Abstract
Interneurones interconnecting the two sides of the spinal cord (commissural interneurones) are critically important for interlimb coordination, but little is known about their organization. We have examined the inputs to commissural interneurones located in the midlumbar segments with projections to contralateral motor nuclei, aiming to determine whether they form distinct subpopulations. Based on intracellular records from 78 interneurones, two major non-overlapping subpopulations were identified: one monosynaptically excited by group II muscle afferents (n = 10), the other monosynaptically excited by reticulospinal neurones (n = 52). Monosynaptic input from group I muscle afferents and/or from vestibulospinal tract neurones was found in those with monosynaptic reticulospinal, but not group II input, and in a few other neurones (n = 6). Only disynaptic input from these sources was found in the remaining 10 interneurones. Disynaptic excitatory input from ipsilateral and contralateral muscle afferents and from descending tracts was distributed less selectively and might mediate coexcitation of interneurones with monosynaptic afferent or descending input. The dominant disynaptic and polysynaptic input was, however, inhibitory. IPSPs were evoked from the descending tracts in a high proportion of the commissural interneurones that were monosynaptically excited by group II afferents (55%) and from group II afferents in a high proportion of the commissural interneurones that were monosynaptically excited by reticulospinal fibres (78%). This distribution suggests that the two subpopulations are activated differentially, rather than being coactivated, in either centrally initiated movements or reflex adjustments. This would be consistent with the previous demonstration that noradrenaline differentially affects commissural neurones of the two subpopulations.
Spinal interneurones with axons that cross the midline, commissural interneurones, play an essential role in coordinating motor activity on the two sides of the body and have been the subject of a number of recent studies (for reviews see Kiehn & Butt, 2003; Lanuza et al. 2004). These neurones are located in two main regions of the spinal grey matter, the majority being located in lamina VIII, but others being located in the base of the dorsal horn in laminae IV–V (Cajal, 1953; Scheibel & Scheibel, 1969; Matsushita, 1970; Eide et al. 1999). Despite the umbrella term ‘commissural interneurones’, the populations of commissural neurones at these two locations are highly non-homogeneous. They include neurones developed from different progenitor groups, which can be distinguished by the expression of specific homeodomain transcription factors (see, e.g. Jessell, 2000; Pierani et al. 2001). These progenitors develop into a variety of neuronal types including many ascending tract cells as well as interneurones with ipsilateral and contralateral projection areas, but even those of the same general type are functionally differentiated. For instance, among lamina VIII commissural interneurones are interneurones with different projection areas (e.g. over long or short distances caudally and/or rostrally; Stokke et al. 2002; Bannatyne et al. 2003; Matsuyama et al. 2004), different target cells (e.g. motoneurones, interneurones or both; Bannatyne et al. 2003; Birinyi et al. 2003; Butt & Kiehn, 2003; Matsuyama et al. 2004), different transmitters and types of actions (e.g. Bannatyne et al. 2003; Butt & Kiehn, 2003) and may be involved in different types of motor behaviour, e.g. locomotion (Kiehn & Butt, 2003; Lanuza et al. 2004; Matsuyama et al. 2004) or various postural adjustments.
Previous studies have shown that commissural interneurones activated under different experimental conditions may contribute to different patterns of muscle coordination, e.g. crossed extension associated with ipsilateral flexion, bilateral extension or bilateral flexion (Sherrington, 1906; Rossignol & Gauthier, 1980; Arya et al. 1991; Aggelopoulos et al. 1996; Rossignol, 1996). Of these, commissural interneurones in the midlumbar segments appear to play a particularly important role in the selection of motor patterns. One indicator of this is that crossed actions of stretch activated muscle afferents are strongest following activation of group II afferents from the quadriceps muscle (Harrison & Zytnicki, 1984; Arya et al. 1991; Aggelopoulos et al. 1996) and that many midlumbar interneurones, including commissural interneurones, are preferentially activated by these afferents (Edgley & Jankowska, 1987b; Jankowska & Noga, 1990). However, previous studies have provided only a preliminary survey of the properties and of the organization of commissural interneurones.
Recordings made directly from lamina VIII commissural interneurones in midlumbar and caudal lumbar segments have revealed that individual interneurones are excited by different combinations of afferent fibres (Harrison et al. 1986; Jankowska & Noga, 1990), by stimuli applied in the cuneiform nuclei (Jankowska & Noga, 1990), the cerebellum and the reticular formation (Jankowska et al. 2003; Matsuyama et al. 2004) and/or the vestibular nuclei (Krutki et al. 2003). How this input is distributed within the population of commissural interneurones is not known. However, major differences have been found in the modulatory effects of noradrenaline (NA) on the monosynaptic activation of midlumbar lamina VIII commissural interneurones by group II afferents (which was depressed), and by descending reticulospinal tract fibres, which was facilitated (Hammar et al. 2004).
The impetus for the current study was the suggestion that these effects of noradrenaline may be postsynaptic, in which case the commissural interneurones that mediate crossed actions of group II afferents and those that mediate the actions of reticulospinal fibres might form distinct subpopulations. To examine this hypothesis we compared input from group II afferents and from reticulospinal tract fibres in the medial longitudinal fascicle (MLF) in the medulla to lamina VIII commissural interneurones.
Methods
Preparation
The experiments were performed on 11 deeply anaesthetized adult cats weighing 2.3–3.5 kg, some of which were also used for other experiments (Hammar et al. 2004). All experimental procedures were approved by Göteborg University Ethics Committee and followed NIH and EU guidelines of animal care. General anaesthesia was induced with sodium pentobarbital (40–44 mg kg−1, i.p.) and maintained with intermittent doses of α-chloralose as required to maintain full anaesthesia (Rhône-Poulenc Santé, France; doses of 5 mg kg−1 administered every 1–2 h, up to 55 mg kg−1, i.v.). During recordings, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden; about 0.2 mg kg−1 h−1i.v.) and the animals were artificially ventilated. Additional doses of α-chloralose were given when increases in the blood pressure or heart rate, which were continuously monitored, were evoked by noxious stimulation, or if the pupils dilated. Mean blood pressure was kept at 100–130 mmHg and the end-tidal concentration of CO2 at about 4% by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 ml h−1 kg−1). Core body temperature was kept at about 37.5°C by servo-controlled infrared lamps. The experiments were terminated by a lethal dose of pentobarbital resulting in cardiac arrest.
A laminectomy exposed the fourth to seventh lumbar (L4–L7), low thoracic (Th11–Th13) and in some experiments also the second to fourth cervical (C2–C4) segments of the spinal cord. In four of the experiments the spinal cord was hemisected at the Th12 level on the side opposite to that of the location of the commissural interneurones. A number of peripheral hindlimb nerves were transected and mounted on stimulating electrodes. Subcutaneous cuff electrodes were used for nerves accessed in the iliac fossa (ipsilateral and contralateral quadriceps, Q; sartorius, Sart; and saphenus, Saph) and for the contralateral gastrocnemius–soleus, GS, nerves). Effects evoked from these nerves were analysed in all of the experiments. The remaining ipsilateral nerves (the posterior biceps and semitendinosus, PBST; anterior biceps and semimembranosus, ABSM; sural, Sur; and gastrocnemius–soleus, GS) were mounted on pairs of silver hook electrodes in a paraffin oil pool (at 36–37°C) created by skin flaps. Effects evoked from these nerves were tested on about half of the interneurones investigated.
The caudal part of the cerebellum was exposed by craniotomy and tungsten electrodes (impedance 40–150 kΩ) were placed in the ipsilateral medial longitudinal fascicle (MLF) and, in seven experiments, also in the ipsilateral lateral vestibular nucleus (LVN). The electrodes were inserted at an angle of 30 deg (with the tip directed rostrally). The initial targets were at Horsley-Clarke co-ordinates P9, L0.6, H-5 for MLF; P7, L4 and H-2 for LVN. However, the final positions of the electrodes were adjusted on the basis of records of descending volleys from the surface of the lateral funiculus at the Th11–Th13 and/or the C4–C5 segments. The electrodes were left at sites from which distinct descending volleys were evoked at stimulus intensities of 20 μA or less. At the end of the experiments these sites were marked with lesions (0.3–0.4 mA constant current passed for 10 s). Their location (Fig. 1A–C) was subsequently verified on 100 μm-thick frontal sections of the brainstem, cut in the plane of insertion of the electrodes using a freezing microtome and counterstained with cresyl violet. The level of location of MLF electrodes corresponded to the rostral border of the inferior olive, of the LVN electrodes to the location of the Nucl. interpositus or about 0.5–1 mm caudal to it. Only data obtained in experiments in which the electrodes were appropriately placed will be reported.
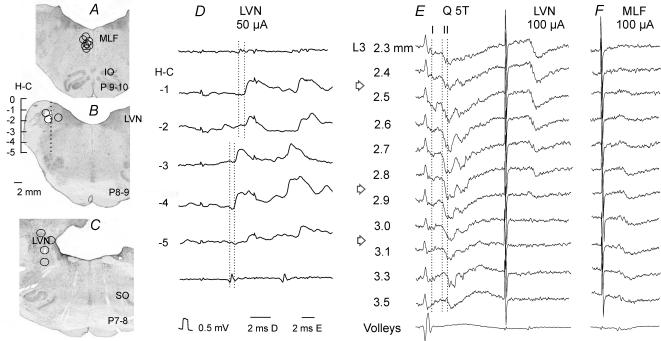
Figure 1. Reconstructions of the locations of the stimulating electrodes.
A–C, locations of the stimulating electrode tips, as defined by the electrolytic lesions made at the end of the experiments, in the ipsilateral medial longitudinal fascicle (MLF) in planes P9–10 and in the ipsilateral lateral vestibular nucleus (LVN) in Horsley-Clarke's planes 8–9 and P7–8, respectively, These are superimposed on representative sections of the brainstem, cut in the plane of the electrode insertions (at the angle of 30 deg). IO, inferior olive; SO, superior olive. D, monosynaptic EPSPs evoked in one of the interneurones in the same experiment as in E, by stimuli applied at different depths at Horsley-Clarke's (H-C) coordinates H0 to H-5 along the electrode track indicated in B and descending volleys from the depth −2 (top trace) and −4 (bottom trace). The two dotted lines indicate the descending volleys and the onset of the EPSPs evoked from the dorsal and ventral stimulation sites (at latencies 0.7 and 0.5 ms from the volleys, respectively). E, field potentials recorded at different depths from the surface of the spinal cord (indicated to the left) along an electrode track crossing grey matter in the L3 segment of the spinal cord at an angle of 10 deg (tip directed lateral). These were evoked by stimulation of the quadriceps nerve (Q) at 5 times threshold (T) and from LVN. The three dotted lines indicate the onset of monosynaptic field potentials from group I afferents and from group II afferents at most dorsal locations and at the depths of 3.3 and 3.5 mm. F, field potentials along another electrode track in the same experiment, 200 μm more medial. Arrows indicate the levels of the maximal field potentials from the LVN, from group II afferents in lamina VIII and from MLF within which interneurones with input from these sources were searched for. In this and the following figures the negativity in the microelectrode (intracellular or extracellular recordings) is downwards and in records from the surface of the spinal cord upwards.
Stimulation and recording
Peripheral nerves were stimulated with constant voltage stimuli (0.1 ms duration, intensity expressed in multiples of threshold, T, for the most sensitive fibres in the nerve). For activation of fibres of the reticulospinal and vestibulospinal tracts, constant current stimuli (0.2 ms, usually 50–100 μA but sometimes up to 200 μA), using a 0.5 mm electrolytically etched tungsten wire, insulated except for its tip (40–200 kΩ), as a cathode. Axons of commissural interneurones were stimulated within the contralateral GS motor nuclei as a means of a functional identification of commissural interneurones projecting to this region in the caudal lumbar segments by antidromic activation. The stimuli (0.2 ms, 5–100 μA) were applied via a thin tungsten electrode (100–300 kΩ). In order to exclude ascending tract neurones from the analysis, ascending axons were stimulated at the level of the Th11–Th12 segments via two pairs of electrodes in contact with the left and right lateral funiculi (via intact dura mater).
Descending volleys were recorded from the cord dorsum (at C4–C5 and Th11–Th12) during the placement of the brainstem stimulating electrodes and from a midlumbar segment during the recording from commissural interneurones, in both cases monopolarly via intact dura mater.
Glass micropipettes filled with either 2 m solution of potassium citrate or with a mixture of equal parts of 5% tetramethylrhodamine-dextran (Molecular Probes, Inc, Eugene, OR, USA) and 5% Neurobiotin (Vector, UK) in saline (pH 6.5) were used for intracellular records from the interneurones. Interneurones were selected for recording if they were antidromically activated from the contralateral GS motor nucleus (at thresholds 10–100 μA) but not by stimuli applied to either the left or right lateral funiculus at a Th11–Th12 level (up to 1 mA). They were sought in the intermediate zone and ventral horn where large monosynaptic field potentials were evoked from group II afferents, MLF or LVN in L3–L5 segments (see Fig. 1E, F). Group II field potentials were evoked by stimulation of Q and Sart nerves at 5 times threshold (T) stimulus intensity (about 0.5 mm deeper than intermediate zone field potentials from both group I and II afferents). MLF and LVN field potentials were evoked by single 100–150 μA stimuli, medioventrally and dorsolaterally of the group II field potentials, respectively. Interneurones penetrated within these areas were located at depths 2.6–3.7 mm from the cord dorsum. Intracellularly labelled commissural interneurones of these groups were located in lamina VIII and the neighbouring part of lamina VII (see Fig. 1Fig. 1 in Hammar et al. 2004).
Analysis
Both the original data and averages of 10–20 single records were stored on-line using a sampling and analysis system of E. Eide, T. Holmström and N. Pihlgren, Göteborg University. Differences between latencies were assessed for statistical significance using Student's t test and distributions of inputs to different populations were tested using χ2 or, if few examples were included, Fisher's exact test.
Criteria of monosynaptic and disynaptic PSPs recorded in commissural interneurones
The sample of 78 intracellularly recorded commissural interneurones analysed in this study includes 23 interneurones with input from group I afferents (10 with monosynaptic EPSPs), 75 with input from group II afferents (10 with monosynaptic EPSPs), 74 with input from MLF (52 with monosynaptic EPSPs) and 28 with input from LVN (10 with monosynaptic EPSPs).
Responses were classified as evoked by group I afferents when they were evoked by stimuli ≤ 1.5T, and/or at latencies too short to be compatible with effects of group II afferents.
Two criteria were used to differentiate between monosynaptically and disynaptically evoked EPSPs. Firstly, monosynaptic EPSPs always appeared after each successive stimulus of a train, with minimal or no temporal facilitation. Disynaptic EPSPs appeared after two or more stimuli in a train and displayed marked temporal facilitation. Secondly, monosynaptic EPSPs were evoked at fixed segmental latencies following the arrival of afferent volleys, not exceeding certain critical ranges. Disynaptic EPSPs were evoked at segmental latencies longer than those of monosynaptic EPSPs to allow time for the additional synaptic delay and intraspinal conduction time.
For monosynaptic EPSPs from group I afferents the latency ranges were 0.5–1 ms from the first positive peak of the afferent volleys in these afferents. For monosynaptic EPSPs from group II afferents the situation is more complex since the conduction velocity of afferents slows considerably within the cord. Accordingly, both the lower and upper limits were higher, 1.8–2.8 ms from afferent volleys in group I afferents, allowing for the longer peripheral conduction time along group II than group I afferents (about 0.7–0.9 ms) and an intraspinal conduction time of 0.6–1 ms between dorsal root entry and lamina VIII (Fu & Schomburg, 1974; Edgley & Jankowska, 1987a,b; Lundberg et al. 1987). The longer latency of field potentials from group II afferents at increasing depths is illustrated in Fig. 1E, those for more dorsal and more ventral locations indicated by the second and third dotted lines. The ranges of latencies of EPSPs attributed to monosynaptic actions of group II afferents corresponded to the latencies of focal field potentials from group II afferents.
For EPSPs evoked from the MLF and the LVN the ranges of the latencies of the earliest descending volleys from MLF were 2.7–3.1 ms from the stimuli and those from LVN were 3.1–3.7 ms; the ranges of latencies compatible with monosynaptic actions were 0.4–0.9 ms and 0.4–1.3 ms after the first positive peak of the descending volleys recorded at a mid-lumbar level, respectively. The reason for considering EPSPs evoked by LVN stimuli with latencies of up to 1.3 ms as being monosynaptic is that LVN neurones may be activated either directly or trans-synaptically (secondarily to stimulation of fibres forming synaptic contacts with them). The trans-synaptic activation involves an additional delay of 0.5–1 ms (see, e.g. Baldissera et al. 1972). The direct and indirect activation of vestibulospinal neurones was sometimes reflected in two distinct components of the descending volleys, separated by about 1 ms and in stepwise changes in the latencies of EPSPs evoked by stimuli applied at different locations in the region of the LVN. Example records of this are shown in Fig. 1D, with the EPSPs from the two most dorsal stimulation sites being evoked at latencies that were about 1 ms longer with respect to the stimuli, but only 0.2 ms longer from the volleys evoked from these sites. We also sought evidence for temporal facilitation following a short train of stimuli as an additional criterion to differentiate between the monosynaptically and disynaptically evoked PSPs. When the EPSPs were evoked by single stimuli and the early components were not temporally facilitated, they were considered to have been evoked monosynaptically, even when their latencies were at the upper limits expected for monosynaptic actions, within the range of overlap between latencies of monosynaptic and disynaptic EPSPs (see shaded regions in Fig. 2B). The converse was true for EPSPs appearing only after the 2nd or 3rd stimulus. By comparing descending volleys and postsynaptic actions evoked by stimuli at different depths in the brainstem we verified that responses were evoked from only a restricted region, as expected for stimulation sites from within the LVN. The differential distribution of monosynaptic field potentials evoked from LVN and from MLF (Fig. 1E, F) indicates that the LVN stimuli did not spread to activate MLF axons.
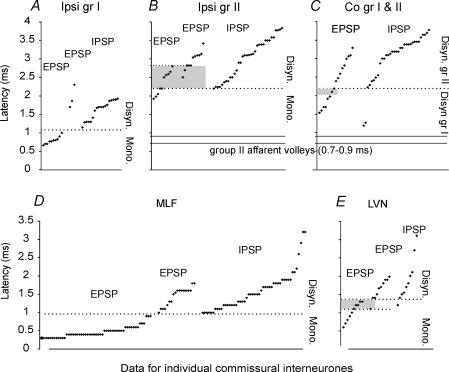
Figure 2. Distribution of latencies of PSPs evoked from group I and II muscle afferents, MLF and LVN.
A–C, the minimal latencies (ordinates) of PSPs evoked from muscle nerves (Q and/or Sart) in individual interneurones, with respect to group I afferent volleys, ranked from the shortest to the longest. A, latencies of monosynaptic and disynaptic EPSPs and disynaptic IPSPs from group I afferents. The dotted line separates the monosynaptically and disynaptically evoked PSPs. B, latencies of monosynaptic and disynaptic EPSPs and disynaptic IPSPs evoked from ipsilateral group II afferents, measured from the group I volleys. The estimated range of delays of the earliest arrival of group II afferent impulses (relative to the group I volleys) is indicated by the two continuous horizontal lines. The grey area indicates EPSPs in a range where additional criteria (see text) had to be used to define the linkage as mono- or disynaptic. C, disynaptic EPSPs and IPSPs evoked from contralateral group I and II afferents, measured as in A. The continuous horizontal lines indicate the timing of group II afferent volleys as in B. The dotted line separates PSPs evoked from group I and group II afferents, defined according to latency and threshold (as in A). The shaded area indicates the overlap in latencies compatible with disynaptic connection from either group I or group II afferents. D and E, latencies of EPSPs and IPSPs evoked from MLF and LVN measured from the first positive components of the descending volleys evoked by the third of a train of three stimuli. The dotted lines separate the monosynaptically and disynaptically evoked PSPs. The shaded area in E indicates latencies of EPSPs evoked from LVN that could be mono- or disynaptically evoked, but which were differentiated on the basis of temporal facilitation (see text).
Results
Are there functionally distinct subpopulations of midlumbar lamina VIII commissural interneurones?
The original hypothesis of this study was that commissural interneurones mediating crossed actions of group II muscle afferents and those mediating centrally initiated movements evoked via reticulospinal tract neurones constitute distinct interneuronal populations. This was based on the finding that noradrenaline modulates the activation of these neurones differently, with some indications that these actions are postsynaptic, which would involve neurones with different properties (Hammar et al. 2004). Here we examine this hypothesis with respect to commissural interneurones mediating actions of group I afferents, group II afferents, and the reticulospinal and vestibulospinal tracts.
Are commissural interneurones coexcited by group II afferents and reticulospinal tract fibres?
Figure 3A shows that commissural interneurones that were monosynaptically excited from group II afferents (1st row from the top) were not monosynaptically excited by stimulation of the MLF. It further shows that some of the commissural interneurones in which monosynaptic EPSPs were evoked from the MLF (2nd row from the top) were also monosynaptically excited by group I afferents and/or from the LVN but that none had monosynaptic group II afferent input. Example intracellular records from interneurones of these groups with exclusive monosynaptic input are shown in Fig. 4. The lack of overlap between monosynaptic input from group II afferents and those with input from the MLF is even more striking in view of the fact that this is not a general feature of midlumbar interneurones; ipsilaterally projecting intermediate zone interneurones with monosynaptic input from both group II and MLF have been described (Davies & Edgley, 1994). Furthermore, the commissural interneurones with monosynaptic input from either group II afferents or from MLF were recorded at similar depths (both at 2.6–3.7 mm from the cord dorsum).
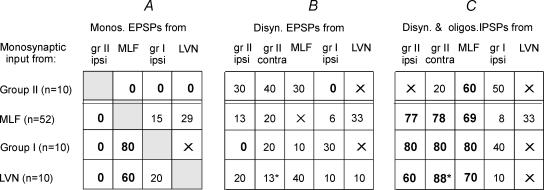
Figure 3. Proportions of intracellularly recorded commissural interneurones with monosynaptic EPSPs, disynaptic EPSPs and disynaptic and/or polysynaptic IPSPs from group I and II muscle afferents, MLF and LVN.
The table has four rows, for interneurones with monosynaptic input from group II muscle afferents, MLF, group I muscle afferents and LVN, as indicated to the left. The figures in the table show the proportions of the cells tested (as percentages) with other inputs, indicated at the head of each column. Note that the sums of the percentages across the rows may exceed 100% because individual interneurones could be coexcited from several sources. The proportions of particular interest, those exceeding 50% or found close to nought, are highlighted in bold. Figures with asterisk in the bottom rows are based on a sample of 8 (rather than 10) interneurones with monosynaptic input from LVN. An X indicates effects that were difficult to quantify, for example when disynaptic PSPs were obscured by being superimposed on monosynaptic EPSPs, and/or when the number of observations was insufficient for calculating proportions.
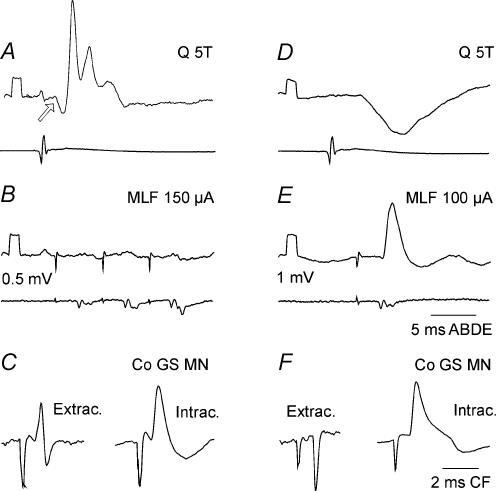
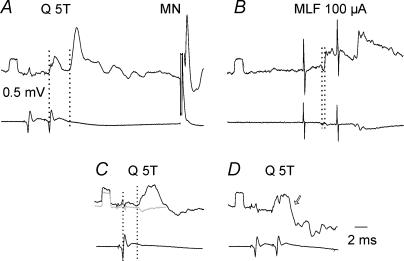
Figure 4. Examples of monosynaptic excitation of commissural interneurones, from either group II muscle afferents or the MLF.
A–C and D–F show records from two different commissural interneurones, both of which were antidromically activated from the contralateral gastrocnemius–soleus motor nuclei and which were recorded in the same experiment. The antidromically evoked responses (C and F) are illustrated with records obtained just before and/or after the penetration of the interneurones. In the upper and middle rows, the lower records in each pair are from the cord dorsum. Voltage calibrations (0.5 mV) are for intracellular records. The arrow in A indicates a disynaptic IPSP evoked from group I afferents (evoked at threshold of < 2T) preceding the group II EPSP.
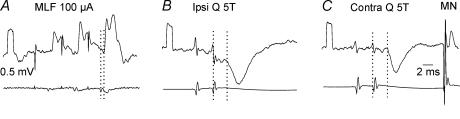
The distribution of disynaptic EPSPs was less restricted. As shown in Fig. 3 disynaptic EPSPs were evoked from group II afferents in 13% of the interneurones which had monosynaptic input from the MLF. Conversely, disynaptic EPSPs were evoked from the MLF in 30% of the interneurones with monosynaptic input from group II afferents (Figs 3B and 5A). Disynaptic EPSPs and IPSPs of group II and MLF origin were also found in a small number of commissural interneurones in which monosynaptic input was not found from any of the sources analysed.
Figure 5. Disynaptic excitation from group II afferents in a commissural interneurone that was monosynaptically excited from the MLF.
A and B, EPSPs evoked by stimulation of ipsilateral group II afferents and from the MLF. Upper (averaged) records are from a commissural interneurone; lower records are from the cord dorsum. The vertical dotted lines in A indicate the onset of the afferent volley and of the temporally facilitated EPSP following the 2nd stimulus applied to the ipsilateral quadriceps nerve. The vertical dotted lines in B indicate the first components of the MLF volley and the onset of the EPSP following this volley. C and D show one limitation of the test for temporal facilitation of group II synaptic actions. Upper traces show PSPs evoked by stimulation of group II afferents. The grey trace in C shows the monosynaptic extracellular field potential from group II afferents; the EPSP was considered to be evoked monosynaptically since its onset had the same latency as the field potential (2.75 ms). The arrow indicates where the monosynaptic EPSP was expected to occur following the second stimulus. It might have failed to appear either because it was masked by the IPSP, or because of strong presynaptic inhibition of transmission from group II afferents following the first stimulus.
In view of the region of overlap between the latencies of monosynaptically and disynaptically evoked EPSPs (see Methods) we would like to stress that in all 10 commissural interneurones in which EPSPs from group II afferents were evoked at latencies of 1.8–2.8 ms, stimulation of the MLF was either ineffective (n = 7) or evoked EPSPs only after the 2nd or 3rd stimulus, or with marked temporal facilitation, at segmental latencies of 1.1–1.54 ms (in the other 3 neurones). In 52 commissural interneurones single stimuli to the MLF evoked EPSPs at latencies 0.4–0.7 ms. In the large majority of these, stimulation of ipsilateral group II afferents failed to evoke any EPSPs (n = 45); in the remaining seven neurones, EPSPs were evoked at latencies of 2.9–3.7 ms (i.e. longer than the range of latencies of monosynaptic EPSPs, 1.7–2.8 ms).
Judgement of the synaptic linkage of the EPSPs is difficult in some cases. If we base the decision on latencies and accept that EPSPs evoked by stimulation of group II afferents at latencies of under 3.0 ms were evoked monosynaptically, then a small proportion of commissural interneurones would be considered to be excited (monosynaptically) by both group II and reticulospinal tract fibres. These proportions would be 2/12 (about 15%) for commissural interneurones with monosynaptic group II input and 2/52 (about 4%) of those with monosynaptic MLF inputs. These proportions are significantly different from the proportions expected if the two inputs were distributed independently (Fisher's exact test, P < 0.001), indicating that there are different populations of neurones with these inputs.
Additional extracellular records were obtained from interneurones that we considered to be monosynaptically activated from the MLF (n = 24, at latencies of 2.8–3.76 ms from the effective near-maximal stimulus; segmental latencies of 0.8–1.5 ms from the descending volleys) or group II afferents (n = 14, at segmental latencies of 1.7–2.9 ms at 5T from group I volleys). In none of these could we find evidence of monosynaptic excitation from both sources. Eleven of these neurones (four with group II input and seven with MLF input) were subsequently penetrated and the intracellularly recorded EPSPs were in agreement with this classification. Ten of the neurones activated from the MLF were activated by stimulation of group II afferents at latencies greater than expected for monosynaptically evoked discharges (> 3.2 ms). In eight of these neurones, discharges were also evoked from contralateral group II afferents, which must have been at least disynaptically evoked, and these had similar latencies to the ipsilaterally evoked discharges. Five commissural interneurones with monosynaptic input from group II afferents were activated following stimulation of the MLF, at latencies between 3.8 and 5.3 ms, which likewise exceeds the range expected for monosynaptic linkage.
Are commissural interneurones coexcited by group I and II afferents?
In view of the previously demonstrated coexcitation of ipsilaterally projecting intermediate zone midlumbar interneurones by group I and group II afferents (Harrison & Jankowska, 1985; Edgley & Jankowska, 1987b; Riddell & Hadian, 2000) we expected that excitatory input to lamina VIII commissural interneurones might likewise arise from both types of afferents. However, no evidence for coexcitation was seen in the present sample; we never saw EPSPs from both group I and group II afferents in the same cell, whether monosynaptic (Fig. 3A) or disynaptic (Fig. 3B).
Are commissural interneurones with monosynaptic input from MLF coexcited by group I afferents and from LVN?
Figure 3 shows that convergent monosynaptic input from MLF, group I afferents and LVN was found in commissural interneurones. The proportions of interneurones with monosynaptic input from MLF in which either monosynaptic or disynaptic EPSPs were evoked (Fig. 3A and B, 2nd row) from the two other sources were rather small (15% and 6% from group I, 29% and 33% from LVN) but higher proportions of interneurones with monosynaptic input from group I afferents (3rd row) or LVN (4th row) were coexcited from MLF; this is particularly true for monosynaptic EPSPs (80% and 60%, respectively). Co-excitation by group I afferents and from MLF and/or LVN is as previously suggested by records from more caudally located commissural interneurones with group I input following stimulation of unspecified descending tract fibres at the Th12–Th13 levels (Harrison et al. 1986).
Inhibitory interactions
One of the major features of input to commissural interneurones summarized in Fig. 3C is that inhibition from muscle afferents often accompanied monosynaptic excitation from the MLF and that, vice versa, inhibition from the MLF often accompanied monosynaptic excitation from group II afferents.
In a high proportion of MLF-excited interneurones, inhibition followed stimulation of either ipsilateral or contralateral or, most often, of both ipsilateral and contralateral group II muscle afferents (46/52 interneurones, 88%). The IPSPs were often very effectively evoked by single stimuli (Fig. 6C and D) or by pairs of stimuli (Fig. 7B and C). Disynaptic IPSPs from ipsilateral or contralateral group II afferents were evoked in similar proportions of interneurones (77% and 78%, respectively) and, as judged by their time course, were most often followed by trisynaptic or polysynaptic IPSPs. In the remaining interneurones they were evoked at longer latencies, apparently only polysynaptically. Disynaptic inhibition from group I afferents was much less frequent (in 8% of commissural interneurones). More than half (60%) of the interneurones with monosynaptic input from group II afferents were inhibited following stimulation of the MLF with IPSPs evoked at latencies indicating disynaptic coupling (1.5–2.2 ms).
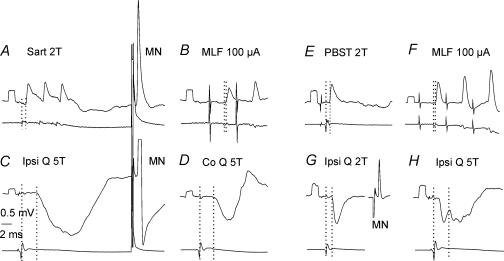
Figure 6. Co-excitation of commissural interneurones by group I afferents and from MLF, associated with inhibition from group II afferents.
Example records from two commissural interneurones (A–D and E–H) in which monosynaptic EPSPs were evoked from group I afferents (A and E) and from MLF (B and F) but not from group II afferents, which instead evoked disynaptic IPSPs (C, latency 3.1 ms; H, latency 2.8 ms). In the first interneurone IPSPs were also evoked from contralateral group II afferents (shown in D) and in the second interneurone IPSPs were also evoked from group I afferents (shown in G). Calibration pulses at the beginning of each trace are 0.5 mV in amplitude. Note that the antidromic spikes in A and G are blocked and that only that in C (taken before the record in A) shows the afterhyperpolarization.
Figure 7. Examples of disynaptic IPSPs evoked from both ipsilateral and contralateral group II muscle afferents in a commissural interneurone that was monosynaptically excited from the MLF.
Upper traces in all panels are intracellular records; lower traces are records from the cord dorsum. A, EPSPs evoked from MLF, classified as comprising an early monosynaptic component (segmental latency 0.65 ms) and a later disynaptic component. The earliest parts of the temporally facilitated disynaptic components were superimposed on the monosynaptic EPSPs. B and C, disynaptic IPSPs evoked from ipsilateral and contralateral group II afferents, at latencies 2.8 and 3.1 ms with respect to group I afferent volleys evoked by the second stimulus. At the extreme right hand side of the records in C is a blocked spike evoked by a stimulus delivered to the contralateral GS motor nuclei (50 μA). Dotted lines indicate the onset of the volleys and the EPSPs or IPSPs following these volleys.
Similar relationships were also found between the excitatory and inhibitory input from group I and II afferents (Fig. 3C). Disynaptic IPSPs evoked from group I afferents were found in half of the commissural interneurones that were monosynaptically excited by group II afferents; examples are shown in Fig. 4A (arrow) and Fig. 6G. An even greater proportion of interneurones that were monosynaptically excited by group I afferents were inhibited by group II afferents. In both cases lack of coactivation was thus associated with a mutual inhibition from the two pairs of input.
The origin of inhibition of individual commissural interneurones was generally wider than of the excitation, e.g. in some interneurones with monosynaptic input from MLF, disynaptic IPSPs were evoked from MLF itself, or from LVN, as well as from muscle afferents. Likewise, in some interneurones with monosynaptic input from group II afferents, disynaptic IPSPs were evoked from MLF, from ipsilateral group I afferents, contralateral group II afferents or LVN.
Discussion
Subpopulations of commissural interneurones with monosynaptic input from either group II afferents or from MLF/LVN/group I afferents
The results described here show that the midlumbar segments of the spinal cord contain two distinct groups of lamina VIII commissural interneurones, one with monosynaptic input from group II afferents and another with monosynaptic input from MLF. Furthermore, they indicate that monosynaptic input from vestibulospinal tract fibres and group I afferents is found in the group with monosynaptic reticulospinal input but not the group with monosynaptic group II input.
While these findings are clear from the data, two issues must be considered in relation to whether the organization applies more broadly. Firstly, we have restricted our recordings to the midlumbar (L3, L4 and L5) segments. We focused on this region partly for functional reasons, in that previous studies have described neurones with group II input in these regions and more recent work has highlighted the role of this region in locomotor behaviour (Rossignol et al. 2004), but also for practical reasons, in that characteristic focal synaptic potentials evoked by both afferent and descending tract fibres provide a guide to recording locations in the deep grey matter of these segments.
Secondly, since intracellular recording from interneurones deep in the grey matter is difficult, the samples of neurones on which the analysis is based are relatively small (samples of 10 neurones with monosynaptic input from each of group I afferents, group II afferents and the LVN and a larger sample of over 50 interneurones with input from MLF). The larger sample of commissural interneurones with input from MLF may indicate that these neurones are more numerous, but also that they are larger and more easilypenetrated: many of the group II activated commissural interneurones from which extracellular recordings were made could not be subsequently penetrated for intracellular recordings. However, the main conclusion that monosynaptic input from group II afferents and MLF is found in separate subpopulations of commissural interneurones is supported by the observations on extracellularly recorded neurones (14 and 24, respectively; see results) which were activated as would be predicted from the intracellular records. Although evaluation of the synaptic linkage of responses in extracellular records is less reliable, the consistency of the data increases the confidence in our conclusions.
Thirdly, in most experiments we investigated group II input only from the Q and Sart nerves, which are the main source of input to laminae VI–VIII interneurones in the midlumbar segments (Edgley & Jankowska, 1987b). Consistent with this, whenever stimulation of other nerves was tested on commissural interneurones with monosynaptic input from MLF, only input from group I afferents was found. Along the same lines, the stimuli we used to activate reticulospinal axons were applied within the ipsilateral MLF at the site from which the largest descending volleys were evoked. These stimuli would be likely to excite a considerable proportion of the reticulospinal tract fibres located in the MLF and the neighbouring part of the reticular formation, given that we routinely used stimuli of 50 or 100 μA, which would act within a radius of 0.5–1 mm (Gustafsson & Jankowska, 1976). However, axons of the reticulospinal tract neurones located in the nucleus gigantocellularis, descend ipsilaterally outside MLF (Basbaum et al. 1978; Matsuyama et al. 1988; Mitani et al. 1988), so they would most likely have escaped activation by stimuli delivered in the MLF. Our conclusions therefore relate to monosynaptic coupling between reticulospinal tract fibres in MLF and commissural interneurones.
Our conclusion that monosynaptic EPSPs from group II afferents and MLF fibres are evoked in different commissural interneurones contrasts with the finding that disynaptic or oligosynaptic actions following the same stimuli were not segregated. The validity of this conclusion depends critically on the reliability of our classification of EPSPs as evoked mono- and disynaptically. As stated in Methods, three features of EPSPs were used to differentiate disynaptic from monosynaptic EPSPs: longer latencies, failure to be evoked by single stimuli and temporal facilitation of effects of successive stimuli in a train.
With respect to EPSPs evoked from group II afferents, we relied mainly on the first two criteria; temporal facilitation tests were often inconclusive, partly because responses to successive stimuli were difficult to estimate on mixtures of EPSPs and IPSPs evoked by the first stimulus, and partly because potent presynaptic inhibition follows stimulation of group II afferents (Edgley et al. 2003) and can counteract even monosynaptic EPSPs (as illustrated in Fig. 5D). The upper limit of the latencies of the EPSPs we classified as monosynaptic (2.8 ms from group I volleys) on the basis of being evoked by single stimuli near threshold for group II afferents, was higher than the minimal latencies of IPSPs evoked by group II afferents, which were unquestionably disynaptic, and could be as low as 2 ms. Thus there was a considerable overlap in latencies of EPSPs evoked monosynaptically and disynaptically from group II afferents (within the grey box in Fig. 2B). However, the latencies of EPSPs evoked from group II afferents in commissural interneurones with monosynaptic input from MLF were all longer than 2.8 ms, i.e. always exceeded the overlapping range of mono- and disynaptic latencies. Conversely, none of the EPSPs of group II origin which we classified as monosynaptic and which had latencies within the overlapping monosynaptic/disynaptic range was accompanied by monosynaptic EPSPs from the MLF.
With respect to EPSPs evoked from the MLF, all of the three criteria were applied and the upper limit of latencies linked with monosynaptic actions (0.9 ms) was found consistently for EPSPs that appeared after a single stimulus and showed only minimal temporal facilitation. We consider therefore the probability of erroneous classification of these EPSPs to be minimal.
Differentiation between monosynaptic and disynaptic EPSPs evoked by LVN stimulation was less reliable since electrical stimuli in the LVN can activate vestibulospinal tract neurones both directly and indirectly (trans-synaptically). We cannot therefore exclude the possibility that some of the EPSPs classified as having been evoked disynaptically resulted from monosynaptic connections of vestibulospinal axons on commissural interneurones. However, none of the EPSPs evoked in commissural interneurones with segmental latencies of < 1 ms were found in interneurones with monosynaptic input from group II afferents.
Given the small samples of interneurones, the patterns of input we describe may represent an oversimplification and not apply to all commissural interneurones. In addition, although the probability of direct synaptic actions of both group II afferents and reticulospinal fibres on midlumbar commissural interneurones appears to be low, the possibility that commissural interneurones located more caudally might be coactivated by group I and II afferents remains an open question. Re-inspection of records from two previous samples (Harrison et al. 1986; Jankowska & Noga, 1990) revealed that six intracellularly recorded commissural interneurones had monosynaptic input from group I but not group II afferents and five commissural interneurones had monosynaptic input from group II but not group I afferents. However, in another five interneurones EPSPs that followed monosynaptic EPSPs from group I afferents, and were not tested for temporal facilitation, could have been evoked either disynaptically by group I afferents or monosynaptically by group II afferents. These EPSPs appeared at latencies of about 2 ms, which would be compatible with monosynaptic actions of group II afferents but in two neurones they were superimposed on disynaptic IPSPs of group I origin, which might have led to the latency of the EPSPs being overestimated. Furthermore, in the remaining neurones, the threshold for inducing these EPSPs was compatible with effects of either the lowest threshold group II afferents or the highest threshold group I afferents (1.6–2T) or was not defined.
Disynaptic and polysynaptic input in subpopulations with selective monosynaptic inputs
An important finding was that di- or oligosynaptic inhibition evoked by stimulation of MLF and ipsi- and contralateral group II afferents was seen in a large majority (> 70%) of the commissural interneurones with monosynaptic EPSPs from the MLF, LVN and group I afferents. Conversely, di- or oligosynaptic inhibition was evoked by MLF stimulation in many (60%) of the commissural interneurones in which monosynaptic EPSPs were evoked by stimulation of group II afferents. This organization suggests that the two groups of commissural interneurones are unlikely to be active coincidentally. In addition, since NA depresses transmission from group II afferents but facilitates transmission from MLF fibres to lamina VIII commissural interneurones (Hammar et al. 2004), local release of NA would further reduce the probability of coactivation of these two subpopulations of interneurones.
Co-activation of commissural interneurones with monosynaptic input from either group II afferents or from MLF might nevertheless be made possible via other neurones. As shown in Fig. 3B, the excitatory disynaptic input from all sources was distributed to all subpopulations of commissural interneurones. The proportions of interneurones in which disynaptic EPSPs were detected were not particularly large but might be greater in behavioural contexts where the interneurones producing the excitation are facilitated.
The actions of commissural interneurones with monosynaptic input from either group II afferents or from the MLF could also depend on postsynaptic inhibitory control of these neurones from other sources, e.g. those listed in Fig. 3C, and by presynaptic GABAergic inhibition (Edgley et al. 2003). The widespread origin of the inhibitory control of commissural interneurones should be interpreted in terms of potential for focusing their activity rather than for indiscriminate reduction of activity. When certain combinations of afferents or descending tract neurones are activated, e.g. in specific behavioural contexts, the inhibition should be much more focused.
A source of postsynaptic inhibition which we would like to single out as being particularly important is disynaptic inhibition evoked from contralateral group II afferents. IPSPs evoked from contralateral group II afferents were evoked at similar minimal latencies as IPSPs from ipsilateral group II afferents and were evoked as easily by single stimuli as by double stimuli (Figs 2C, 6D and 7C). They were found in both subpopulations of commissural interneurones but were most common in commissural interneurones monosynaptically activated from MLF/LVN and by group I afferents, as shown in Fig. 3C. Actions mediated by either group of commissural interneurones could thus be coordinated on the basis of information from muscles on both sides of the body.
However, postural adjustments could also be based on peripheral afferent information from muscle, skin and joint receptors as well as from vestibular and neck receptors and on ongoing central commands that are forwarded to commissural interneurones via reticulospinal neurones (Peterson & Felpel, 1971; Kasper et al. 1989; Bolton et al. 1992). The shared use of at least some commissural interneurones by reticulospinal and vestibulospinal neuronal systems appears to parallel the mutual interactions between these systems at medullary level (Peterson & Felpel, 1971; Bolton et al. 1992). The question might therefore be asked whether facilitation of reticulospinal actions on commissural interneurones by vestibular neurones occurs at the spinal level, or only in the medulla. Previous control experiments demonstrated that the bulk of effects of stimuli applied in MLF and LVN can be attributed to separate actions of descending reticulospinal and vestibulospinal fibres (Jankowska et al. 2003; Krutki et al. 2003; Matsuyama & Jankowska, 2004). In addition, the effects of stimuli evoked from MLF and LVN in this study differed considerably, for example monosynaptic field potentials were evoked from LVN and MLF in different regions of the spinal grey matter (Fig. 1E, F), monosynaptic EPSPs from one, but not the other, were found in a number of commissural interneurones (Fig. 2), and there were opposite effects (EPSPs and IPSPs) evoked by these systems in some commissural interneurones. Mutual facilitation between synaptic actions evoked from MLF and LVN may therefore occur in the commissural interneurones.
Functional roles of the different subpopulations of commissural interneurones
Bilateral coordination is fundamental to locomotion and spinal commissural neurones form essential elements of locomotor networks in fish (Buchanan & McPherson, 1995; Grillner & Wallen, 2002) and tadpoles (Roberts, 2000). In mammals many commissural interneurones are rhythmically active during locomotor-like activity (Kiehn & Butt, 2003; Matsuyama et al. 2004) and have been considered to be fundamental parts of the locomotor central pattern generating network (Kiehn & Butt, 2003). On the other hand, it has been reported that alternating activation of flexors and extensors on the left and right sides may be preserved even when the spinal cord is split along almost the whole length of the lumbosacral enlargement (L2–S1 in the chronic cat (Kato, 1988); L1–the cone in the neonatal rat in vitro (Cowley & Schmidt, 1997)).
Commissural interneurones with monosynaptic input from MLF and/or LVN are likely to be involved in locomotion in view of the importance of reticulospinal neurones in the initiation of locomotion (Armstrong, 1988; Jordan, 1991; Mori et al. 2001; Deliagina et al. 2002; Noga et al. 2003) and the short latencies of postsynaptic potentials evoked in motoneurones during centrally initiated locomotion (Shefchyk & Jordan, 1985; Noga et al. 2003). Furthermore, commissural interneurones monosynaptically activated from the reticular formation are rhythmically active during fictive locomotion and some are disynaptically excited from the cuneiform nucleus (MLR; Matsuyama et al. 2004). Disynaptic excitation from MLR has also been found in two commissural interneurones described by Jankowska & Noga (1990) in which monosynaptic EPSPs were evoked from ipsilateral group I afferents and oligosynaptic IPSPs from group II afferents. The observation that the activation of these commissural interneurones is facilitated by locally applied noradrenaline (Hammar et al. 2004) is also consistent with involvement in locomotion since noradrenergic receptor antagonists delivered in the midlumbar segments block locomotion generated by the lumbosacral segments (Rossignol et al. 2001).
Ipsilaterally projecting midlumbar interneurones with monosynaptic input from group II muscle afferents may be disynaptically excited (Edgley et al. 1988) by stimulation of brainstem structures used to evoke fictive locomotion (the cuneiform nucleus, MLR), in agreement with connections from reticulospinal fibres in these ipsilaterally projecting neurones (Davies & Edgley, 1994). Two-thirds of these neurones were rhythmically active during one of the phases of the step cycle (Shefchyk et al. 1990). However, during MLR-evoked locomotion in decerebrate preparations responses to stimulation of group II afferents are generally depressed. If the ipsilaterally and contralaterally projecting interneurones behave in the same way, activity of the lamina VIII commissural interneurones with group II input could also be modulated during locomotion. Critically, during real locomotion additional excitatory drives might come from descending systems, e.g. the LVN and the rubrospinal or pyramidal tracts (Davies & Edgley, 1994) as well as from both group Ia and group II afferents.
Another major function of commissural interneurones with group II input is in determining different patterns of crossed reflexes. Marked differences in the expression of crossed actions of group II afferents are found with descending tracts intact, in which case the actions are strongly biased to inhibition, and after spinalization when the inhibition is less predominant (Arya et al. 1991). These patterns depend on the spinal actions of monoamines (Aggelopoulos et al. 1996), and it may be important that monoamines have differential effects on the groups of commissural interneurones with input from the MLF and from group II afferents (Hammar et al. 2004) indicating that these groups of interneurones have different roles in these crossed reflex actions. Interactions between different subpopulations of commissural interneurones and other interneurones remain to be assessed but, importantly, their actions should be strictly contralateral since commissural interneurones of both populations have only crossed terminal projection areas (Bannatyne et al. 2003).
Acknowledgments
We wish to thank Mrs Rauni Larsson for her invaluable assistance during the experiments and with histological verifications. The study was supported by a grant from NIH (NS 40 863).
References
- Aggelopoulos NC, Burton MJ, Clarke RW, Edgley SA. Characterization of a descending system that enables crossed group II inhibitory reflex pathways in the cat spinal cord. J Neurosci. 1996;16:723–729. doi: 10.1523/JNEUROSCI.16-02-00723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM. The supraspinal control of mammalian locomotion. J Physiol. 1988;405:1–37. doi: 10.1113/jphysiol.1988.sp017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya T, Bajwa S, Edgley SA. Crossed reflex actions from group II muscle afferents in the lumbar spinal cord of the anaesthetized cat. J Physiol. 1991;444:117–131. doi: 10.1113/jphysiol.1991.sp018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Lundberg A, Udo M. Stimulation of pre- and postsynaptic elements in the red nucleus. Exp Brain Res. 1972;15:151–167. doi: 10.1007/BF00235579. [DOI] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions identified by immunocytochemistry. Eur J Neurosci. 2003;18:2273–2284. doi: 10.1046/j.l460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Clanton CH, Fields HL. Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J Comp Neurol. 1978;178:209–224. doi: 10.1002/cne.901780203. [DOI] [PubMed] [Google Scholar]
- Birinyi A, Viszokay K, Weber I, Kiehn O, Antal M. Synaptic targets of commissural interneurons in the lumbar spinal cord of neonatal rats. J Comp Neurol. 2003;461:429–440. doi: 10.1002/cne.10696. [DOI] [PubMed] [Google Scholar]
- Bolton PS, Goto T, Schor RH, Wilson VJ, Yamagata Y, Yates BJ. Response of pontomedullary reticulospinal neurons to vestibular stimuli in vertical planes. Role in vertical vestibulospinal reflexes of the decerebrate cat. J Neurophysiol. 1992;67:639–647. doi: 10.1152/jn.1992.67.3.639. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, McPherson DR. The neuronal network for locomotion in the lamprey spinal cord: evidence for the involvement of commissural interneurons. J Physiol (Paris) 1995;89:221–233. doi: 10.1016/0928-4257(96)83638-2. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Kiehn O. Functional identification of interneurons responsible for left-right coordination of hindlimbs in mammals. Neuron. 2003;38:953–963. doi: 10.1016/s0896-6273(03)00353-2. [DOI] [PubMed] [Google Scholar]
- Cajal SR. Histologie Du Systeme Nerveux de L'homme et Des Vertebres. Madrid: Instituto Ramon y Cajal; 1953. [Google Scholar]
- Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J Neurophysiol. 1997;77:247–259. doi: 10.1152/jn.1997.77.1.247. [DOI] [PubMed] [Google Scholar]
- Davies HE, Edgley SA. Inputs to group II-activated midlumbar interneurones from descending motor pathways in the cat. J Physiol. 1994;479:463–473. doi: 10.1113/jphysiol.1994.sp020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina TG, Zelenin PV, Orlovsky GN. Encoding and decoding of reticulospinal commands. Brain Res Rev. 2002;40:166–177. doi: 10.1016/s0165-0173(02)00199-6. 10.1016/S0165-0173(02)00199-6. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987a;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol. 1987b;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Krutki P, Hammar I. Both dorsal horn and lamina VIII interneurones contribute to crossed reflexes from group II muscle afferents. J Physiol. 2003;552:961–974. doi: 10.1113/jphysiol.2003.048009. 10.1113/jphysiol.2003.048009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Shefchyk S. Evidence that mid-lumbar neurones in reflex pathways from group II afferents are involved in locomotion in the cat. J Physiol. 1988;403:57–71. doi: 10.1113/jphysiol.1988.sp017238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide AL, Glover J, Kjaerulff O, Kiehn O. Characterization of commissural interneurons in the lumbar region of the neonatal rat spinal cord. J Comp Neurol. 1999;403:332–345. 10.1002/(SICI)1096-9861(19990118)403:3<332::AID-CNE4>3.0.CO;2-R. [PubMed] [Google Scholar]
- Fu TC, Schomburg ED. Electrophysiological investigation of the projection of secondary muscle spindle afferents in the cat spinal cord. Acta Physiol Scand. 1974;91:314–329. doi: 10.1111/j.1748-1716.1974.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallen P. Cellular bases of a vertebrate locomotor system-steering, intersegmental and segmental co-ordination and sensory control. Brain Res Rev. 2002;40:92–106. doi: 10.1016/s0165-0173(02)00193-5. 10.1016/S0165-0173(02)00193-5. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol. 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar I, Bannatyne BA, Maxwell DJ, Edgley SA, Jankowska E. The actions of monoamines and distribution of noradrenergic and serotoninergic contacts on different subpopulations of commissural interneurons in the cat spinal cord. Eur J Neurosci. 2004;19:1305–1316. doi: 10.1111/j.l460-9568.2004.03239.x. 10.1111/j.1460-9568.2004.03239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. Organization of input to the interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol. 1985;361:403–418. doi: 10.1113/jphysiol.1985.sp015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E, Zytnicki D. Lamina VIII interneurones interposed in crossed reflex pathways in the cat. J Physiol. 1986;371:147–166. doi: 10.1113/jphysiol.1986.sp015965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Zytnicki D. Crossed actions of group I muscle afferents in the cat. J Physiol. 1984;356:263–273. doi: 10.1113/jphysiol.1984.sp015463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Noga BR. Contralaterally projecting lamina VIII interneurones in middle lumbar segments in the cat. Brain Res. 1990;535:327–330. doi: 10.1016/0006-8993(90)91618-q. 10.1016/0006-8993(90)91618-Q. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signal and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jordan LM. Brainstem and spinal cord mechanisms for the initiation of locomotion. In: Shimamura M, Grillner S, Edgerton VR, editors. Neurobiological Basis of Human Locomotion. Tokyo: Scientific Societies Press; 1991. pp. 3–20. [Google Scholar]
- Kasper J, Schor RH, Wilson VJ. Neck-vestibular interaction in the vestibular nuclei. A dynamic, two-dimensional study. Acta Otolaryngol Supplement. 1989;468:137–139. doi: 10.3109/00016488909139033. [DOI] [PubMed] [Google Scholar]
- Kato M. Longitudinal myelotomy of lumbar spinal cord has little effect on coordinated locomotor activities of bilateral hindlimbs of the chronic cats. Neurosci Lett. 1988;93:259–263. doi: 10.1016/0304-3940(88)90092-4. 10.1016/0304-3940(88)90092-4. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Butt SJ. Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord. Progr Neurobiol. 2003;70:347–361. doi: 10.1016/s0301-0082(03)00091-1. 10.1016/S0301-0082(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Krutki P, Jankowska E, Edgley SA. Are crossed actions of reticulospinal and vestibulospinal neurons on feline motoneurons mediated by the same or separate commissural neurons? J Neurosci. 2003;23:8041–8050. doi: 10.1523/JNEUROSCI.23-22-08041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42:375–386. doi: 10.1016/s0896-6273(04)00249-1. 10.1016/S0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Exp Brain Res. 1987;65:271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Matsushita M. The axonal pathways of spinal neurons in the cat. J Comp Neurol. 1970;138:391–417. doi: 10.1002/cne.901380402. 10.1002/cne.901380402. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Jankowska E. Coupling between feline cerebellum (fastigial neurons) and motoneurons innervating hindlimb muscles. J Neurophysiol. 2004;91:1183–1192. doi: 10.1152/jn.00896.2003. 10.1152/jn.00896.2003. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Nakajima K, Mori F, Aoki M, Mori S. Lumbar commissural interneurons with reticulospinal inputs in the cat: Morphology and discharge patterns during fictive locomotion. J Comp Neurol. 2004;474:546–561. doi: 10.1002/cne.20131. 10.1002/cne.20131. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Ohta Y, Mori S. Ascending and descending projections of the nucleus reticularis gigantocellularis in the cat demonstrated by the anterograde neural tracer, Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Res. 1988;460:124–141. doi: 10.1016/0006-8993(88)91212-7. 10.1016/0006-8993(88)91212-7. [DOI] [PubMed] [Google Scholar]
- Mitani A, Ito K, Mitani Y, McCarley RW. Descending projections from the gigantocellular tegmental field in the cat: cells of origin and their brainstem and spinal cord trajectories. J Comp Neurol. 1988;268:546–566. doi: 10.1002/cne.902680406. 10.1002/cne.902680406. [DOI] [PubMed] [Google Scholar]
- Mori S, Matsuyama K, Mori F, Nakajima K. Supraspinal sites that induce locomotion in the vertebrate central nervous system. Adv Neurol. 2001;87:25–40. [PubMed] [Google Scholar]
- Noga BR, Kriellaars DJ, Brownstone RM, Jordan LM. Mechanism for activation of locomotor centers in the spinal cord by stimulation of the mesencephalic locomotor region. J Neurophysiol. 2003;90:1464–1478. doi: 10.1152/jn.00034.2003. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Felpel LP. Excitation and inhibition of reticulospinal neurons by vestibular, cortical and cutaneous stimulation. Brain Res. 1971;27:373–376. doi: 10.1016/0006-8993(71)90264-2. 10.1016/0006-8993(71)90264-2. [DOI] [PubMed] [Google Scholar]
- Pierani A, Moran-Rivard L, Sunshine MJ, Littman DR, Goulding M, Jessell TM. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron. 2001;29:367–384. doi: 10.1016/s0896-6273(01)00212-4. 10.1016/S0896-6273(01)00212-4. [DOI] [PubMed] [Google Scholar]
- Riddell JS, Hadian M. Interneurones in pathways from group II muscle afferents in the lower-lumbar segments of the feline spinal cord. J Physiol. 2000;522:109–123. doi: 10.1111/j.1469-7793.2000.t01-2-00109.xm. 10.1111/j.1469-7793.2000.t01-2-00109.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. Early functional organization of spinal neurons in developing lower vertebrates. Brain Res Bull. 2000;53:585–593. doi: 10.1016/s0361-9230(00)00392-0. 10.1016/S0361-9230(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Neural control of stereotypic limb movements. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 173–216. [Google Scholar]
- Rossignol S, Bouyer L, Langlet C, Barthelemy D, Chau C, Giroux N, et al. Determinants of locomotor recovery after spinal injury in the cat. Progr Brain Res. 2004;143:163–172. doi: 10.1016/S0079-6123(03)43016-1. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Gauthier L. An analysis of mechanisms controlling the reversal of crossed spinal reflexes. Brain Res. 1980;182:31–45. doi: 10.1016/0006-8993(80)90828-8. 10.1016/0006-8993(80)90828-8. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Giroux N, Chau C, Marcoux J, Brustein E, Reader TA. Pharmacological aids to locomotor training after spinal injury in the cat. J Physiol. 2001;533:65–74. doi: 10.1111/j.1469-7793.2001.0065b.x. 10.1111/j.1469-7793.2001.0065b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. A structural analysis of spinal interneurons and Renshaw cells. UCLA Forum Med Sci. 1969;11:159–208. [PubMed] [Google Scholar]
- Shefchyk SJ, Jordan LM. Excitatory and inhibitory postsynaptic potentials in alpha-motoneurons produced during fictive locomotion by stimulation of the mesencephalic locomotor region. J Neurophysiol. 1985;53:1345–1355. doi: 10.1152/jn.1985.53.6.1345. [DOI] [PubMed] [Google Scholar]
- Shefchyk S, McCrea D, Kriellaars D, Fortier P, Jordan L. Activity of interneurons within the L4 spinal segment of the cat during brainstem-evoked fictive locomotion. Exp Brain Res. 1990;80:290–295. doi: 10.1007/BF00228156. [DOI] [PubMed] [Google Scholar]
- Sherrington CS, editor. The Integrative Action of the Nervous System. New Haven: Yale University Press; 1906. [Google Scholar]
- Stokke MF, Nissen UV, Glover JC, Kiehn O. Projection patterns of commissural interneurons in the lumbar spinal cord of the neonatal rat. J Comp Neurol. 2002;446:349–359. doi: 10.1002/cne.10211. 10.1002/cne.10211. [DOI] [PubMed] [Google Scholar]