBF response showed signs of saturation with increasing synaptic activity (Fig. 4C and D). The graphs in Fig. 4C and D show a non-linear relationship between synaptic activity and CBF, and positive Ptiss,O2 responses, respectively. They also illustrate that the positive Ptiss,O2 response followed the increase in CBF only at higher levels of synaptic activity.
The pattern of these responses suggested strong interaction between the evoked CBF responses and the magnitudes of the negative and positive Ptiss,O2 responses. The negative Ptiss,O2 response area increased as a function of ΣLFP up to the level of synaptic activity at which the magnitude of the CBF response increased sharply. At this point also, the positive Ptiss,O2 response appeared (Fig. 4B–D). We hypothesized that the activity-induced, biphasic Ptiss,O2 signal originates from increased oxygen consumption followed by increased oxygen supply due to the rise in CBF, and that this increase in oxygen supply is responsible for attenuating the negative Ptiss,O2 response.
Effect of attenuated CBF rise on Ptiss,O2 responses and synaptic activity
To validate the above hypothesis, we tried to clamp CBF during activation using 7-NI (40 mg kg−1), which is a relatively selective, irreversible nNOS inhibitor. 7-NI effectively attenuates stimulation-induced CBF responses (Cholet et al. 1996; Yang et al. 1999; Lindauer et al. 1999), without affecting either resting or stimulation-induced cerebral glucose utilization (Cholet et al. 1997). Furthermore, NOS inhibition has no effect on stimulation-induced neuronal activity (Akgoren et al. 1996; Lindauer et al. 1996) or on the basal CMRO2 (Iadecola et al. 1994; Chi et al. 2003). Thus 7-NI is a suitable pharmacological tool to investigate the effect of neuronal activation on tissue oxygenation changes under conditions of an attenuated CBF response.
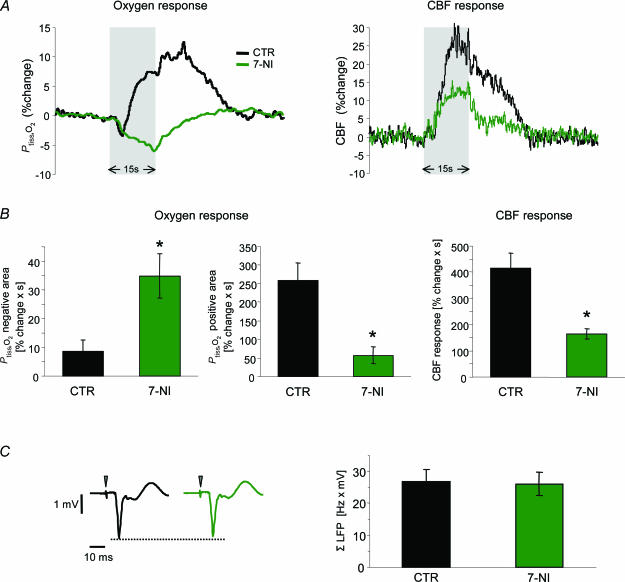
In the present study, 7-NI did not result in significant changes of arterial blood gas parameters, MABP (+1.6 ± 1.6%) baseline CBF (−11.4 ± 6.3%), or baseline Ptiss,O2 (−0.8 ± 2.7%). In the following experiments, 10 Hz stimulations were given repeatedly under control conditions and in the presence of 7-NI (n = 8). This stimulation frequency of 10 Hz was chosen as it was found to produce robust biphasic Ptiss,O2 responses. Figure 5A shows an example of Ptiss,O2 and CBF responses before (CTR) and after administration of 7-NI. 7-NI attenuated the stimulation-induced CBF increase by 60.3 ± 4.6% (P= 0.003; Fig. 5B). At the same time, the negative Ptiss,O2 response increased fourfold (+403.4 ± 22.1%; P= 0.01), while the positive Ptiss,O2 response decreased by 78.1 ± 8.8% (P= 0.003; Fig. 5B). These findings confirmed our hypothesis, which predicted that 7-NI would enlarge the negative and reduce the positive Ptiss,O2 responses.
Figure 5. Effect of attenuated CBF rise on Ptiss,O2 responses and synaptic activity.
Responses to climbing fibre stimulation at 10 Hz were compared before (CTR) and after i.p. application of the nNOS inhibitor 7-nitroindazol (7-NI; n = 8 rats). A, shows an example of Ptiss,O2 and corresponding CBF responses before (black) and after application of 7-NI (green). Grey bars denote the stimulation period. The biphasic Ptiss,O2 response at this frequency was calculated as negative and positive Ptiss,O2 response area. B, 7-NI enlarged the negative Ptiss,O2 response by ∼400% (P < 0.05) and reduced the positive Ptiss,O2 response by ∼78% (P < 0.05). These changes accompanied the attenuation of the evoked CBF rise of ∼60% (P < 0.05) B, all values in B are given as mean ± s.e.m.C, demonstrates that the observed changes were not due to effects on synaptic activity, as ΣLFP was not significantly altered by 7-NI. C, left panel shows averaged LFPs before (black) and after (green) application of 7-NI from the same animal as in A. Time points of stimulation are marked by grey arrowheads. *P < 0.05.
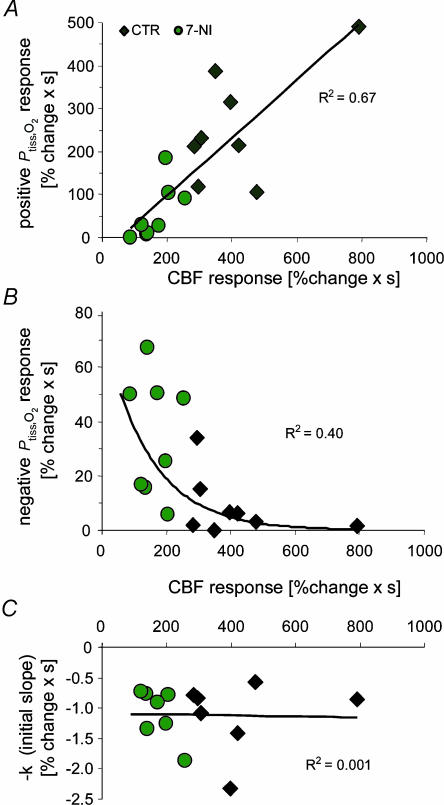
Interestingly, −k was not affected by 7-NI (+2.7 ± 6.9%; P= 0.71). This indicated that the initial slope of the negative Ptiss,O2 response was independent of the associated CBF rise (Fig. 6C). In comparison, both the positive and the negative Ptiss,O2 areas depended on the magnitude of the evoked CBF response. The positive Ptiss,O2 response was linearly correlated to the evoked CBF increase (Fig. 6A; linear regression analysis: y= 0.673x−37.2; R2= 0.67, P= 0.0001) which demonstrated that the positive Ptiss,O2 response in the cerebellum is indeed a consequence of increased oxygen supply. However, CBF had to rise above a certain threshold before a positive Ptiss,O2 response was evident. The negative Ptiss,O2 response decayed exponentially with increasing CBF (Fig. 6B). Of most importance, ΣLFP was not affected by NOS inhibition (−2.5%, P= 0.26; Fig. 5C). Thus, the observed changes in the Ptiss,O2 signal were not due to reduced synaptic activity. So far, our findings indicated that the origin of the positive Ptiss,O2 response is an increase in O2 supply derived from the activation-induced increase in CBF. At the same time, the negative Ptiss,O2 response is modulated by the increment in CBF.
Figure 6. Tissue PO2 responses in relation to CBF increases before and after application of 7-NI.
A, plotting the areas of positive Ptiss,O2 responses to 10-Hz stimulations against corresponding CBF responses shows that small positive Ptiss,O2 responses correspond to small increases in CBF obtained after 7-NI application (green circles), while large positive Ptiss,O2 responses correspond to large increases in CBF obtained during control conditions (♦) (n = 8 rats). This indicates a linear relationship between positive Ptiss,O2 signals and increases in CBF (linear regression analysis, P < 0.05). B, the areas of negative Ptiss,O2 responses decayed exponentially with increasing magnitude of CBF responses (y= 75.012e − 0.0069x). C, in contrast to the positive and negative Ptiss,O2 responses, the disappearance rate of oxygen in the tissue (−k) was independent of the magnitude of evoked CBF responses.
Effect of glutamate receptor blockade on synaptic transmission, Ptiss,O2 and CBF responses
Here we examined whether postsynaptic activity in Purkinje cells could explain oxygen consumption during activation. Therefore, we studied the effect of AMPA receptor blockade on activation-induced Ptiss,O2 changes. AMPA receptor blockade has previously been shown to effectively inhibit glutamate-mediated synaptic transmission at the climbing fibre–Purkinje cell synapse (Mathiesen et al. 1998). Beside this direct action of CNQX on postsynaptic excitation, indirect actions might increase inhibition (Brickley et al. 2001) or decrease disinhibition of Purkinje cells (Satake et al. 2000) which could also affect the Purkinje cell output (spiking) activity.
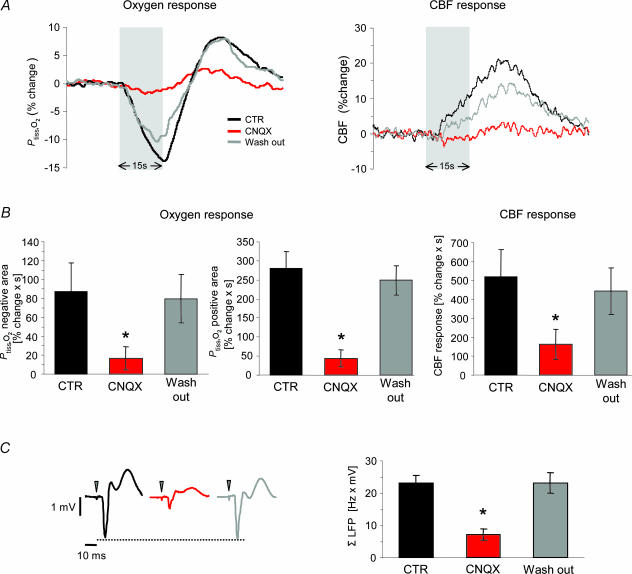
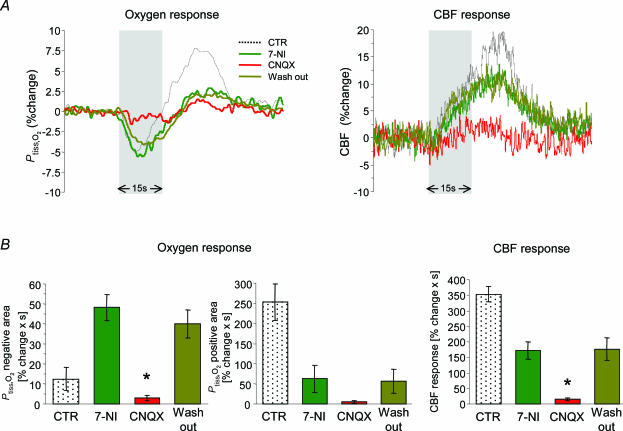
We found that superfusion of the cerebellum with the AMPA receptor antagonist, CNQX (0.5 mm), had no effect on MABP, baseline CBF (+2.8 ± 2.7%) or baseline Ptiss,O2 (+2.2 ± 2.3%; n = 8). CNQX attenuated ΣLFP by 68.9 ± 7% (P < 0.001; Fig. 7C) and CBF responses by 69.0 ± 15.3% (P < 0.001; Fig. 7A and B; n = 8). At the same time, CNQX significantly inhibited both the negative (−81.3 ± 13.9%; P= 0.004) and the positive Ptiss,O2 responses (−84.4 ± 8.1%, P < 0.001; Fig. 7A and B). All effects of CNQX were reversible with no significant differences between control conditions and washout (Fig. 7). These findings supported the idea that the decrease in tissue oxygenation seen during activation depends upon postsynaptic activity in Purkinje cells. This was confirmed by additional experiments in which CNQX was applied after pretreatment with 7-NI (n = 5; Fig. 8).
Figure 7. Effect of AMPA receptor blockade on synaptic transmission, and evoked Ptiss,O2 and CBF responses.
The effect of topically applied CNQX on responses evoked by climbing fibre stimulation at 10 Hz is illustrated (n = 8 rats). Responses obtained before (CTR), during (CNQX) and after wash out (Wash out) were compared. A, shows an example of Ptiss,O2 and corresponding CBF responses before (black trace), during (red trace) and after washout of CNQX (grey trace). Grey bars denote the stimulation period. B, AMPA receptor blockade reversibly attenuated both phases of the Ptiss,O2 response and the CBF increase (negative Ptiss,O2 response area, −81%; positive Ptiss,O2 response area, −84%; CBF response, −69%). All values in B are given as mean ± s.e.m.C, the concomitant inhibition of ΣLFP during AMPA receptor blockade (−69%, P < 0.05, right panel) indicated that the reduction of the Ptiss,O2 responses in the presence of CNQX was due to reduced postsynaptic activity. C, left panel shows averaged LFPs before (black), during (red) and after wash out (grey) of CNQX from the same animal as in A. Time points of stimulation are marked by grey arrowheads. *P < 0.05 compared to CTR.
Figure 8. Effect of AMPA receptor blockade after pretreatment with 7-NI.
CNQX (0.5 mm) was topically applied in five out of eight animals treated with 7-NI and responses evoked by climbing fibre stimulation at 10 Hz were compared. A, shows an example of Ptiss,O2 and corresponding CBF responses from one animal under control conditions (black trace), after application of 7-NI (dark green), in the presence of 7-NI + CNQX (red) and after washout of CNQX (bright green). Grey bars denote the stimulation period. B, shows the averaged responses before (CTR) and after application of 7-NI (7-NI) and illustrates the temporary effect of CNQX (CNQX and Wash out). As 7-NI is an irreversible nNOS inhibitor, it cannot be washed out. Compared to 7-NI values, the negative Ptiss,O2 response during application of CNQX was reduced by ∼94%. The already diminished positive Ptiss,O2 and CBF responses were both further reduced by ∼91%. However, the decrease in the positive Ptiss,O2 response did not reach significance. CNQX application attenuated the ΣLFP by 85% (compared to 7-NI; P < 0.05; data not shown). All CNQX effects were reversible with no significant differences to 7-NI. All values are given as mean ± s.e.m.*P < 0.05 compared to 7-NI.
Discussion
The direct assessment of local changes in Ptiss,O2 in conjunction with CBF and electrophysiological recordings revealed a linear relationship between the disappearance rate of oxygen in the tissue and synaptic activity during the initial phase of neuronal activation in the cerebellar cortex. This increase in oxygen metabolism was evident at all levels of synaptic activity and depended on activation of glutamatergic AMPA receptors localized postsynaptically on Purkinje cell dendrites. Hence in vivo, early increases in neuronal aerobic metabolism depend on postsynaptic activity as previously shown in vitro for the hippocampus (Kasischke et al. 2004). During the initial phase of activation we observed a temporal uncoupling of oxidative metabolism and CBF as a decline in Ptiss,O2 was observed preceding the rise in CBF. Following the initial oxygen ‘dip’, Ptiss,O2 increased due to the rise in CBF. Application of 7-NI attenuated the CBF rise, while increasing the negative Ptiss,O2 response and decreasing the positive Ptiss,O2 response. These findings imply that, in the cerebellum, the activity-induced reduction in Ptiss,O2 is counteracted by an increase in oxygen supply due to the rise in CBF. The data also support the hypothesis that the positive Ptiss,O2 response is an overshoot caused by the evoked increase in CBF. The fact that oxygen consumption increased before CBF suggests a reserve capacity of oxygen in the tissue as proposed previously (Buxton, 2001; Mintun et al. 2001; Sheth et al. 2004).
Negative Ptiss,O2 and CBF response
In the present study, the typical Ptiss,O2 response was biphasic with an initial decrease followed by an increase. The magnitude of both phases varied with the level of activity and differed between animals, reflecting the well-known spatial heterogeneity of Ptiss,O2 profiles at rest and during activation (Gijsbers & Melzack, 1967; Silver, 1978; Lubbers et al. 1994; Erecinska & Silver, 2001; Masamoto et al. 2004). Despite this heterogeneity, initial Ptiss,O2 reductions preceding evoked CBF responses were observed at all stimulation frequencies. This is consistent with previous studies in the somatosensory and visual cortices in which early decreases in tissue PO2 (Ances et al. 2001) or intravascular oxygen content (Malonek et al. 1997; Jones et al. 2001) occurred before significant CBF responses. The present study confirmed this response pattern in the cerebellar cortex. Our findings strengthen the concept that early activity-induced increases in deoxyhaemoglobin in optical imaging studies (Jones et al. 2001; Devor et al. 2003) and the initial dip seen in functional MRI (fMRI) studies (Menon et al. 1995; Kim et al. 2000) reflect local increases in oxygen consumption. The area of the negative Ptiss,O2 response is thought to reflect changes in oxygen consumption and supply over time. The influence of activity-dependent increases in CBF for the Ptiss,O2 response has been hypothesized previously (Silver, 1978; Ances et al. 2001; Masamoto et al. 2003a; Thompson et al. 2003) but has not been proven by simultaneous measurements of Ptiss,O2, neuronal activity and CBF over a wide range of activities. For example Thompson et al. (2004) showed that activation of a small neuronal population induced a negative Ptiss,O2 change that was partly cancelled out by a positive Ptiss,O2 signal, which arose when a larger population of neurones was activated. They suggested that this was due to a rise in CBF, but CBF was not measured. We demonstrated by varying the synaptic input to a given population of neurones that the level of activity at which the negative Ptiss,O2 area no longer increases coincided with the level of activity at which the CBF response curve escalated (Fig. 4B and C). The non-linear relationship found between evoked synaptic activity and CBF responses in the cerebellum was similar to those previously found in the cerebral cortex (Devor et al. 2003; Sheth et al. 2004).
The hypothesis that the reduction of the negative Ptiss,O2 area was caused by increased oxygen supply due to greatly increased CBF responses was further tested in the 7-NI experiments. We found that the negative Ptiss,O2 area increased and the positive Ptiss,O2 area decreased as the magnitude of the activity-evoked CBF responses declined in the presence of 7-NI. Thus, this study clearly demonstrated an inverse relationship between the negative Ptiss,O2 area and the CBF response, and that at high levels of activation, the negative Ptiss,O2 area, and thereby estimates of oxygen consumption based upon this parameter, are confounded by increased O2 supply. We conclude that the non-linear relationship between the negative Ptiss,O2 area and synaptic activity is explained by increased CBF and oxygen supply and not by a reduction in oxygen consumption.
Tissue and intravascular deoxygenation during activation
The non-linearity between synaptic activity and negative Ptiss,O2 area in the present study raises important issues concerning the initial dip observed with optical imaging techniques and fMRI and whether it can accurately reflect the magnitude of underlying neuronal activity (Nemoto et al. 2004). PO2 in the tissue lies between capillary and mitochondrial PO2 levels and shows a high susceptibility to changes in oxygen consumption (Secomb et al. 2000). The increased oxygen concentration gradient in the tissue during activation (Vanzetta & Grinvald, 1999; Ances et al. 2001; Thompson et al. 2003) results in increased conversion of oxy-to deoxyhaemoglobin. Hence, deoxygenation signals from tissue and vasculature are linked in their response to neuronal activation. We found that the magnitude of the negative Ptiss,O2 area, representing tissue deoxygenation, reaches a plateau at high levels of neuronal activity (Fig. 4B). Thus, the initial dip representing intravascular deoxygenation cannot be expected to increase at high levels of neuronal activity when tissue deoxygenation does not. This implies that the initial dip calculated as peak response or area might not be capable of indicating increases in neuronal activity beyond a given threshold, which could explain some of the inconsistent findings in optical imaging studies correlating neuronal activity to the magnitude of the initial dip (Mayhew et al. 2000; Devor et al. 2003; Sheth et al. 2003, 2004; Nemoto et al. 2004).
Initial slope and activity-dependent increases in early oxygen consumption
As the relationship between synaptic activity and the area of the negative Ptiss,O2 response was confounded by CBF, we then assessed the initial slope of the negative Ptiss,O2 response (−k) to obtain a relative measure of activity-induced oxygen consumption. Local oxygen consumption has previously been determined in absolute terms in the brain cortex and hippocampus by using O2-disappearance measurements during brief periods of total brain ischaemia (Leniger-Follert, 1977; Buerk & Nair, 1993). In the present study, −k was independent of the associated CBF rises, implying that −k is a reliable indicator of early increases in oxygen consumption during activation in the cerebellum. Unlike the negative Ptiss,O2 response area, −k did increase as a linear function of synaptic activity (i.e. ΣLFP; Fig. 4A). This confirms previous findings using optical imaging techniques, which calculated that peak CMRO2 increased with growing stimulation intensities (Jones et al. 2001) and that the relationship between ΣLFP and increases in CMRO2 was linear (Sheth et al. 2004). Sheth et al. (2004) calculated that increased oxygen extraction from the vasculature occurs only above a given threshold of synaptic activity. In comparison, we found no such threshold in the cerebellum, indicating that tissue oxygen consumption increased at even the lowest levels of synaptic activity. This, in conjunction with the observed non-linear increase of CBF with synaptic activity, supports the idea that at rest a significant amount of readily available oxygen in the tissue exists which might serve as a tissue oxygen reserve (Buxton, 2001; Mintun et al. 2001). Importantly, the present study indicated that an increase in local oxygen consumption occurs in response to activation and that this increase is linearly related to the level of synaptic activity.
Using the initial slope (−k) to represent oxygen consumption during activation, we found that the relationship between evoked oxygen consumption and CBF responses was non-linear (Fig. 4A and C). Our data are at variance with other studies reporting a 2 : 1 linear relationship between CBF and CMRO2 changes (Hoge et al. 1999; Sheth et al. 2004), but are consistent with mathematical models of tissue oxygen delivery predicting a supra-linear relationship (Buxton & Frank, 1997; Hudetz, 1999; Vafaee & Gjedde, 2000; Secomb et al. 2000).
Neurometabolic coupling
Activity-induced changes in Ptiss,O2 have been explored in a number of studies (Travis & Clark, 1965; Gijsbers & Melzack, 1967; Sick & Kreisman, 1979; Ances et al. 2001) but recently the relationship between neuronal activity and oxygen consumption has been assessed more directly by recording the two variables simultaneously in the same location. This has shown that negative Ptiss,O2 changes are highly specific for local increases in neuronal activity and quantitatively related to the amount of underlying neuronal activity (Thompson et al. 2003; Masamoto et al. 2003a), although the type of neuronal activity has not been established. Masamoto et al. (2003a) related neuronal activity induced by single activation tasks to voltage-sensitive dye recordings which, however, allowed no discrimination between pre-and postsynaptic activation. Thompson et al. (2003, 2004) focused on the local specificity of tissue deoxygenation signals in relation to spike recordings and confirmed a correlation between spiking activity and negative tissue PO2 responses during activation (Sick & Kreisman, 1979). As neurotransmission and synaptic input in most neuronal circuits are prerequisites for spike generation and are often linearly correlated to changes in output spiking activity (Devor et al. 2003; Jones et al. 2004), it is not possible to deduce by spike measurements alone whether glutamate release, postsynaptic processing or output firing is responsible for the increased oxygen demand during activation.
During climbing fibre stimulation, we observed a linear relationship between synaptic activity and −k as discussed above. Topical application of CNQX almost abolished synaptic transmission as well as the evoked negative Ptiss,O2 response, identifying postsynaptic AMPA receptor activation as necessary for early negative Ptiss,O2 changes. Astrocytes are not activated during electrical stimulation of Purkinje cell afferents as reported in an immunohistochemical study (Tian & Bishop, 2002). Thus, in the climbing fibre–Purkinje cell pathway, increases in oxygen consumption are coupled to activity in postsynaptic neurones. As the climbing fibre–Purkinje cell synapse is one of the most powerful excitatory synapses where glutamatergic transmission leads to activation of AMPA receptors on one of the widest dendritic trees, it has to be determined whether oxygen consumption in other brain regions is also mainly driven by postsynaptic activation. Our findings are consistent with a nuclear magnetic resonance spectroscopy study by Sibson et al. (1998), who showed that rates of neuronal CMRO2 and glutamate release and cycling are stoichiometrically related. However, our study cannot be used to differentiate between oxygen used for postsynaptic processing and oxygen used for generation of spikes. We conclude that the observed early increase in oxygen consumption during activation in vivo is of neuronal origin as has been previously shown in the hippocampus in vitro (Kasischke et al. 2004). Furthermore, the results of the present study demonstrate that neuronal oxygen consumption in the cerebellar cortex increases together with synaptic activity.
Neuronal activity and oxygen transport to tissue
There is still debate concerning the coupling between blood flow responses and oxygen consumption during activation in the brain. Oxygen-limitation models designed to explain this coupling have been based on the assumption that at rest all oxygen in the tissue is consumed and PO2 at the level of the mitochondria is zero (Buxton & Frank, 1997; Hyder et al. 1998; Vafaee & Gjedde, 2000). However during activation, we observed that oxygen consumption in the tissue increased before CBF did, and that in the presence of 7-NI, the magnitude of the negative Ptiss,O2 area increased despite the strong attenuation of the CBF response. These observations suggest that resting Ptiss,O2 is far from zero. Our findings support the existence of a tissue oxygen reserve and are in line with human positron emission tomography data (Mintun et al. 2001), calculations of resting tissue oxygenation levels in the brain (Secomb et al. 2000) and recent modelling of tissue oxygen delivery (Hudetz, 1999; Mintun et al. 2001). The results from our study imply that in the anaesthetized state, the cerebellum has an excess oxygen delivery at rest and that this tissue oxygen reserve is used during the initial phase and at low grades of activation. We suggest that up to a given level of neuronal activity, oxygen consumption can increase even under conditions of constant CBF. On the other hand, the finding of a supra-linear relationship between CBF and CMRO2 changes during activation provides experimental confirmation of the predictions derived from the oxygen-limitation models (Buxton & Frank, 1997; Vafaee & Gjedde, 2000). According to these models, oxygen diffusivity is limited (Gjedde et al. 1991) and during activation, increases in oxygen consumption are supported by disproportionately large increases in CBF (Buxton & Frank, 1997). Combined, our data support the recently proposed hypothesis that a buffer of oxygen availability in the tissue exists and that CBF increases during activation are regulated to maintain this buffer (Buxton, 2001).
Conclusion
In this study we examined the physiological basis of activity-induced changes in tissue oxygen tension in rat cerebellar cortex. We showed that Ptiss,O2 responses are the result of a temporally staggered interplay between neuronal oxygen consumption and evoked CBF increments both driven by postsynaptic activity. At the level of the climbing fibre–Purkinje cell synapse, tissue oxygen consumption is linearly coupled to synaptic excitation with no evidence of a threshold; thus, we found that tissue energy demands are supplied by oxidative metabolism at all levels of synaptic activity. We have also quantitatively assessed the relationships between synaptic activity and tissue oxygenation signals and CBF responses. The findings indicate a supralinear relationship between increases in oxygen consumption and CBF during activation and support the existence of a buffer of oxygen availability in brain tissue.
Acknowledgments
We thank Lillian Grondahl for expert technical assistance, and Jesper Grondahl for his sophisticated MatLab programming. This study was supported by The Lundbeck Foundation, the Danish Medical Research Council, Vera and Carl Michelsens legat, and The NOVO-Nordisk Foundation.
References
- Akgoren N, Dalgaard P, Lauritzen M. Cerebral blood flow increases evoked by electrical stimulation of rat cerebellar cortex: relation to excitatory synaptic activity and nitric oxide synthesis. Brain Res. 1996;710:204–214. doi: 10.1016/0006-8993(95)01354-7. 10.1016/0006-8993(95)01354-7. [DOI] [PubMed] [Google Scholar]
- Ances BM, Buerk DG, Greenberg JH, Detre JA. Temporal dynamics of the partial pressure of brain tissue oxygen during functional forepaw stimulation in rats. Neurosci Lett. 2001;306:106–110. doi: 10.1016/s0304-3940(01)01868-7. 10.1016/S0304-3940(01)01868-7. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Baumgartl H, Zimelka W, Lubbers DW. Evaluation of PO2 profiles to describe the oxygen pressure field within the tissue. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:75–85. doi: 10.1016/s1095-6433(01)00532-3. 10.1016/S1095-6433(01)00532-3. [DOI] [PubMed] [Google Scholar]
- Beitz AJ, Saxon D. Harmaline-induced climbing fiber activation causes amino acid and peptide release in the rodent cerebellar cortex and a unique temporal pattern of Fos expression in the olivo-cerebellar pathway. J Neurocytol. 2004;33:49–74. doi: 10.1023/B:NEUR.0000029648.81071.20. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Farrant M, Swanson GT, Cull-Candy SG. CNQX increases GABA-mediated synaptic transmission in the cerebellum by an AMPA/kainate receptor-independent mechanism. Neuropharmacology. 2001;41:730–736. doi: 10.1016/s0028-3908(01)00135-6. [DOI] [PubMed] [Google Scholar]
- Buerk DG, Nair P. PtiO2 and CMRO2 changes in cortex and hippocampus of aging gerbil brain. J Appl Physiol. 1993;74:1723–1728. doi: 10.1152/jappl.1993.74.4.1723. [DOI] [PubMed] [Google Scholar]
- Buxton RB. The elusive initial dip. Neuroimage. 2001;13:953–958. doi: 10.1006/nimg.2001.0814. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab. 1997;17:64–72. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- Chi OZ, Liu X, Weiss HR. Effects of inhibition of neuronal nitric oxide synthase on NMDA-induced changes in cerebral blood flow and oxygen consumption. Exp Brain Res. 2003;148:256–260. doi: 10.1007/s00221-002-1310-7. [DOI] [PubMed] [Google Scholar]
- Cholet N, Bonvento G, Seylaz J. Effect of neuronal NO synthase inhibition on the cerebral vasodilatory response to somatosensory stimulation. Brain Res. 1996;708:197–200. doi: 10.1016/0006-8993(95)01387-3. [DOI] [PubMed] [Google Scholar]
- Cholet N, Seylaz J, Lacombe P, Bonvento G. Local uncoupling of the cerebrovascular and metabolic responses to somatosensory stimulation after neuronal nitric oxide synthase inhibition. J Cereb Blood Flow Metab. 1997;17:1191–1201. doi: 10.1097/00004647-199711000-00008. [DOI] [PubMed] [Google Scholar]
- Clarke DD, Sokoloff L. Circulation and energy metabolism of the brain. In: Siegel GJ, Agranoff BW, Albers RW, Molinoff PB, editors. Basic Neurochemistry: Molecular, Cellular, and Medical. New York: Raven Press; 1994. pp. 645–680. [Google Scholar]
- Devor A, Dunn AK, Andermann ML, Ulbert I, Boas DA, Dale AM. Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron. 2003;39:353–359. doi: 10.1016/s0896-6273(03)00403-3. 10.1016/S0896-6273(03)00403-3. [DOI] [PubMed] [Google Scholar]
- Dunbar RL, Chen G, Gao W, Reinert KC, Feddersen R, Ebner TJ. Imaging parallel fiber and climbing fiber responses and their short-term interactions in the mouse cerebellar cortex in vivo. Neuroscience. 2004;126:213–227. doi: 10.1016/j.neuroscience.2004.02.020. 10.1016/j.neuroscience.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentågothai J. The Cerebellum as a Neuronal Machine. New York: Springer; 1967. [Google Scholar]
- Eccles JC, Llinas R, Sasaki K. The excitatory synaptic action of climbing fibres on the purkinje cells of the cerebellum. J Physiol. 1966;182:268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol. 2001;128:263–276. doi: 10.1016/s0034-5687(01)00306-1. 10.1016/S0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- Foster KA, Regehr WG. Variance-mean analysis in the presence of a rapid antagonist indicates vesicle depletion underlies depression at the climbing fiber synapse. Neuron. 2004;43:119–131. doi: 10.1016/j.neuron.2004.06.022. 10.1016/j.neuron.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Yamamoto T, Llinas R. The isochronic band hypothesis and climbing fibre regulation of motricity: an experimental study. Eur J Neurosci. 2001;13:315–326. doi: 10.1046/j.0953-816x.2000.01394.x. 10.1046/j.0953-816X.2000.01394.x. [DOI] [PubMed] [Google Scholar]
- Gijsbers KJ, Melzack R. Oxygen tension changes evoked in the brain by visual stimulation. Science. 1967;156:1392–1393. doi: 10.1126/science.156.3780.1392. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Ohta S, Kuwabara H, Meyer E. Is oxygen diffusion limiting for blood-brain transfer of oxygen? In: Lassen NA, Ingvar DH, Raichle ME, Friberg L, editors. Brain Work and Mental Activity. Copenhagen: Munksgaard; 1991. pp. 177–184. [Google Scholar]
- Hanson CL, Chen G, Ebner TJ. Role of climbing fibers in determining the spatial patterns of activation in the cerebellar cortex to peripheral stimulation: an optical imaging study. Neuroscience. 2000;96:317–331. doi: 10.1016/s0306-4522(99)00470-4. 10.1016/S0306-4522(99)00470-4. [DOI] [PubMed] [Google Scholar]
- Harrison J, Jahr CE. Receptor occupancy limits synaptic depression at climbing fiber synapses. J Neurosci. 2003;23:377–383. doi: 10.1523/JNEUROSCI.23-02-00377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci U S A. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz AG. Mathematical model of oxygen transport in the cerebral cortex. Brain Res. 1999;817:75–83. doi: 10.1016/s0006-8993(98)01200-1. 10.1016/S0006-8993(98)01200-1. [DOI] [PubMed] [Google Scholar]
- Hyder F, Shulman RG, Rothman DL. A model for the regulation of cerebral oxygen delivery. J Appl Physiol. 1998;85:554–564. doi: 10.1152/jappl.1998.85.2.554. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Pelligrino DA, Moskowitz MA, Lassen NA. Nitric oxide synthase inhibition and cerebrovascular regulation. J Cereb Blood Flow Metab. 1994;14:175–192. doi: 10.1038/jcbfm.1994.25. [DOI] [PubMed] [Google Scholar]
- Jones M, Berwick J, Johnston D, Mayhew J. Concurrent optical imaging spectroscopy and laser-Doppler flowmetry: the relationship between blood flow, oxygenation, and volume in rodent barrel cortex. Neuroimage. 2001;13:1002–1015. doi: 10.1006/nimg.2001.0808. 10.1006/nimg.2001.0808. [DOI] [PubMed] [Google Scholar]
- Jones M, Hewson-Stoate N, Martindale J, Redgrave P, Mayhew J. Nonlinear coupling of neural activity and CBF in rodent barrel cortex. Neuroimage. 2004;22:956–965. doi: 10.1016/j.neuroimage.2004.02.007. 10.1016/j.neuroimage.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Kim DS, Duong TQ, Kim SG. High-resolution mapping of iso-orientation columns by fMRI. Nat Neurosci. 2000;3:164–169. doi: 10.1038/72109. 10.1038/72109. [DOI] [PubMed] [Google Scholar]
- Konnerth A, Llano I, Armstrong CM. Synaptic currents in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1990;87:2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen M. Opinion: Reading vascular changes in brain imaging: is dendritic calcium the key? Nat Rev Neurosci. 2005;6:77–85. doi: 10.1038/nrn1589. 10.1038/nrn1589. [DOI] [PubMed] [Google Scholar]
- Leniger-Follert E. Direct determination of local oxygen consumption of the brain cortex in vivo. Pflugers Arch. 1977;372:175–179. doi: 10.1007/BF00585333. 10.1007/BF00585333. [DOI] [PubMed] [Google Scholar]
- Lennie P. The cost of cortical computation. Curr Biol. 2003;13:493–497. doi: 10.1016/s0960-9822(03)00135-0. 10.1016/S0960-9822(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Megow D, Matsuda H, Dirnagl U. Nitric oxide: a modulator, but not a mediator, of neurovascular coupling in rat somatosensory cortex. Am J Physiol. 1999;277:H799–H811. doi: 10.1152/ajpheart.1999.277.2.H799. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Megow D, Schultze J, Weber JR, Dirnagl U. Nitric oxide synthase inhibition does not affect somatosensory evoked potentials in the rat. Neurosci Lett. 1996;216:207–210. doi: 10.1016/0304-3940(96)13044-5. 10.1016/0304-3940(96)13044-5. [DOI] [PubMed] [Google Scholar]
- Llano I, Marty A, Armstrong CM, Konnerth A. Synaptic-and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Leznik E, Makarenko VI. On the amazing olivocerebellar system. Ann N Y Acad Sci. 2002;978:258–272. doi: 10.1111/j.1749-6632.2002.tb07573.x. [DOI] [PubMed] [Google Scholar]
- Llinas R, Nicholson C. Analysis of field potentials in the central nervous system. In: Remond A, editor. Handbook of Electroencephalography and Clinical Neurophysiology,Vol.2. Amsterdam: Elsevier Scientific Publishing Co; 1974. pp. 61–85. [Google Scholar]
- Llinas R, Volkind RA. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res. 1973;18:69–87. doi: 10.1007/BF00236557. [DOI] [PubMed] [Google Scholar]
- Lubbers DW, Baumgartl H, Zimelka W. Heterogeneity and stability of local PO2 distribution within the brain tissue. Adv Exp Med. 1994;345:567–574. doi: 10.1007/978-1-4615-2468-7_75. [DOI] [PubMed] [Google Scholar]
- Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc Natl Acad Sci U S A. 1997;94:14826–14831. doi: 10.1073/pnas.94.26.14826. 10.1073/pnas.94.26.14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K, Kurachi T, Takizawa N, Kobayashi H, Tanishita K. Successive depth variations in microvascular distribution of rat somatosensory cortex. Brain Res. 2004;995:66–75. doi: 10.1016/j.brainres.2003.09.055. 10.1016/j.brainres.2003.09.055. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Omura T, Takizawa N, Kobayashi H, Katura T, Maki A, Kawaguchi H, Tanishita K. Biphasic changes in tissue partial pressure of oxygen closely related to localized neural activity in guinea pig auditory cortex. J Cereb Blood Flow Metab. 2003a;23:1075–1084. doi: 10.1097/01.WCB.0000084248.20114.B3. 10.1097/01.WCB.0000084248.20114.B3. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Takizawa N, Kobayashi H, Oka K, Tanishita K. Dual responses of tissue partial pressure of oxygen after functional stimulation in rat somatosensory cortex. Brain Res. 2003b;979:104–113. doi: 10.1016/s0006-8993(03)02882-8. 10.1016/S0006-8993(03)02882-8. [DOI] [PubMed] [Google Scholar]
- Mathiesen C, Caesar K, Akgoren N, Lauritzen M. Modification of activity-dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J Physiol. 1998;512:555–566. doi: 10.1111/j.1469-7793.1998.555be.x. 10.1111/j.1469-7793.1998.555be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew J, Johnston D, Berwick J, Jones M, Coffey P, Zheng Y. Spectroscopic analysis of neural activity in brain: increased oxygen consumption following activation of barrel cortex. Neuroimage. 2000;12:664–675. doi: 10.1006/nimg.2000.0656. 10.1006/nimg.2000.0656. [DOI] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Hu X, Strupp JP, Anderson P, Ugurbil K. BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: echo-planar imaging correlates with previous optical imaging using intrinsic signals. Magn Reson Med. 1995;33:453–459. doi: 10.1002/mrm.1910330323. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Lundstrom BN, Snyder AZ, Vlassenko AG, Shulman GL, Raichle ME. Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proc Natl Acad Sci U S A. 2001;98:6859–6864. doi: 10.1073/pnas.111164398. 10.1073/pnas.111164398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto M, Sheth S, Guiou M, Pouratian N, Chen JW, Toga AW. Functional signal-and paradigm-dependent linear relationships between synaptic activity and hemodynamic responses in rat somatosensory cortex. J Neurosci. 2004;24:3850–3861. doi: 10.1523/JNEUROSCI.4870-03.2004. 10.1523/JNEUROSCI.4870-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C, Llinas R. Field potentials in the alligator cerebellum and theory of their relationship to Purkinje cell dendritic spikes. J Neurophysiol. 1971;34:509–531. doi: 10.1152/jn.1971.34.4.509. [DOI] [PubMed] [Google Scholar]
- Nielsen A, Lauritzen M. Coupling and uncoupling of activity-dependent increases of neuronal activity and blood flow in rat somatosensory cortex. J Physiol. 2001;533:773–785. doi: 10.1111/j.1469-7793.2001.00773.x. 10.1111/j.1469-7793.2001.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T, Liu TT, Miller KL, Luh WM, Wong EC, Frank LR, Buxton RB. Discrepancies between BOLD and flow dynamics in primary and supplementary motor areas: application of the balloon model to the interpretation of BOLD transients. Neuroimage. 2004;21:144–153. doi: 10.1016/j.neuroimage.2003.08.040. 10.1016/j.neuroimage.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci U S A. 1998;95:765–772. doi: 10.1073/pnas.95.3.765. 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. Functional brain imaging and human brain function. J Neurosci. 2003;23:3959–3962. doi: 10.1523/JNEUROSCI.23-10-03959.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsbech NP. An oxygen microsensor with a guard cathode. Limnol Oceanogr. 1989;34:474–478. [Google Scholar]
- Satake S, Saitow F, Yamada J, Konishi S. Synaptic activation of AMPA receptors inhibits GABA release from cerebellar interneurons. Nat Neurosci. 2000;3:551–558. doi: 10.1038/75718. 10.1038/75718. [DOI] [PubMed] [Google Scholar]
- Secomb TW, Hsu R, Beamer NB, Coull BM. Theoretical simulation of oxygen transport to brain by networks of microvessels: effects of oxygen supply and demand on tissue hypoxia. Microcirculation. 2000;7:237–247. 10.1038/sj.mn.7300114. [PubMed] [Google Scholar]
- Sheth S, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Evaluation of coupling between optical intrinsic signals and neuronal activity in rat somatosensory cortex. Neuroimage. 2003;19:884–894. doi: 10.1016/s1053-8119(03)00086-7. 10.1016/S1053-8119(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and hemodynamic responses. Neuron. 2004;42:347–355. doi: 10.1016/s0896-6273(04)00221-1. 10.1016/S0896-6273(04)00221-1. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sick TJ, Kreisman NR. Local tissue oxygen tension as an index of changes in oxidative metabolism in the bullfrog optic tectum. Brain Res. 1979;169:575–579. doi: 10.1016/0006-8993(79)90407-4. 10.1016/0006-8993(79)90407-4. [DOI] [PubMed] [Google Scholar]
- Siesjo BK. Brain Energy Metabolism. New York: Wiley; 1978. [Google Scholar]
- Silver IA. Cerebral Vascular Smooth Muscle and its Control, CIBA Foundation Symposium. Vol. 56. Excerpta-Medica,North Holland: Elsevier; 1978. Cellular microenvironment in relation to local blood flow; pp. 49–67. [DOI] [PubMed] [Google Scholar]
- Silver RA, Momiyama A, Cull-Candy SG. Locus of frequency-dependent depression identified with multiple-probability fluctuation analysis at rat climbing fibre-Purkinje cell synapses. J Physiol. 1998;510:881–902. doi: 10.1111/j.1469-7793.1998.881bj.x. 10.1111/j.1469-7793.1998.881bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JK, Peterson MR, Freeman RD. Single-neuron activity and tissue oxygenation in the cerebral cortex. Science. 2003;299:1070–1072. doi: 10.1126/science.1079220. 10.1126/science.1079220. [DOI] [PubMed] [Google Scholar]
- Thompson JK, Peterson MR, Freeman RD. High-resolution neurometabolic coupling revealed by focal activation of visual neurons. Nat Neurosci. 2004;7:919–920. doi: 10.1038/nn1308. 10.1038/nn1308. [DOI] [PubMed] [Google Scholar]
- Thomsen K, Offenhauser N, Lauritzen M. Principle neuron spiking: neither necessary nor sufficient for cerebral blood flow in rat cerebellum. J Physiol. 2004;560:181–189. doi: 10.1113/jphysiol.2004.068072. 10.1113/jphysiol.2004.068072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JB, Bishop GA. Stimulus-dependent activation of c-Fos in neurons and glia in the rat cerebellum. J Chem Neuroanat. 2002;23:157–170. doi: 10.1016/s0891-0618(01)00153-3. 10.1016/S0891-0618(01)00153-3. [DOI] [PubMed] [Google Scholar]
- Travis RP, Jr, Clark LC., Jr Changes in evoked brain oxygen during sensory stimulation and conditioning. Electroencephalogr Clin Neurophysiol. 1965;19:484–491. doi: 10.1016/0013-4694(65)90188-4. 10.1016/0013-4694(65)90188-4. [DOI] [PubMed] [Google Scholar]
- Vafaee MS, Gjedde A. Model of blood-brain transfer of oxygen explains nonlinear flow-metabolism coupling during stimulation of visual cortex. J Cereb Blood Flow Metab. 2000;20:747–754. doi: 10.1097/00004647-200004000-00012. [DOI] [PubMed] [Google Scholar]
- Vanzetta I, Grinvald A. Increased cortical oxidative metabolism due to sensory stimulation: implications for functional brain imaging. Science. 1999;286:1555–1558. doi: 10.1126/science.286.5444.1555. 10.1126/science.286.5444.1555. [DOI] [PubMed] [Google Scholar]
- Yang G, Chen G, Ebner TJ, Iadecola C. Nitric oxide is the predominant mediator of cerebellar hyperemia during somatosensory activation in rats. Am J Physiol. 1999;277:R1760–R1770. doi: 10.1152/ajpregu.1999.277.6.R1760. [DOI] [PubMed] [Google Scholar]