Abstract
Increases in Ca2+ concentration in the nucleus of neurones modulate gene transcription and may be involved in activity-dependent long-term plasticity, apoptosis, and neurotoxicity. Little is currently known about the regulation of Ca2+ in the nuclei of neurones. Investigation of neuronal nuclei is hampered by the cellular heterogeneity of the brain where neurones comprise no more than 10% of the cells. The situation is further complicated by large differences in properties of different neurones. Here we report a method for isolating nuclei from identified central neurones. We employed this technique to study nuclei from rat cerebellar Purkinje and granule neurones. Patch-clamp recording from the nuclear membrane of Purkinje neurones revealed numerous large-conductance channels selective for monovalent cations. The nuclear membrane of Purkinje neurones also contained multiple InsP3- activated ion channels localized exclusively in the inner nuclear membrane with their receptor loci facing the nucleoplasm. In contrast, the nuclear membrane of granule neurones contained only a small number of mainly anion channels. Nuclear InsP3 receptors (InsP3Rs) were activated by InsP3 with EC50= 0.67 μm and a Hill coefficient of 2.5. Ca2+ exhibited a biphasic effect on the receptors elevating its activity at low concentrations and inhibiting it at micromolar concentrations. InsP3 in saturating concentrations did not prevent the inhibitory effect of Ca2+, but strongly increased InsP3R activity at resting Ca2+ concentrations. These data are the first evidence for the presence of intranuclear sources of Ca2+ in neurones. Ca2+ release from the nuclear envelope may amplify Ca2+ transients penetrating the nucleus from the cytoplasm or generate Ca2+ transients in the nucleus independently of the cytoplasm.
Synaptic activity can evoke a rise in Ca2+ concentration in the nucleus of neurones either by generating a regenerative Ca2+ wave, spreading from activated synapses to the cell body or by depolarizing the neurone and activating voltage-operating Ca2+ channels in the somatic plasma membrane (Berridge, 1998; Power & Sah, 2002). Increases in Ca2+ concentration in neuronal nuclei regulate gene transcription by activating nuclear Ca2+-sensitive kinases and phosphatases or by directly affecting Ca2+-binding transcription factors. The two regulatory mechanisms may be exemplified by the transcription factors cAMP responsive element binding protein (CREB) and downstream response element-antagonist modulator (DREAM), respectively (Hardingham et al. 2001; West et al. 2002). Nuclear Ca2+ transients have been implicated in a number of physiological phenomena including neuronal development, survival, and activity-dependent long-term neuronal plasticity (Berridge, 1998; Bading, 2000; West et al. 2002).
Little is currently known about Ca2+ regulation in the nuclei of neurones. In non-neuronal cells this issue has been addressed in numerous studies, which have yielded conflicting results (reviewed in Santella & Carafoli, 1997; Bootman et al. 2000). There are two potential sources of Ca2+ that may generate an increase inside the nucleus. Ca2+ ions can penetrate the nucleus from the cytoplasm through numerous nuclear pores or they can be released into the nucleoplasm from the nuclear envelope. The nuclear envelope is a flattened cistern morphologically and biogenetically related to the endoplasmic reticulum. It has been shown that the nuclear envelopes of Xenopus oocytes, hepatocytes and a number of other cells are functional Ca2+ stores (Nicotera et al. 1990; Gerasimenko et al. 1995; Stehno-Bittel et al. 1995). The role of the nuclear envelope in the regulation of nuclear Ca2+ is the main issue of the current controversy. The reason for this controversy may at least partly result from different mechanisms of Ca2+ regulation in the nuclei of different cells.
One of the difficulties in studying neuronal nuclei is the heterogeneous cellular composition of the nervous system. Neurones constitute no more than 10% of the total number of brain cells (Kendal et al. 2000). The standard method of nucleus isolation includes homogenization of a tissue and subsequent differential centrifugation of the homogenate (Blobel & Potter, 1966). When applied to nervous tissue this method yields a mixture where neuronal nuclei comprise a minority group. Isolation of nuclei from certain parts of the brain, such as the cerebral or cerebellar cortex, can enrich the preparation with neuronal nuclei, but does not solve the problem. Further complication arises from the extreme diversity of the neurones themselves.
We have developed a method for isolating nuclei from identified central neurones. Using this technique we have studied ion channels in the nuclei of cerebellar Purkinje and granule neurones and found that they have different properties.
Methods
Isolation of neuronal nuclei
All experimental procedures involving animals and their care were conducted in conformity with the Animals (Scientific Procedures) Act 1986 and the guidelines of the Ministry of Public Health of Ukraine. Male Wistar rats (3- to 4-weeks old) were anaesthetized with ether and decapitated. The cerebellum was rapidly removed and placed in ice-cold (1–3°C) artificial cerebrospinal fluid (ACSF), containing (mm): NaCl 120, KCl 2.5, MgSO4 1.3, CaCl2 1, NaH2PO4 1.3, NaHCO3 26, glucose 10 and equilibrated with 95% O2 and 5% CO2. Coronal cerebellar slices (300 μm; see Fig. S1 of the Supplemental material available with this article online) were cut by hand or using a Vibratome (Campden Instruments, Sileby, UK). Slices were placed in a chamber on the stage of a Leica DMLFS upright microscope (Leica Microsystems, Germany) and continuously perfused with ACSF continuously oxygenated with 95% O2 and 5% CO2 at room temperature. Neurones were visualized using a × 40 long working distance water-immersion objective and a VX 45 Microscope CCD camera (PCO Computer Optics, Kelheim, Germany) with DIC/infrared optics.
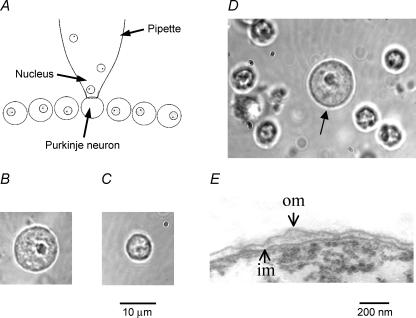
Nuclei were collected from neurones with glass pipettes (Fig. 1A). The opening of the pipette varied depending on the size of the nucleus and was 20–30% larger than the nucleus diameter. The cytoplasm from a Purkinje or granule neurone was aspirated under visual control into the pipette until the nucleus was sucked into the pipette. This procedure was applied to several other nuclei until the collected nuclei with remains of the cytoplasm were ejected into a microtube of homogenization solution containing (mm): potassium gluconate 150, Hepes-KOH 10 (pH 7.3). Protease Inhibitor Cocktail (Roche Diagnostics UK Ltd) was added to the solution according to the manufacturer's instructions. The nuclei were cleared from remains of the cytoplasm by four to five strokes in a 2 ml Dounce tissue homogenizer (Bellco Glass, Vineland, NJ, USA). Comparison of nuclei isolated from Purkinje neurones (Fig. 1B) with nuclei isolated from the rest of the cerebellar cortex showed that because of their much larger size and distinct morphology Purkinje neurone nuclei could be easily and unequivocally identified in crude homogenates of the cerebellar cortex (Fig. 1D). This allowed us to simplify the procedure considerably. In the later stages of the work we picked out Purkinje neurone nuclei directly from homogenates of the cerebellar cortex. The homogenate was placed into a working chamber on the stage of the Leica DMIRB inverted microscope. The nuclei were allowed to attach to the glass bottom of the chamber and then washed from debris with solution (further referred to as KCl solution) containing (mm): KCl 150, Na2ATP 0.5, K2EGTA 0.53, CaEGTA 1.47 (calculated free Ca2+ concentration 250 nm) and Hepes-KOH 10 (pH 7.3). To obtain access to the inner nuclear membrane the isolated nuclei were treated with sodium citrate as follows. The homogenate was centrifuged for 15 min at 1000 g, and the resulting pellet was suspended in homogenization medium with 1% (w/v) sodium citrate added. The suspension was incubated for 30–60 min on ice while stirring gently and then used in experiments as described above.
Figure 1. Isolated nuclei from neurones of rat cerebellar cortex.
A, the method of isolating nuclei from central neurones. The cytoplasm from a neurone (here a Purkinje neurone) in a brain slice was aspirated into a glass micropipette with a relatively large opening until the nucleus was sucked into the pipette. The same pipette was used to collect nuclei from several neurones. B and C, individual nuclei isolated from a Purkinje neurone (B) and a granule neurone (C). D, a rough homogenate of the cerebellar cortex. The large nucleus from a Purkinje neurone (arrow) clearly differs from all other nuclei in the homogenate. E, a transmission electron micrograph of the periphery of the nucleus isolated from a Purkinje neurone. The nucleus had well-preserved inner (im) and outer (om) nuclear membranes.
Electrophysiology
Single ion channels were recorded from nucleus-attached and excised patches of the nuclear membrane in the voltage-clamp mode of the patch-clamp technique. Patch pipettes were prepared from borosilicate glass (Sutter Instrument, Novato, CA, USA) and filled, unless stated otherwise, with KCl solution. Pipette resistances ranged from 7 to 15 MΩ. Data acquisition was carried out using a Visual Patch VP-500 amplifier (Bio-Logic, Claix, France). Currents were filtered with a low-pass Bessel filter at 1 kHz, digitized at 5 kHz and stored on a computer disk. The indifferent electrode was an Ag–AgCl plug connected to a bath chamber via an agar bridge. In all recordings the potential of the bath solution was considered to be 0 mV. The permeability ratio Pa/Pb of ion species a and b was calculated using the following formula (see, e.g. Miedema, 2002):
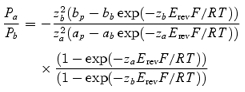 |
where ap, bp and ab, bb refer to the activities of ion species a and b in, respectively, pipette and bath solution; za and zb are valences of a and b, Erev is the reversal potential of the single channel current, and R, T and F are standard thermodynamic parameters.
The dependence of InsP3-activated channels on InsP3 concentration was fitted with the Hill equation:
where Po is the open probability of the channel at a given InsP3 concentration x, Pmax is its maximum open probability, K is half-maximum activating InsP3 concentration (EC50) and h is the Hill coefficient.
Electron microscopy
Nuclei were pelleted at 1000 g in homogenization medium. The pellet was fixed for 18 h in 2% glutaraldehyde–PBS and postfixed in 1% osmium tetraoxide–PBS, pH 7.4. Samples were dehydrated in ethanol and propylene oxide and then embedded in epon.
Results
Morphology of isolated neuronal nuclei
Nuclei isolated from Purkinje neurones had a spherical or slightly ellipsoidal shape with an average diameter of 11.8 ± 1.0 μm (n = 34; Fig. 1B). The nuclei had characteristically light nucleoplasm with a clearly visible large dark nucleolus. The nuclei from granule neurones were much smaller with an average diameter of 6.8 ± 0.7 μm (n = 78; Fig. 1C). Because of their large size and distinct morphology, nuclei from Purkinje neurones were quite different from nuclei of any other cerebellar cells and could be easily identified in rough homogenates of the cerebellar cortex (Fig. 1D). Transmission electron microscopy showed that the nuclear envelopes of isolated nuclei had a typical double-membrane structure with well-preserved outer and inner nuclear membranes (Fig. 1E). The integrity of isolated nuclear envelopes was also confirmed by their staining with fluo-3 AM (see Fig. S1 in Supplemental material). Therefore the outer nuclear membrane was readily accessible for patch-clamp recording in this preparation.
Spontaneously active ion channels in the nuclear membrane of Purkinje neurones
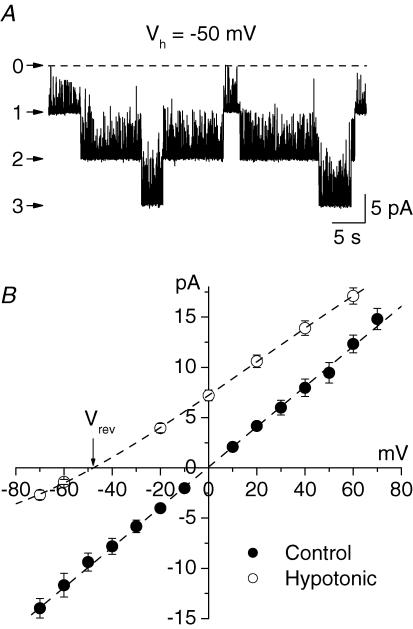
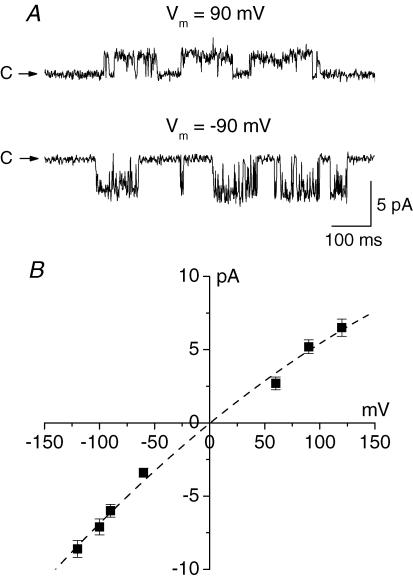
The majority of membrane patches (137 out of 189) from the nuclear membrane of Purkinje neurones contained large conductance ion channels. The channels were characterized by high density (usually 3–5 channels per patch), high open probability and slow kinetics (Fig. 2A). In symmetrical KCl solutions the channels had a linear current–voltage relationship (Fig. 2B, •) with a slope conductance of 198 ± 27 pS (n = 58).
Figure 2. Large conductance channel in the nuclear membrane of Purkinje neurones.
A, large conductance channels in an excised patch from the outer nuclear membrane of a Purkinje cell. The patch contained three channels; the zero current level is indicated by a dotted line. Note the slow kinetics and bursting activity of the channel. B, current–voltage relationships of the large conductance channel. In symmetrical standard KCl solution the slope conductance of the channel was 198 ± 27 pS (n = 58, •). After substitution of standard KCl bath solution with low (15 mm) KCl solution the reversal potential of the current shifted to −48.6 mV (Vrev) suggesting cationic selectivity of the channel (O).
To determine the selectivity of this channel to K+ and Cl− we substituted the isotonic KCl solution in the bath with hypotonic solutions, which contained reduced concentrations of KCl. In these conditions the equilibrium potential for K+ was shifted to the left and that for Cl− to the right. Consequently the current–voltage relationship of an anionic channel would shift to the right and that of a cationic channel to the left. The magnitude of the shift depends on the ratio of the channel permeability to K+ and Cl−. The reversal potential of a non-selective channel would not change.
When standard KCl bath solution was substituted with a hypotonic solution (in different experiments the concentration of KCl in the bath solution varied from 10 to 40 mm) the current–voltage relationship of the channel shifted to the left toward the reversal potential for K+ (Fig. 2B, O). When the pipette solution contained 155 mm K+ and 150 mm Cl− and the bath solution contained 15 mm K+ and 10 mm Cl− the calculated equilibrium potential for K+ was −59 mV. In these experimental conditions the reversal potential of the large conductance channel was −48 ± 1 mV (n = 3). The calculated ratio of the channel permeabilities for Cl− and K+ (PCl/PK) was 0.05. Therefore the large-conductance channel in the nuclear membrane of Purkinje neurones was selective to cations.
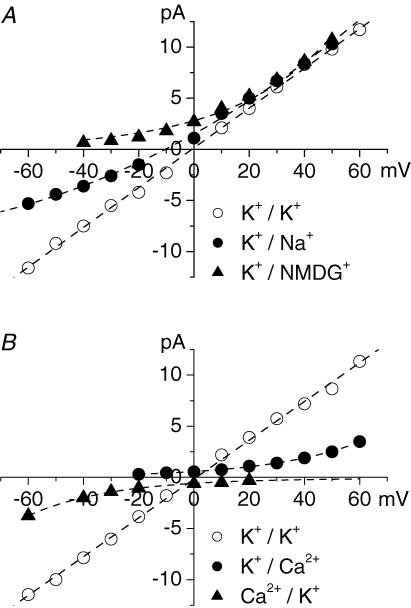
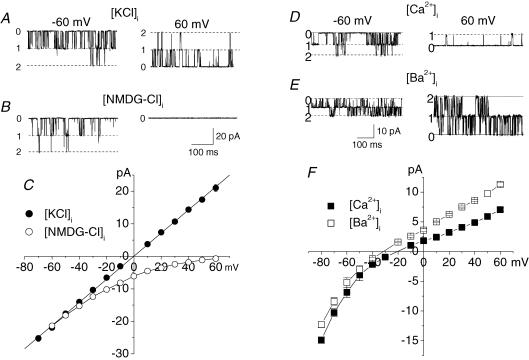
To study the permeability of the large conductance channel to other monovalent cations, K+ in the bath solution was replaced with an equimolar amount of the test cation (Fig. 3A). The substitution of K+ in the bath solution with Na+ shifted the reversal potential of the channels to −10.5 ± 1 mV (n = 3) indicating that the channel is permeable to Na+ with the permeability ratio PNa/PK= 0.65 (Fig. 3A, •). The slope conductance for inward current, carried by Na+ through the channel, was 94 pS (n = 3). The channel was also permeable to Cs+ (not illustrated). When K+ in the bath solution was replaced with the large organic monovalent cation N-methyl-d-glucamine (NMDG) no inward current through the channels was recorded (Fig. 3A, ▴). Therefore the large conductance channel was a poorly selective K+ channel.
Figure 3. The large conductance channel is a poorly selective K+ channel.
A, permeability of the large conductance channel to monovalent cations. Substitution of KCl in the bath solution with equimolar NaCl (•) shifted the reversal potential of the current through the channel to −10.4 mV suggesting that the channel is permeable to Na+ with PNa/PK= 0.65. When K+ in the bath solution was substituted with NMDG+ (▴) no inward current was observed. B, when CaCl2 solution in the bath (▴) or in patch pipettes (•) was substituted for KCl solution no Ca2+ inward or outward current, respectively, was observed suggesting that the channel is impermeable to Ca2+.
To study the permeability of the large conductance channels to Ca2+ either the working chamber (n = 6) or patch pipettes (n = 4) were filled with isotonic CaCl2 solution with standard KCl solution at the opposite side of the membrane (Fig. 3B). In both cases only K+ currents through the channels were recorded. In symmetric CaCl2 solution no channel activity was observed in the range from −150 to 150 mV. These data suggest that the large conductance channel is practically impermeable to Ca2+. Similar experiments with BaCl2 solutions suggest that the channels are also impermeable to Ba2+ (not illustrated).
The potassium channel blockers tetraethylammonium (10 mm) and 4-aminopyridine (2 mm) had no effect on either the conductance or gating of the large conductance channel. The non-selective blocker of cationic channels La3+ at a concentration of 10–100 μm was also ineffective. The ryanodine receptor blocker ruthenium red (10 μm), the ryanodine receptor agonist/blocker ryanodine (1–10 μm), InsP3 (10 μm), and the InsP3 receptor blocker heparin did not affect the large conductance cation channel. Large conductance channels were also unaffected by ATP, Ca2+ and Mg2+ at physiological concentrations (0.5–5 mm, 0.05–50 μm and 1–5 mm, respectively). At very high concentrations (100 mm) Ca2+ and Ba2+ on either side of the membrane reduced K+ currents through the channel (Fig. 3B).
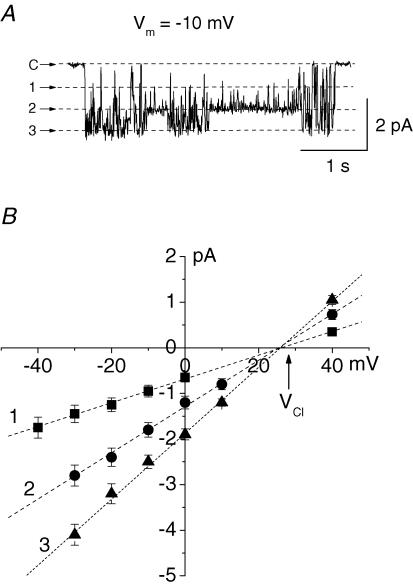
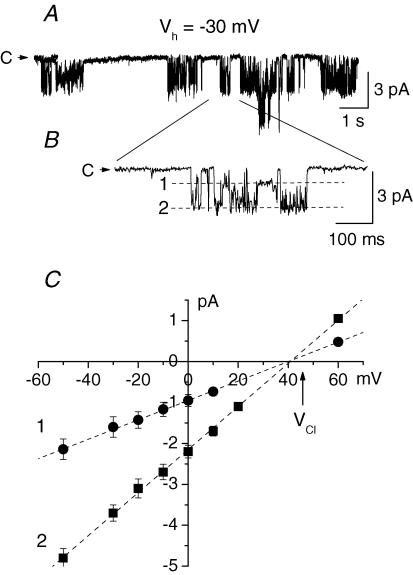
The large conductance channels were by far the most abundant type of channel in the nuclear membrane of Purkinje cells. Nevertheless, in a small fraction of patches (11 out of 189 patches) of the nuclear membrane from Purkinje neurones a channel with smaller amplitude and very different pattern of activity was observed (Fig. 4A). The relative rarity of the channel and the abundance of the large conductance cationic channels prevented us from studying this channel in detail. The channel activity was characterized by fast fluctuations between multiple subconductance states. The slope conductances of the more frequently occurring subconductance states were 67 ± 5, 52 ± 4 and 27 ± 3 pS (n = 6). To determine the ionic selectivity of the channel, KCl in the bath solution was partly replaced with equimolar amounts of potassium gluconate. In these conditions the reversal potential of the channel shifted to positive values close to the equilibrium potential for Cl− (Fig. 4B). These data suggest that the channel is selective for Cl− with little if any permeability to K+ and gluconate.
Figure 4. Anion channels in the nuclear membrane of Purkinje neurones.
A, the channel demonstrated fast transitions between multiple subconductance states. Three of the subconductance states and the closed state (C) are shown by dashed lines. B, current–voltage relationships of the three subconductance states of the anion channel. The arrow indicates the equilibrium potential for Cl− (VCl). Pipettes contained standard KCl solution, in bath solution 100 mm KCl was substituted with potassium gluconate.
InsP3-activated ion channels in the nuclear membrane of Purkinje neurones
To record InsP3-activated channels the patch pipette or the bath was filled with KCl solution containing 10 μm InsP3. This solution also contained Na2ATP and free Ca2+ in concentrations reported to be optimal for cerebellar InsP3R stimulation (0.5 mm and 250 nm, respectively; Bezprozvanny et al. 1991). Patch-clamp recording from the outer nuclear membrane of Purkinje neurones has revealed the presence of large-conductance spontaneously active ion channels, but InsP3-containing solution did not activate any additional ion channels in excised as well as nucleus-attached nuclear patches (n≫ 153).
To access the inner nuclear membrane isolated nuclei were treated with sodium citrate (see Methods). It has previously been reported that citrate selectively removes the outer membrane of the nuclear envelope leaving the inner nuclear membrane intact (Humbert et al. 1996).
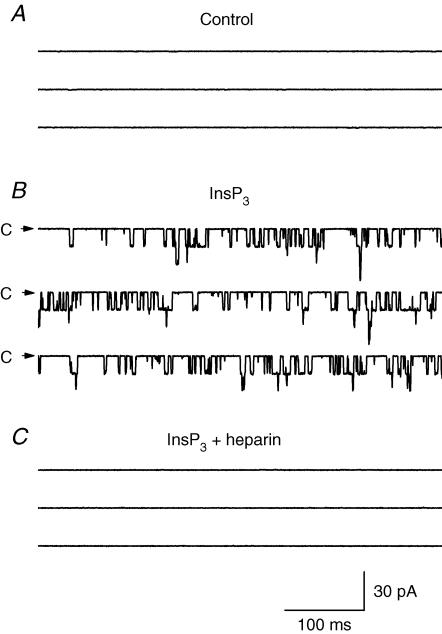
The application of InsP3 via bath solution and therefore to the nucleoplasmic side of the nuclear membrane of citric acid-treated nuclei activated multiple ion channels in most excised patches (136 out of 153 patches, Fig. 5). The same response was also achieved with nucleus-attached patches. The competitive blocker of InsP3Rs, heparin (0.02–0.4 mg ml−1) added to the nucleoplasmic side of the patch, suppressed the channel activity (n = 3). InsP3 in the pipette solution (at the luminal side of the membrane) was ineffective. Therefore the internal nuclear membrane of Purkinje neurones contained InsP3Rs with their receptor loci directed toward the nucleoplasm. In symmetric KCl solutions the InsP3-activated channels had a linear current–voltage relationship with a slope conductance of 356 ± 4 pS (n = 7, Fig. 6A and C, •).
Figure 5. InsP3-activated ion channels in the internal nuclear membrane of Purkinje cells.
A, in the absence of InsP3 no channels were recorded. B, InsP3 (10 μm) added to bath solution (the nucleoplasmic side of the membrane) activated at least 3 channels, shown as downward deflections from the zero current level indicated by arrows. C, the blocker of InsP3Rs, heparin (0.4 mg ml−1), inhibited the channel activity. The bath and pipette contained standard KCl solution with Na2ATP (0.5 mm) and Ca2+ (250 nm). Holding potential was −40 mV.
Figure 6. InsP3-activated channels are selective to Ca2+.
In symmetrical KCl solution (A and C, •) the current–voltage relationship of InsP3-activated channels had a slope conductance of 356 ± 4 pS. After K+ in the pipette solution was substituted by NMDG+ (B and C, O) only inward currents were recorded indicating that the channels were permeable to K+ and impermeable to Cl−. Single InsP3-activated currents when patch pipettes were filled with high Ca2+ (D and F, ▪) or Ba2+ (E and F, □) solution indicate that the channel was permeable to Ca2+ and Ba2+ with PCa/PK= 5.2 and PBa/PK= 5.7.
InsP3 receptors in the endoplasmic reticulum are Ca2+-selective channels, which are also permeable to other cations such as K+ and Ba2+ (Bezprozvanny & Ehrlich, 1994; Boehning et al. 2001). An InsP3-activated Na+ channel impermeable to Ca2+ has also been reported (Somasundaram & Mahaut-Smith, 1995). To determine the permeability of the channel to K+ and Cl− we substituted the large organic monovalent cation NMDG+ mole for mole for K+ in the patch pipette solution. Under these conditions only inward InsP3-activated currents were recorded, suggesting that the InsP3-activated channels were permeable to K+ with little if any permeability to Cl− and NMDG+ (n = 4, Fig. 6B and C, O). To determine the Ca2+ permeability of the channel, patch pipettes were filled with solution containing 50 mm CaCl2 and 30 mm KCl. The working chamber contained standard KCl solution with 10 μm InsP3 and 200 nm free Ca2+. Under these conditions the reversal potential of the InsP3-activated channels was −20.7 ± 0.5 mV (n = 4; Fig. 6D and F, ▪). The calculated permeability ratio for Ca2+ and K+ was PCa/PK= 5.2. The InsP3-activated channels were also permeable to Ba2+ (Fig. 6E and F, □). When patch pipettes were filled with solution containing 100 mm BaCl2 and 10 mm Hepes-KOH (pH 7.3) the current through InsP3-activated channels had a reversal potential of −34 ± 0.8 mV (n = 3), corresponding to PBa/PK= 5.7. The single-channel conductance of InsP3Rs with Ba2+ as the current carrier in the potential range from −20 mV to 60 mV was 121 ± 2 pS (n = 3). Therefore nuclear InsP3Rs were Ca2+ channels with conductance and selectivity similar to those previously reported for cerebellar InsP3Rs incorporated into artificial lipid bilayers and recombinant type 1 InsP3Rs expressed in mammalian cells (Bezprozvanny & Ehrlich, 1994; Boehning et al. 2001).
The open probability of the InsP3-activated channels (Po) depended on the ion species carrying current through the channel (Fig. S4 of Supplemental material). At a membrane potential of 60 mV in symmetrical KCl solution containing 10 μm InsP3, 250 nm free Ca2+ and 0.5 mm ATP, Po was 0.036 ± 0.04 (n = 3). With Ba2+ as a current carrier (patch pipettes were filled with 100 mm BaCl2) under the same conditions Po increased to 0.32 ± 0.03 (n = 3). The presence of Ca2+ in the pipette solution in concentrations ≥ 10 mm strongly decreased the Po of outward currents through InsP3Rs (Fig. 6D). When patch pipettes contained 50 mm CaCl2 and 30 mm KCl, the Po of single outward InsP3-activated currents was reduced to 0.0023 ± 0.0003 (n = 4). The decrease in Po apparently results from the inhibitory effect of high [Ca2+] in the vicinity of the receptor produced by Ca2+ current through the channel rather than an effect of the presence of Ca2+ at the luminal surface of the membrane, because the Po of single inward K+ currents remained practically unchanged (0.038 ± 0.006, n = 4, Vh=−60 mV.
Most patches of the inner nuclear membrane contained both InsP3Rs and large conductance cationic channels (see Figs S3 and S4 of Supplemental material). The superposition of multiple large conductance cationic channels complicated our study of InsP3Rs. To separate the two channels in the following experiments Ba2+ was used as the current carrier. Patch pipettes were filled with solution containing (mm): BaCl2, 100 and Hepes-KOH, 10 (pH 7.3). InsP3-activated currents were recorded at positive membrane potentials. The bath contained standard KCl solution with InsP3 and free Ca2+ as indicated. As we mention above, 198 pS channels were impermeable to Ba2+ and partly inhibited by large concentrations of Ba2+. Thus at positive membrane potentials currents through these channels were negligible.
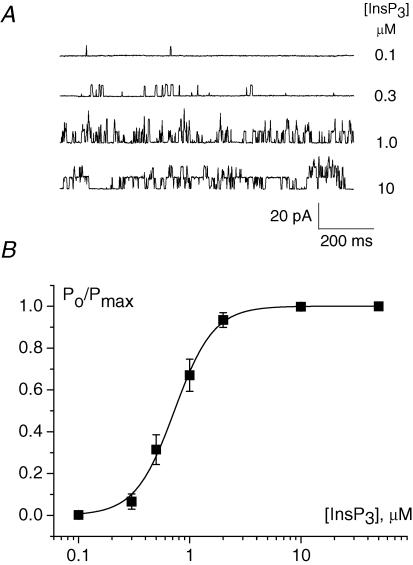
In the absence of InsP3, Ca2+ alone (20 nm–50 μm) was unable to activate the channels. In the presence of 250 nm free Ca2+ InsP3 evoked a noticeable activity of the channels in concentrations > 0.1 μm and fully activated InsP3Rs in concentrations ≥ 2–3 μm (n = 5, Fig. 7). The experimental data can be fitted by the Hill equation with EC50= 0.68 μm and a Hill coefficient of 2.5.
Figure 7. Dependence of nuclear InsP3-activated channels on InsP3 concentration.
A, InsP3-activated channel activity at different InsP3 concentrations. B, dependence of the normalized open probability of InsP3-activated channels on InsP3 concentration. Data points represent mean ± s.e.m. of five experiments. The continuous curve is the Hill equation fit with EC50= 0.68 μm and Hill coefficient = 2.5. Patch pipettes were filled with BaCl2 solution, bath contained standard KCl solution with [Ca2+]i= 250 nm. Holding potential was 40 mV.
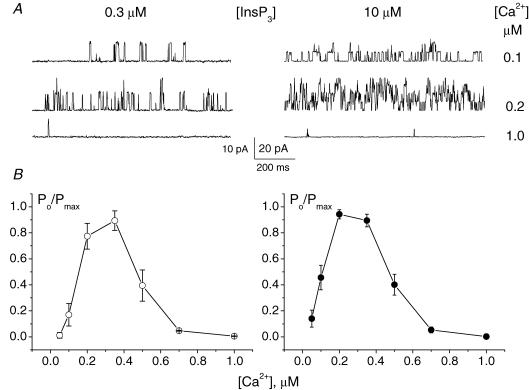
It has previously been reported that Ca2+ can both activate and, at higher concentrations, inhibit InsP3Rs (Iino, 1990; Bezprozvanny et al. 1991; Finch et al. 1991). In Xenopus oocytes the inhibitory effect of Ca2+ on InsP3Rs was eliminated by treatment with saturating concentrations of InsP3 (Mak et al. 1998). Therefore we studied the Ca2+ dependence of InsP3Rs at both low (0.3 μm, about 7% of the maximum response; n = 5) and saturated (10 μm, n = 7) concentrations of InsP3 (Fig. 8 and Fig. S5 of Supplemental material). At both InsP3 concentrations the Ca2+ dependence of the InsP3-activated channels was very similar with the maximum activity of the channel observed at [Ca2+]i in the range from 200 to 400 nm and strong inhibition of InsP3Rs by [Ca2+]i≥ 1 μm.
Figure 8. Dependence of InsP3-activated channels on Ca2+ concentration at the nucleoplasmic side of the membrane.
Channel activity (A) and normalized open probability of InsP3-activated channels (B) at different Ca2+ concentrations in the presence of low (0.3 μm, left; n = 5) and saturated (10 μm, right; n = 7) InsP3 concentrations. At both InsP3 concentrations 1 μm of Ca2+ almost completely inhibited the channel activity. Patch pipettes were filled with BaCl2 solution, bath contained standard KCl solution. Holding potential was 40 mV.
Apart from InsP3Rs, ryanodine receptors have also been reported in Purkinje neurones (Pozzan et al. 1994). Therefore we tried to detect these channels in the nuclear membrane of Purkinje neurones. The agonists of ryanodine receptors, Ca2+ (10–50 μm) and cyclic adenosine 5′-diphosphate-ribose (cADPR, 10 μm), activated channels neither in the outer (n = 56 and 17, respectively) nor in the inner (n = 23 and 12) nuclear membrane of Purkinje neurones. The agonist of putative Ca2+-releasing channels, nicotinic acid adenine dinucleotide phosphate (NAADP, 20–50 nm), was also ineffective (n = 15).
Ion channels in the nuclear membrane of granule neurones
In sharp contrast to Purkinje neurones no large-conductance cationic channels were recorded in the nuclear membrane of cerebellar granule neurones. The total density of the channels was also much lower. The most common channel in the nuclear membrane of granule neurones (17 out of 78 patches) had a pattern of activity very similar to that of the anionic channel in Purkinje neurones (Fig. 9A and B). The channel quickly fluctuated between multiple subconductance states. The conductances of the more common subconductance states were 46 ± 5 and 18.8 ± 2.1 pS (n = 4). When Cl− in the bath solution was replaced with equimolar amount of gluconate the current–voltage relationship of the channel shifted to the right and was close to the reversal potential for Cl− (Fig. 9C). When 125 mm of the total 150 mm KCl was replaced with potassium gluconate the reversal potential of the current was 40.25 ± 1.3 mV (n = 4). These data indicate that the channel is selectively permeable to Cl− with PK/PCl and PGlu/PCl < 0.05.
Figure 9. Anion channel in the nuclear membrane of a granule neurone.
A and B, the anion channel quickly fluctuated between multiple subconductance states. Two of the substates are shown by dashed lines (B). C, current–voltage relationships of the main conductance substates of the anion channel. The arrow indicates the equilibrium potential for Cl− (VCl). Pipettes contained standard KCl solution, in bath solution 125 mm KCl was substituted with potassium gluconate.
The nuclear membranes of granule neurones also contained a channel with a different pattern of activity (Fig. 10A). It was found in 6 out of 78 patches. In symmetrical KCl solution the channel demonstrated small inward rectification with the slope conductance at negative membrane potentials of 53 ± 4 pS (n = 5, Fig. 10B). The substitution of isotonic KCl solution with hypotonic solutions with reduced concentration of KCl shifted the reversal potential of the channel to the left suggesting cationic selectivity of the channel (not illustrated).
Figure 10. Cation channels in the nuclear membrane of granule cells.
A, currents through a single channel at two different holding potentials. B, the current–voltage relationship of the cation channel in symmetric KCl solution demonstrated small inward rectification.
In experimental conditions identical to those used for recording InsP3Rs in nuclear membranes of Purkinje neurones, InsP3 (10 μm) in KCl solution containing 0.5 mm ATP and 250 nm free Ca2+ failed to activated InsP3Rs in either the outer (n = 32) or the inner (n = 26) nuclear membrane of granule neurones.
Discussion
Here we have presented a method for isolating nuclei from identified neurones of the brain. We have employed this technique to isolate nuclei from cerebellar Purkinje and granule neurones, but the same approach can be readily used for isolating nuclei from any other brain cells (see Fig. S2 of Supplemental material). The significance of studying properties of nuclei from particular types of brain cells is illustrated by the findings of this work.
The major finding is that cerebellar Purkinje and granule cells express different sets of ion channels in their nuclear membranes and therefore their nuclear envelopes may play distinct functional roles. Distinct types of spontaneously active ion channels have also been reported in the outer nuclear membrane of B- and T-lymphocyte cell lines (Franco-Obregon et al. 2000). The inner, but not the outer, nuclear membrane of Purkinje neurones contained multiple InsP3-activated channels with their receptor loci facing the nucleoplasm. No InsP3Rs were found in the nuclear membrane of granule neurones. Although in vitro expression of InsP3Rs in cerebellar granule neurones can be induced artificially (Choi et al. 2004), in vivo these neurones express little if any InsP3Rs (Pozzan et al. 1994; Taylor et al. 1999). Therefore the absence of InsP3-activated channels in the nuclear membranes of granule neurones in our experiments is hardly surprising. On the other hand, Purkinje neurones express the highest level of InsP3Rs among mammalian cells and their absence in the outer nuclear membrane needs some discussion.
It is well-established that the endoplasmic reticulum is a functionally heterogeneous organelle (reviewed by Meldolesi & Pozzan, 1998; Papp et al. 2003). Ca2+-releasing channels as well as other components of Ca2+-signalling machinery are distributed very unevenly throughout the endoplasmic reticulum. In particular in Purkinje neurones InsP3Rs can be packed in stacks of parallel cisternae at a density about 100 times higher than in the rest of the endoplasmic reticulum and are practically absent in some other regions (Ross et al. 1989; Satoh et al. 1990). InsP3Rs in Purkinje neurones were found in perinuclear cisternae although the exact localization of the receptors to the inner or outer nuclear membrane was uncertain.
The localization and orientation of the Ca2+-release channels in the nuclear membrane that we report suggest that the nuclear envelope in Purkinje neurones is specialized to release Ca2+ directly into the nucleoplasm. This is the first evidence for the existence of intranuclear sources of Ca2+ in neurones. Ca2+ release from the nuclear envelope may amplify Ca2+ signals penetrating into the nucleus from the cytoplasm or generate Ca2+ transients in the nucleus independently from the cytoplasm. This hypothesis also makes it possible to explain the distribution of InsP3Rs in the nuclear membrane – InsP3Rs in the outer nuclear membrane would release Ca2+ into the cytoplasm and therefore would reduce Ca2+ transients inside the nucleus.
Purkinje neurones predominantly express type 1 InsP3Rs although small amounts (∼1–3%) of type 2 and 3 receptors have also been reported (De Smedt et al. 1994; Taylor et al. 1999). Nuclear receptors comprise only a small fraction of the total pool of InsP3Rs in Purkinje neurones and therefore their identity may differ from InsP3Rs of the rest of the cell. Different types of InsP3Rs have similar ion selectivity and conductance, but greatly vary in their sensitivity to InsP3 (Thrower et al. 2001). The InsP3Rs studied here had an EC50= 0.68 μm that is close to values (0.194–0.5 μm) reported for cerebellar type 1 InsP3Rs incorporated into artificial lipid bilayers, but differs from the EC50 of type 2 and 3 receptors (58 nm and 3.2 μm, respectively; Ramos-Franco et al. 1998; Hagar & Ehrlich, 2000). These data suggest that the InsP3Rs expressed in the inner nuclear membrane of Purkinje neurones are likely to be type 1.
Our values for EC50 (0.68 μm) and the Hill coefficient (2.5) of InsP3Rs in Purkinje neurone nuclei were somewhat higher than those reported for cerebellar InsP3Rs incorporated into artificial lipid bilayers (0.194 μm and 0.96 reported by Ramos-Franco et al. 1998; 0.5 μm and 1.7 reported by Hagar & Ehrlich, 2000). InsP3Rs have several putative phosphorylation sites (Thrower et al. 2001). It has been reported that phosphorylation by protein kinase A of recombinant type 1 InsP3Rs expressed in insect cells increased the Po of the channels incorporated into artificial lipid bilayers more than 10-fold (from < 2–3% to 30–40%) and increased their sensitivity to InsP3 about 4-fold (Tang et al. 2003). Different level of phosphorylation may in principle explain differences in EC50 reported by different authors. Tang et al. (2003) estimated the EC50 of phosphorylated InsP3Rs to be < 50 nm. If the values obtained for heterologously expressed InsP3Rs hold for native mammalian receptors then because of the high EC50 of the nuclear InsP3Rs they must have been dephosphorylated. On the other hand, the Po of the nuclear InsP3Rs with Ba2+ as the current carrier was 0.32 which is close to the Po of phosphorylated recombinant InsP3Rs. Therefore most of the nuclear InsP3Rs were apparently phosphorylated. These data suggest that the properties of heterologously expressed and native InsP3Rs may, at least quantitatively, differ. The cause of this discrepancy is unknown and the effects of phosphorylation on native InsP3Rs need further investigation.
Experiments on permeabilized cells and with flash photolysis of caged InsP3 in cerebellar slices have demonstrated that InsP3Rs in the cytoplasm of Purkinje neurones are 20–50 times less sensitive to InsP3 than the same receptors incorporated into artificial lipid bilayers (Khodakhah & Ogden, 1993; Fujiwara et al. 2001). The reason for this difference is not known and may result from the presence in Purkinje neurones of intrinsic inhibitors of InsP3Rs (Yang et al. 2002; Haynes et al. 2004; Kasri et al. 2004). In contrast to cytoplasmic receptors, InsP3Rs in the native nuclear membrane of Purkinje neurones were highly sensitive to InsP3. These data suggest that the nuclear Ca2+ store of Purkinje neurones has a much lower threshold for activation by InsP3 than the rest of the endoplasmic reticulum. Similar differences in the sensitivity of nuclear and endoplasmic reticulum InsP3Rs have been reported in a liver cell line, but in that case it was achieved by expression of different types of InsP3Rs (Leite et al. 2003).
Several groups have reported that mammalian type 1 InsP3Rs were activated by low (< 300 nm) and inhibited by higher Ca2+ concentrations (Iino, 1990; Bezprozvanny et al. 1991; Finch et al. 1991). In experiments on Xenopus oocytes Mak et al. (1998) found that an increase in InsP3 concentration about completely eliminated the inhibitory effect of Ca2+. In our experiments Ca2+ inhibited InsP3Rs with the same efficiency both at low (0.3 μm, ∼7% of Pmax) and saturated (10 μm) InsP3 concentrations. Therefore the inhibitory effect of Ca2+ on Purkinje neurone nuclear InsP3Rs did not depend on the InsP3 concentration. Experiments with flash photolysis of caged InsP3 in Purkinje neurones support these data. It has been shown that Ca2+ entry through plasmalemmal Ca2+ channels strongly suppressed Ca2+ release from stores induced by high (25 μm) InsP3 concentrations (Khodakhah & Ogden, 1995). Recombinant type 1 InsP3Rs expressed in insect cells also demonstrated a bell-shaped Ca2+ dependence at saturated InsP3 concentrations and it was not affected by phosphorylation (Tang et al. 2003).
There is also other evidence that Xenopus InsP3Rs are not functionally identical to mammalian type 1 receptors. Data presented by Mak et al. (1998) suggest that Xenopus oocyte InsP3Rs are much more sensitive to InsP3 than mammalian type 1 receptors. The authors estimated the EC50 of Xenopus InsP3Rs to be 50 nm, less by about an order of magnitude than the EC50 of mammalian type 1 InsP3Rs. Another difference between the two receptors is the very high open probability of Xenopus InsP3Rs (Pmax∼ 0.8) with long (> 1 s) open bursts, whereas in our experiments in similar ion conditions (K+ as the current carrier) Pmax= 0.034 and no long-lasting bursts of activity were observed. Studies on type 1 InsP3Rs incorporated into artificial lipid bilayers also report Pmax in the range of a few per cent, although these data were obtained in non-physiological ionic conditions (Ramos-Franco et al. 1998; Hagar & Ehrlich, 2000).
Xenopus oocyte InsP3R genes are highly homologous although not identical to mammalian type 1 InsP3Rs (∼93%; Taylor et al. 1999). Therefore the discrepancies in properties of InsP3Rs in Xenopus oocytes and Purkinje neurones may result from structural differences between the receptors. An alternative explanation of the differences is suggested by the report that purified cerebellar InsP3Rs incorporated into artificial lipid bilayers were not inhibited by Ca2+ and therefore the inhibition of InsP3Rs by Ca2+ may depend on accessory proteins (Michikawa et al. 1999). The assortment of these proteins may be different in Xenopus and mammalian cells and this may be the reason for the differences in the properties of InsP3Rs in these cells.
A decrease in the inhibitory effect of Ca2+ was also reported in cerebellar InsP3Rs incorporated into artificial lipid bilayers, but this effect was observed at unusually high InsP3 concentrations (180 μm) and the physiological relevance of this observation is unclear (Kaftan et al. 1997; Thrower et al. 2001).
An important effect of saturated InsP3 concentrations was the high activity of InsP3Rs at resting levels of [Ca2+]i. At [Ca2+]i= 100 nm InsP3R activity reached about 46% of Pmax at 10 μm InsP3, compared with about 17% of Pmax at 0.3 μm InsP3. Taking into account that Pmax at 0.3 μm InsP3 comprised only 7% of that at 10 μm[InsP3] the absolute activity of InsP3Rs at resting [Ca2+]i rose about 40 times with the rise in InsP3 concentration. The bell-shaped Ca2+ dependence of InsP3Rs is considered to be the cause of Ca2+ oscillations seen in many cells (Bezprozvanny et al. 1991; Thrower et al. 2001). Ca2+ oscillations are usually observed at low levels of agonist stimulation whereas stronger stimuli evoke a biphasic Ca2+ response (a Ca2+ spike followed by a smaller steady-state rise in [Ca2+]i). This effect was explained by Mak et al. (1998) by the so called ‘tuning’ of the receptor, i.e. the elimination of the inhibitory effect of Ca2+ on InsP3Rs at saturated InsP3 concentrations generated by strong stimuli. Our data suggest an alternative explanation of this phenomenon – at large InsP3 concentrations high activity of InsP3Rs even at low [Ca2+]i makes the sequestration of Ca2+ in the endoplasmic reticulum ineffective and produces biphasic rather than oscillatory responses to agonists.
Yang et al. (2002) reported that a family of neuronal Ca2+-binding proteins (CaBP) interacts with InsP3-binding region of InsP3Rs and showed that a member of this CaBP1 family is an agonist of InsP3Rs of the outer nuclear membrane of Xenopus oocytes. They also demonstrated that CaBP1 is highly expressed and co-localized with InsP3Rs in Purkinje neurones. The authors proposed that CaBP1 is a protein agonist of InsP3Rs that enables the channel to be involved in Ca2+-induced Ca2+ release in the absence of InsP3 in a manner similar to ryanodine receptors. In our experiments Ca2+ alone, in the absence of InsP3, was unable to activate InsP3Rs. CaBP1 is a membrane-localized protein and it is unlikely that it was washed-out during nucleus isolation. These data suggest that CaBP1 is not involved in regulation of nuclear InsP3Rs in Purkinje neurones. There are several other reports that indicate that InsP3-independent activation of InsP3Rs is not a universal property of cerebellar InsP3Rs. A local rise in InsP3 in Purkinje neurone dendrites due to synaptic activation of metabotropic glutamate receptors or flash photolysis of caged InsP3 evokes an increase in [Ca2+]i restricted to the area of stimulation without a significant regenerative propagation of the Ca2+ signal, in spite of the reported presence of high levels of CaBP1 throughout the dendritic tree (Finch & Augustine, 1998; Takechi et al. 1998). In experiments on other mammalian cells CaBP1 inhibited agonist-evoked InsP3-mediated Ca2+ signalling and no rise in InsP3-independent Ca2+-induced Ca2+ release was reported by the authors (Haynes et al. 2004; Kasri et al. 2004). Therefore we, and other authors also, have not obtained any data confirming the agonist action of CaBP1, and its role in InsP3R regulation remains contentious.
Nuclear envelopes from Purkinje neurones also contained numerous spontaneously active large-conductance channels. The channels had high open probability and were permeable to K+ and some other monovalent cations, but impermeable to Ca2+. Large-conductance cationic channels resembling the nuclear channels of Purkinje neurones have previously been reported in the sarcoplasmic reticulum of different cells (see, e.g. Picard et al. 2002). High permeability to K+ is generally characteristic of the membrane of endo(sarco)plasmic reticulum Ca2+ stores. These channels are thought to provide a route for counterflow of K+ to prevent changes in the membrane potential that may arise due to movements of Ca2+ through the endoplasmic reticulum membrane. The large-conductance cationic channels in the nuclear membrane of Purkinje neurones may have the same function. This assumption is consistent with the fact that the channels are expressed in InsP3-sensitive nuclear envelopes of Purkinje neurones, but not in the nuclear envelopes of granule neurones.
As we have already mentioned above, InsP3-activated Ca2+ channels have been recorded in the outer nuclear membrane of Xenopus oocytes (Stehno-Bittel et al. 1995; Mak et al. 1998). No native InsP3-activated channels have until now been recorded in the nuclear membrane of mammalian cells (Mazzanti et al. 2001). On the other hand fluorescent Ca2+ measurements indicate that the nuclear envelope of liver and a number of other mammalian cells are InsP3-sensitive Ca2+ stores (Nicotera et al. 1990; Gerasimenko et al. 1995). Incorporation of the nuclear membranes from a hepatocyte cell line into artificial lipid bilayers also revealed the presence of InsP3Rs (Leite et al. 2003). The distribution of InsP3Rs in mammalian nuclei has not been unequivocally established. InsP3 binding sites were found in the internal nuclear membrane of hepatocytes (Humbert et al. 1996). It has recently been reported that InsP3 can release Ca2+ from protrusions of inner nuclear membrane in the nucleoplasm of a hepatocellular carcinoma cell line, indicating the presence of functional InsP3Rs inside the nucleus (Echevarría et al. 2003). On the other hand, careful analysis of Ca2+ signalling in HeLa cells showed no sources of Ca2+ inside the nucleus (Lipp et al. 1997).
These discrepancies may, at least partially, be explained by different mechanisms of Ca2+ regulation in the nuclei of different cells. In our experiments we were unable to record Ca2+ channels in the nuclear membrane of cerebellar granule neurones. Therefore different types of neurones may have distinct mechanisms of regulating Ca2+ in the nucleoplasm. The reason for these differences between neurones is unknown. One possibility is that Ca2+ channels in the internal nuclear membrane are expressed only in neurones where nuclear Ca2+ signalling plays an important role and larger Ca2+ transients may be required to trigger downstream events. Alternatively, the existence of a source of Ca2+ inside the nucleus of Purkinje neurones may result from the large size of these nuclei. The nucleus of Purkinje neurones is about twice as large in diameter and 8 times larger in volume than the nucleus of granule neurones. The inflow from the cytoplasm may by itself be insufficient to fill this volume with the necessary amount of Ca2+.
InsP3 for modulating the Ca2+ excitability of InsP3Rs in the inner nuclear membrane may come from two sources. It may be synthesized in the plasma membrane and enter the nucleus from the cytoplasm through nuclear pores. In neurones this possibility is limited by the fact that only a small fraction of InsP3 synthesized in neurones can reach the nucleus, because the vast majority of the InsP3-synthesizing metabotropic receptors are located in dendrites and produce only a local rise in InsP3 concentration (Finch & Augustine, 1998; Takechi et al. 1998). The alternative possibility is that InsP3 may be synthesized inside the nucleus. It has been reported that the nucleus contains a complete set of enzymes of InsP3 metabolism (D'Santos et al. 1998; Irvine, 2000). The regulation of these enzymes is poorly understood. In Swiss 3T3 cells stimulation of insulin-like growth factor I (IGF-I) receptors activates inositide metabolism in the nucleus. IGF-I is present in climbing fibres, is released by their electrical stimulation and Purkinje neurones express IGF-I receptors (Ito, 2001). Therefore InsP3 levels in the nucleus of Purkinje neurones may be regulated by IGF-I and/or other neuromodulators independently from the cytoplasm, switching on and off the nuclear envelope Ca2+ store.
The physiological role of nuclear Ca2+ signalling has not been unequivocally established and may be different in different cells. As we have already mentioned in the introduction, nuclei contain Ca2+-sensitive transcription factors and therefore a rise in nuclear Ca2+ can affect gene transcription. It has been hypothesized that nuclear Ca2+ may be involved in the formation of transcription-dependent neuronal plasticity (Bading, 2000). A rise in nuclear Ca2+ controls CREB-mediated gene expression triggered by synaptic activity (Hardingham et al. 2001). In Purkinje neurones associated stimulation of synaptic inputs from climbing and parallel fibres evokes long-term depression (LTD) of synaptic transmission at excitatory parallel fibre synapses (reviewed by Ito, 2001). LTD induction is Ca2+ dependent. Mice with a disrupted type 1 InsP3R gene completely lack LTD suggesting an important role of InsP3Rs in LTD formation (Inoue et al. 1998). It has been shown that the late phase of cerebellar LTD requires protein synthesis (Linden, 1996). Inhibition of CREB or CaMKIV function eliminated or strongly suppressed the late phase of LTD (Ahn et al. 1999; Ho et al. 2000). Therefore the formation of the late phase of LTD depends on Ca2+-sensitive transcription factors. InsP3Rs in the internal nuclear membrane of Purkinje neurones may be involved in regulation of nuclear Ca2+ metabolism and induction of late phase of LTD.
Acknowledgments
We thank Dr Christof J. Schwiening for image analysis software and Jon Holdich for expert technical help. This work was supported by a grant from the Wellcome Trust (S.M. and R.C.T.).
Supplemental material
The online version of this paper can be accessed at: 10.1113/jphysiol.2004.081299 http://jp.physoc.org/cgi/content/full/jphysiol.2004.081299/DC1 and contains five supplemental figures.
This material can also be found as part of the full-text HTMl version available from http://www.blackwell-synergy.com
References
- Ahn S, Ginty DD, Linden DJ. A late phase of cerebellar long-term depression requires activation of CaMKIV and CREB. Neuron. 1999;23:559–568. doi: 10.1016/s0896-6273(00)80808-9. [DOI] [PubMed] [Google Scholar]
- Bading H. Transcription-dependent neuronal plasticity. Eur J Biochem. 2000;267:5280–5283. doi: 10.1046/j.1432-1327.2000.01565.x. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Ehrlich BE. Inositol 1,4,5-trisphosphate InsP3-gated Ca2+ channels from cerebellum, conduction properties for divalent cations and regulation by intraluminal calcium. J General Physiol. 1994;104:821–856. doi: 10.1085/jgp.104.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins (1,4,5) P3 and calcium gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Blobel G, Potter VR. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966;154:1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Boehning D, Mak DO, Foskett JK, Joseph SK. Molecular determinants of ion permeation and selectivity in inositol 1,4,5-trisphosphate receptor Ca2+ channels. J Biol Chem. 2001;276:13509–13512. doi: 10.1074/jbc.C100094200. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Thomas D, Tovey SC, Berridge MJ, Lipp P. Nuclear calcium signalling. Cell Mol Life Sci. 2000;57:371–378. doi: 10.1007/PL00000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Beaman-Hall CM, Vallano ML. Granule neurons in cerebellum express distinct splice variants of the inositol trisphosphate receptor that are modulated by calcium. Am J Physiol Cell Physiol. 2004;287:C971–980. doi: 10.1152/ajpcell.00571.2003. [DOI] [PubMed] [Google Scholar]
- De Smedt H, Missiaen L, Parysn JB, Bootman MD, Mertenss L, Van Den Boschn L, Casteels R. Determination of relative amounts of inositol trisphosphate receptor mRNA isoforms by ratio polymerase chain reaction. J Biol Chem. 1994;269:21691–21698. [PubMed] [Google Scholar]
- D'Santos CS, Clarke JH, Divecha N. Phospholipid signaling in the nucleus. Biochim Biophys Acta. 1998;1436:201–232. doi: 10.1016/s0005-2760(98)00146-5. [DOI] [PubMed] [Google Scholar]
- Echevarría W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nature Cell Biol. 2003;5:440–446. doi: 10.1038/ncb980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- Finch EA, Turner TJ, Goldin SM. Calcium as a co-agonist of inositol trisphosphate induced calcium release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Franco-Obregon A, Wang HW, Clapham DE. Distinct ion channel classes are expressed on the outer nuclear envelope of T- and B-lymphocyte cell lines. Biophys J. 2000;79:202–214. doi: 10.1016/S0006-3495(00)76284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara A, Hirose K, Yamazawa T, Iino M. Reduced IP3 sensitivity of IP3 receptor in Purkinje neurons. NeuroReport. 2001;12:2647–2651. doi: 10.1097/00001756-200108280-00012. [DOI] [PubMed] [Google Scholar]
- Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995;80:439–444. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]
- Hagar RE, Ehrlich BE. Regulation of the type III InsP3 receptor by InsP3 and ATP. Biophys J. 2000;79:271–278. doi: 10.1016/S0006-3495(00)76289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- Haynes LP, Tepikin AV, Burgoyne RD. Calcium-binding protein 1 is an inhibitor of agonist-evoked, inositol 1,4,5-trisphosphate-mediated calcium signaling. J Biol Chem. 2004;279:547–555. doi: 10.1074/jbc.M309617200. [DOI] [PubMed] [Google Scholar]
- Ho N, Liauw JA, Blaeser F, Wei F, Hanissian S, Muglia LM, et al. Impaired synaptic plasticity and cAMP response element-binding protein activation in Ca2+/calmodulin-dependent protein kinase type IV/Gr-deficient mice. J Neurosci. 2000;20:6459–6472. doi: 10.1523/JNEUROSCI.20-17-06459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert J-P, Matter N, Artault J-C, Köppler P, Malviya AN. Inositol 1,4,5-trisphosphate receptor is located to the inner nuclear membrane vindicating regulation of nuclear calcium signaling by inositol 1,4,5-trisphosphate. J Biol Chem. 1996;271:478–485. doi: 10.1074/jbc.271.1.478. [DOI] [PubMed] [Google Scholar]
- Iino M. Biphasic calcium dependence of inositol trisphosphate induced calcium release in smooth muscle cells of the guinea pig Taenia caeci. J General Physiol. 1990;95:1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Kato K, Kohda K, Mikoshiba K. Type 1 inositol 1,4,5-trisphosphate receptor is required for induction of long-term depression in cerebellar Purkinje neurons. J Neurosci. 1998;18:5366–5373. doi: 10.1523/JNEUROSCI.18-14-05366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. Nuclear lipid signaling. Sci STKE. 2000;48:RE1. doi: 10.1126/stke.2000.48.re1. 10.1126/stke.2002.150.re13. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Kaftan EJ, Ehrlich BE, Watras J. Inositol 1,4,5-trisphosphate (InsP3) and calcium interact to increase the dynamic range of InsP3 receptor-dependent calcium signaling. J General Physiol. 1997;110:529–538. doi: 10.1085/jgp.110.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasri NN, Holmes AM, Bultynck G, Parys JB, Bootman MD, Rietdorf K, et al. Regulation of InsP3 receptor activity by neuronal Ca2+-binding proteins. EMBO J. 2004;23:312–321. doi: 10.1038/sj.emboj.7600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal ER, Schwartz JH, Jessel TM. Principles of Neuroscience. USA: McGraw-Hill/Appleton & Lange; 2000. [Google Scholar]
- Khodakhah K, Ogden D. Functional heterogeneity of calcium release by inositol trisphosphate in single Purkinje neurones, cultured cerebellar astrocytes, and peripheral tissues. Proc Natl Acad Sci U S A. 1993;90:4976–4980. doi: 10.1073/pnas.90.11.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodakhah K, Ogden D. Fast activation and inactivation of inositol trisphosphate-evoked Ca2+ release in rat cerebellar Purkinje neurones. J Physiol. 1995;487:343–358. doi: 10.1113/jphysiol.1995.sp020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite MF, Thrower EC, Echevarria W, Koulen P, Hirata K, Bennett AM, et al. Nuclear and cytosolic calcium are regulated independently. Proc Natl Acad Sci U S A. 2003;100:2975–2980. doi: 10.1073/pnas.0536590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DJ. A protein synthesis-dependent late phase of cerebellar long-term depression. Neuron. 1996;17:483–490. doi: 10.1016/s0896-6273(00)80180-4. [DOI] [PubMed] [Google Scholar]
- Lipp P, Thomas D, Berridge M, Bootman M. Nuclear calcium signalling by individual cytoplasmic calcium puffs. EMBO J. 1997;16:7166–7173. doi: 10.1093/emboj/16.23.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak DO, McBride S, Foskett JK. Inositol 1,4,5-trisphosphate activation of inositol tris-phosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci U S A. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M, Bustamante JO, Oberleithner H. Electrical dimension of the nuclear envelope. Physiol Rev. 2001;81:1–19. doi: 10.1152/physrev.2001.81.1.1. [DOI] [PubMed] [Google Scholar]
- Meldolesi J, Pozzan T. The heterogeneity of ER Ca2+ stores has a key role in nonmuscle cell signalling and function. J Cell Biol. 1998;142:1395–1398. doi: 10.1083/jcb.142.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michikawa T, Hirota J, Kawano S, Hiraoka M, Yamada M, Furuichi T, Mikoshiba K. Calmodulin mediates calcium-dependent inactivation of the cerebellar type 1 inositol 1,4,5-trisphosphate receptor. Neuron. 1999;23:799–808. doi: 10.1016/s0896-6273(01)80037-4. [DOI] [PubMed] [Google Scholar]
- Miedema H. Surface potentials and the calculated selectivity of ion channels. Biophys J. 2002;82:156–159. doi: 10.1016/S0006-3495(02)75382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P, Orrenius S, Nilsson T, Berggren PO. An inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in liver nuclei. Proc Natl Acad Sci U S A. 1990;87:6858–6862. doi: 10.1073/pnas.87.17.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp S, Dziak E, Michalak M, Opas M. Is all of the endoplasmic reticulum created equal? The effects of the heterogeneous distribution of endoplasmic reticulum Ca2+-handling proteins. J Cell Biol. 2003;160:475–479. doi: 10.1083/jcb.200207136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard L, Cote K, Teijeira J, Greentree D, Rousseau E. Sarcoplasmic reticulum K+ channels from human and sheep atrial cells display a specific electro-pharmacological profile. J Mol Cell Cardiol. 2002;34:1163–1172. doi: 10.1006/jmcc.2002.2041. [DOI] [PubMed] [Google Scholar]
- Power JM, Sah P. Nuclear calcium signaling evoked by cholinergic stimulation in hippocampal CA1 pyramidal neurons. J Neurosci. 2002;22:3454–3462. doi: 10.1523/JNEUROSCI.22-09-03454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–637. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Ramos-Franco J, Fill M, Mignery GA. Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophys J. 1998;75:834–839. doi: 10.1016/S0006-3495(98)77572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Meldolesi J, Milner TA, Satoh T, Supattapone S, Snyder SH. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature. 1989;339:468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- Santella L, Carafoli E. Calcium signaling in the cell nucleus. FASEB J. 1997;11:1091–1109. [PubMed] [Google Scholar]
- Satoh T, Ross CA, Villa A, Supattapone S, Pozzan T, Snyder SH, Meldolesi J. The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cells: quantitative immunogold labeling reveals concentration in an ER subcompartment. J Cell Biol. 1990;111:615–624. doi: 10.1083/jcb.111.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram B, Mahaut-Smith MP. A novel monovalent cation channel activated by inositol trisphosphate in the plasma membrane of rat megakaryocytes. J Biol Chem. 1995;270:16638–16644. doi: 10.1074/jbc.270.28.16638. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L, Luckhoff A, Clapham DE. Calcium release from the nucleus by InsP3 receptor channels. Neuron. 1995;14:163–167. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- Takechi H, Eilers J, Konnerth A. A new class of synaptic response involving calcium release in dendritic spines. Nature. 1998;396:757–760. doi: 10.1038/25547. [DOI] [PubMed] [Google Scholar]
- Tang TS, Tu H, Wang Z, Bezprozvanny I. Modulation of type 1 inositol (1,4,5) -trisphosphate receptor function by protein kinase A and protein phosphatase 1α. J Neurosci. 2003;23:403–415. doi: 10.1523/JNEUROSCI.23-02-00403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Genazzani AA, Morris SA. Expression of inositol trisphosphate receptors. Cell Calcium. 1999;26:237–251. doi: 10.1054/ceca.1999.0090. [DOI] [PubMed] [Google Scholar]
- Thrower EC, Hagar RE, Ehrlich BE. Regulation of Ins (1,4,5) P3 receptor isoforms by endogenous modulators. Trends Pharmacol Sci. 2001;22:580–586. doi: 10.1016/s0165-6147(00)01809-5. [DOI] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nature Rev Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- Yang J, McBride S, Mak DD, Vardi N, Palczewski K, Haeseleer F, Foskett JK. Identification of a family of calcium sensors as protein ligands of inositol trisphosphate receptor Ca2+ release channels. Proc Natl Acad Sci U S A. 2002;99:7711–7716. doi: 10.1073/pnas.102006299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.