Abstract
To reveal the role of clock genes in generating the circadian rhythm of baroreflexes, we continuously measured mean arterial pressure and baroreflex sensitivity in free-moving normal wild-type mice, and in Cry-deficient mice which lack a circadian rhythm, in constant darkness for 24 h. In wild-type mice the mean arterial pressure was higher at night than during the day, and was accompanied by a significantly enhanced baroreflex sensitivity of −13.6 ± 0.8 at night compared with −9.7 ± 0.7 beats min−1 mmHg−1 during the day (P < 0.001). On the other hand, diurnal changes in arterial pressure disappeared in Cry-deficient mice with remarkably enhanced baroreflex sensitivity compared with wild-type mice (P < 0.001): −21.9 ± 1.6 at night and −23.1 ± 2.1 beats min−1 mmHg−1 during the day. Moreover, the mean arterial pressure response to 10 μg kg−1 of phenylephrine, an α1-adrenoceptor agonist, was severely suppressed in Cry-deficient mice regardless of time, while that for the wild-type mice was 10.1 ± 1.9 mmHg in the night, significantly lower than 22.0 ± 3.5 mmHg in the day (P < 0.01). These results suggest that CRY genes are involved in generating the circadian rhythm of baroreflex sensitivity, partially by regulating α1-adrenoceptor-mediated vasoconstriction in peripheral vessels.
It has been reported that baroreflexes are involved in generating the circadian rhythm of cardiovascular variability (Makino et al. 1997; Di Rienzo et al. 2001). On the other hand, in mammals, the oscillation of circadian clock genes has been discovered not only in the suprachiasmatic nucleus of the hypothalamus (Okamura et al. 1999) but also in peripheral tissues (Maemura et al. 2000; Nonaka et al. 2001; Yagita et al. 2001; Oishi et al. 2003); however, there have been few studies demonstrating how these genes are associated with the circadian rhythm of baroreflex sensitivity.
Arterial pressure is controlled by baroreflexes in the cardiovascular centre of the medulla regulating efferent signals to the heart and the peripheral vessels according to input from the baroreceptors in the systemic circulation. Since the feedback gain of baroreflexes is further controlled by the upper brain regions (Rowell et al. 1996), and since clock genes are expressed in these regions (Masubuchi et al. 2000; Okamura et al. 2002), the central clocks are likely to be involved in the circadian rhythm of baroreflex sensitivity. On the other hand, it has been suggested that α-adrenoceptor responsiveness in the peripheral vessels shows circadian rhythm (Gohar et al. 1992; Keskil et al. 1996), probably associated with the expression of peripheral clock genes (Nonaka et al. 2001). However, there have been no attempts to assess whether central or peripheral clock genes, or both, are involved in the circadian rhythm of baroreflex sensitivity.
Recently, we reported that the baroreflex control of heart rate (HR) was enhanced to stabilize arterial pressure in mice that were genetically deficient in calponin in the vascular smooth muscles, calponin being an intracellular mediator of α-agonist-induced vasoconstriction (Masuki et al. 2003a), but with normal clock genes. More recently, Masuki et al. (2005) reported in human subjects that the baroreflex control of HR was enhanced with attenuated α-adrenoceptor responsiveness at a given time of day. These enhancements compensated for the reduced sensitivity of α-adrenoceptor responsiveness in the peripheral vessels. Therefore, we postulated that the circadian rhythm of baroreflex sensitivity would be a secondary adaptation to the primary change in α-adrenoceptor-mediated vasoconstrictor responsiveness, which may be caused by the oscillatory expression of peripheral clock genes.
In this study, we hypothesized that if the circadian rhythm of α-adrenoceptor responsiveness is controlled by clock genes in the peripheral vessels, and if that of baroreflex sensitivity is controlled by other mechanisms unrelated to clock genes, the α-adrenoceptor responsiveness would be severely suppressed in animals genetically deficient in a biological clock while arterial pressure would be well maintained by enhanced baroreflex sensitivity. To examine these hypotheses, we measured the circadian rhythms of baroreflex sensitivity and α-adrenoceptor responsiveness in free-moving Cry1−/−Cry2−/− mice, genetically deficient in Cry1 and Cry2, since Cry1 and Cry2 genes are indispensable for the molecular core oscillator function of circadian clocks in peripheral tissues (Yagita et al. 2001) as well as in the suprachiasmatic nuclei (Okamura et al. 1999).
Methods
Animals
We used C57BL/6J mice lacking Cry1 and Cry2 genes (Cry-deficient (Cry1−/−Cry2−/−) mice) and mice carrying normal Cry1 and Cry2 genes (wild-type mice). Cry1−/− mice were generated as previously described (Vitaterna et al. 1999). To generate Cry2−/− mice, a phage clone carrying a genomic fragment overlapping Cry2 was isolated by screening a lambda dash mouse genomic library for the 129/Sv strain. The clone carries the C-terminal half of Cry2 from exons 6 to 11. The nucleotide sequence of the genomic fragment after digestion with a restriction enzyme revealed that a 2.1 kb Sph I/Kpn I fragment carried exons 7, 8 and 9. We constructed a targeting vector that lacked the 2.1 kb region by subcloning the entire genomic fragment into the Not I site of pBluescript and then replacing the 2.1 kb region with a 6 kb Sph I/Kpn I fragment containing an En2-derived splice acceptor and Ires LacZ-Neo fusion gene (Mountford et al. 1994). The resultant targeting vector was used to transform E14tg2a mouse embryonic stem (ES) cells (Hooper et al. 1987) as previously described (Vitaterna et al. 1999).
Adult male mice were used, aged 9–11 weeks. Body weight was 28.0 ± 0.3 g in wild-type mice (n = 6) and 22.3 ± 1.6 g in Cry1−/−Cry2−/− mice (n = 5). The Cry1−/−Cry2−/− mice were significantly lighter than the wild-type mice (P < 0.01). They were housed at 25°C with food and water ad libitum under conditions of light from 7:00 to 19:00 h. The procedures used were approved by the Animal Ethics Committee of Shinshu University School of Medicine.
Catheterization used to measure arterial pressure
As reported previously (Masuki et al. 2003a), after anaesthetization with pentobarbital sodium (50 mg (kg body wt)−1, i.p.), a polyethylene catheter to measure mean arterial pressure (MAP) and HR was inserted into the left femoral artery so that the tip was positioned 5 mm below the left renal artery. The catheter was secured to the surrounding leg muscles, tunnelled subcutaneously and then exteriorized between the scapulae. The exteriorized catheter was connected to a cannula swivel (model TCS2-21; Tsumura, Tokyo, Japan), and the mouse was placed in a cage with a free-moving system (model FM-1121, Tsumura). The arterial catheter was flushed every day with 100 i.u. heparin in 0.2 ml saline.
Measurements
Cry1−/−Cry2−/− mice lose periodicity in wheel-running behaviour (van der Horst et al. 1999) as well as electrophysiological activity in the suprachiasmatic nucleus cells under constant darkness (Bonnefont et al. 2003). However, lights have a masking effect on the behaviour: the wheel-running activity is suppressed in the light, and a light–dark cycle results in daily rhythmicity. To exclude any effects of light on the rhythms and to assess mere effects of the circadian clock, we performed the experiments under constant darkness in both Cry1−/−Cry2−/− mice and wild-type mice.
Before the experiments, animals were housed in 12 h light: 12 h dark conditions for at least 1 week after implantation with a polyethylene catheter in the femoral artery to measure MAP and HR (Masuki et al. 2003a). After 36 h they were transferred to constant darkness and MAP and HR were measured for the next 24 h. Here we used the terms ‘day’ (7:00–19:00 h or circadian time (CT) 0–CT12) and ‘night’ (19:00–7:00 h or CT12–CT24) as ‘subjective day’ and ‘subjective night’, respectively, according to the normal biological clock in wild-type mice. Although Cry1−/−Cry2−/− mice completely lack a biological clock, we used ‘36–48 hours’ and ‘48–60 hours’ for day and night after the transition into constant darkness, respectively.
MAP was measured through a catheter connected to a pressure transducer (model TP-400T; Nihon Kohden, Tokyo, Japan). HR was counted from the arterial pressure pulse with a tachometer (model AT-601G, Nihon Kohden). They were recorded with a computer (OptiPlex GX260; Dell, Kawasaki, Japan) every 100 ms through a low-pass filter with an edge frequency of 1.5 Hz to remove pulsatile arterial pressure signals. Activity was monitored with locomotion sensors (model LCM-10M; Melquest, Toyama, Japan) and recorded at 2 min intervals. The criterion for adopting the resting data (Table 1) was activity of less than 5 counts (2 min)−1 for more than 20 min. The data were also used to calculate spontaneous baroreflex sensitivity (Table 2).
Table 1.
Mean arterial blood pressure (MAP) and heart rate (HR) in wild-type and Cry1−/–Cry2−/– mice at rest
| Wild-type (n = 6) | Cry1−/–Cry2−/– (n = 5) | |||
|---|---|---|---|---|
| Day | Night | Day | Night | |
| MAP | ||||
| Mean (mmHg) | 79.6 ± 3.6 | 85.5 ± 3.6** | 91.8 ± 4.0* | 89.9 ± 3.8* |
| aVariance (mmHg2) | 2.0 ± 0.4 | 1.7 ± 0.3 | 1.2 ± 0.2 | 1.2 ± 0.2 |
| HR | ||||
| Mean (beats min−1) | 435 ± 35 | 481 ± 27** | 529 ± 17* | 515 ± 23* |
| aVariance (beats2 min−2) | 167 ± 16 | 299 ± 30*** | 794 ± 225** | 614 ± 155* |
| Number of data (×104) | 18.8 ± 0.8 | 8.5 ± 1.0*** | 13.0 ± 1.7** | 13.1 ± 1.3** |
Values are means ± s.e.m.
Variance was calculated from the change in MAP (ΔMAP) or HR (ΔHR) from mean values every 4 s. Number of data, the number meeting the criterion for resting. Total number of data during the day or night was 43.2 × 104. Significant differences from wild-type mice during the day:
P < 0.05
P < 0.01
P < 0.001.
Table 2.
Spontaneous baroreflex sensitivity (ΔHR/ΔMAP) in wild-type and Cry1−/−Cry2−/− mice
| Day | Night | ||||||
|---|---|---|---|---|---|---|---|
| Mouse no. | ΔHR/ΔMAP | R2 | Number of data (×104) | ΔHR/ΔMAP | R2 | Number of data (×104) | |
| Wild-type | |||||||
| 1 | −9.0 | 0.29 | 12.1 | −14.4 | 0.28 | 5.7 | |
| 2 | −11.7 | 0.32 | 14.0 | −16.3 | 0.33 | 4.4 | |
| 3 | −10.6 | 0.33 | 10.1 | −14.5 | 0.25 | 3.5 | |
| 4 | −6.7 | 0.31 | 13.3 | −10.6 | 0.30 | 5.3 | |
| 5 | −9.8 | 0.39 | 14.4 | −12.8 | 0.33 | 6.1 | |
| 6 | −10.3 | 0.41 | 11.9 | −13.1 | 0.35 | 9.3 | |
| Mean ± s.e.m. | −9.7 ± 0.7 | 12.6 ± 0.6 | −13.6 ± 0.8*** | 5.7 ± 0.8*** | |||
| Cry1−/−Cry2−/− | |||||||
| 1 | −28.6 | 0.36 | 4.6 | −26.8 | 0.26 | 4.2 | |
| 2 | −25.1 | 0.32 | 8.9 | −22.4 | 0.27 | 8.5 | |
| 3 | −15.9 | 0.33 | 7.1 | −16.7 | 0.30 | 7.6 | |
| 4 | −22.3 | 0.23 | 8.6 | −21.5 | 0.24 | 6.8 | |
| 5 | −23.6 | 0.24 | 8.7 | −22.2 | 0.21 | 8.3 | |
| Mean ± s.e.m. | −23.1 ± 2.1*** | 7.6 ± 0.8*** | −21.9 ± 1.6*** | 7.1 ± 0.8*** | |||
ΔHR/ΔMAP, heart rate response to the spontaneous change in mean arterial pressure (beats min−1 mmHg−1). Number of data, the number meeting the criteria for inclusion in spontaneous baroreflex analyses. Total number of data during the day or night was 43.2 × 104. R2, square of correlation coefficient; ΔHR was highly correlated with ΔMAP in all mice at the level of P < 0.00001.
Significant differences from wild-type mice during the day, P < 0.001.
Spontaneous baroreflex sensitivity analyses
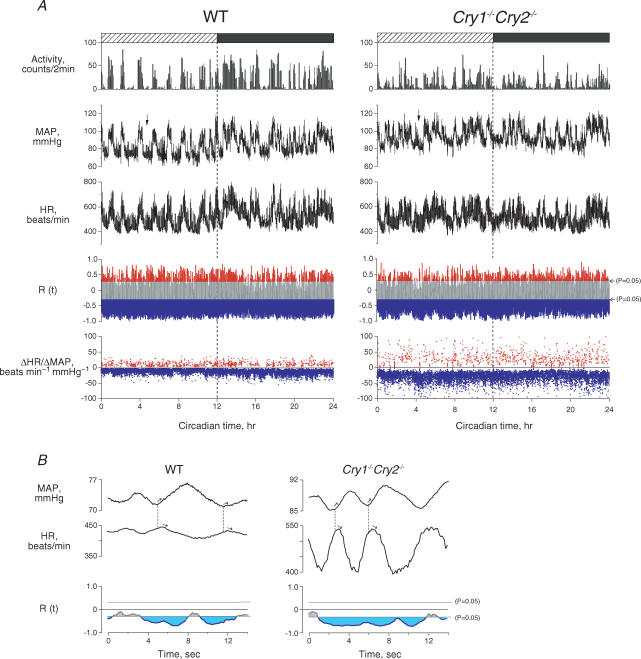
Since movement can reduce baroreflex sensitivity in mice (Masuki et al. 2003c), it was determined only when animals were resting. As in Fig. 1A, spontaneous baroreflex sensitivity was determined from HR change in response to the spontaneous change in MAP (ΔHR/ΔMAP) every 4 s, where a cross-correlation function (R(t)) between ΔMAP and ΔHR was below the line of P = 0.05 (blue), indicating significant negative correlation. Figure 1B shows ΔMAP and ΔHR for the parts indicated by the arrows in Fig. 1A on an enlarged scale. We found that a rise in MAP caused a fall in HR and inversely a fall in MAP caused a rise in HR after a 0.6 s delay, and the amplitude of the HR response to spontaneous change in MAP was enhanced in the Cry1−/−Cry2−/− mouse.
Figure 1. Twenty-four hour profiles of activity, MAP, HR and spontaneous baroreflex sensitivity (ΔHR/ΔMAP) in wild-type (WT) and Cry1−/−Cry2−/− mice.
A, typical example of measurements, from top to bottom: activity, MAP, HR, cross-correlation function (R(t)) between ΔMAP and ΔHR, ΔHR/ΔMAP in a WT (left) and a Cry1−/−Cry2−/− mice (right). R(t) above (red) and below (blue) the lines of P = 0.05 indicate significantly positive and negative correlations, respectively, which were used to determine positive (red) and negative (blue) ΔHR/ΔMAP. B, the areas indicated by arrows in A were enlarged to show dynamic change in MAP and HR. The HR response to a given change in MAP was greater in the Cry1−/−Cry2−/− mouse than in the WT mouse. R(t) in blue indicates a significantly negative correlation at P < 0.05, used to determine spontaneous baroreflex sensitivity (ΔHR/ΔMAP).
HR and MAP during the total resting period of ∼223 min (n =∼134 000) were used to determine spontaneous baroreflex sensitivity in each mouse at rest during the day and night. As spontaneous changes in MAP and consecutive changes in HR were observed at 5–15 cycles min−1 (4–12 s cycle−1) as shown in Fig. 1B, we analysed the relation between ΔMAP and ΔHR from the baselines every 4 s of the highest resolution time (τ) using the cross-correlation function given in the following formulas:
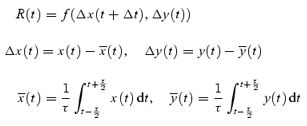 |
where R(t) is the cross-correlation coefficient between x (= MAP) and y (= HR) at the given time of t after correction for the delay time (Δt = 0.6 s) in response to HR change. The 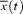 and
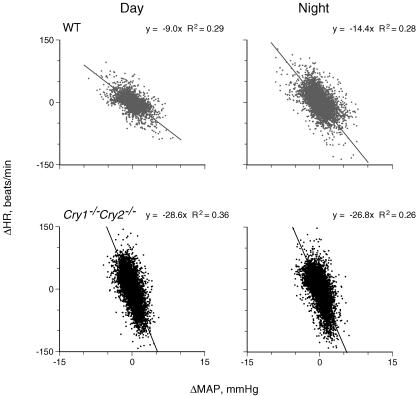
and 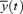 were averaged values of MAP and HR, respectively, from time t−τ/2 to t+τ/2 (τ= 4 s). Since the cross-correlation function between ΔMAP and ΔHR marked the highest value after correction for the delay time of 0.6 s in all mice, we used this time for the analyses. The detailed numerical analyses were reported previously (Masuki et al. 2003a). A regression equation was determined from the pooled data during rest in each mouse. The slope of the regression line was used as an index of spontaneous baroreflex sensitivity (Fig. 2).
were averaged values of MAP and HR, respectively, from time t−τ/2 to t+τ/2 (τ= 4 s). Since the cross-correlation function between ΔMAP and ΔHR marked the highest value after correction for the delay time of 0.6 s in all mice, we used this time for the analyses. The detailed numerical analyses were reported previously (Masuki et al. 2003a). A regression equation was determined from the pooled data during rest in each mouse. The slope of the regression line was used as an index of spontaneous baroreflex sensitivity (Fig. 2).
Figure 2. Spontaneous baroreflex sensitivity in wild-type (WT) and Cry1−/−Cry2−/− mice.
The slope (ΔHR/ΔMAP), the spontaneous baroreflex sensitivity, was determined from regression analyses (Brace, 1977) on data during periods where ΔMAP was negatively correlated with ΔHR (P < 0.05). The number of data was ∼83 000 in each graph. Note that ΔHR/ΔMAP is enhanced during the night in a WT mouse while the diurnal difference disappeared in a Cry1−/−Cry2−/− mouse with enhanced ΔHR/ΔMAP.
Variances for ΔMAP and ΔHR were also determined as an index of variability for MAP and HR (Table 1), where ΔMAP and ΔHR were calculated from the same data and equations as in the determination of spontaneous baroreflex sensitivity described above.
Drug-induced baroreflex sensitivity analyses and MAP responses to phenylephrine or sodium nitroprusside
Drug-induced baroreflex sensitivity was determined from the sigmoid response of HR to changes in MAP after an intra-arterial injection of phenylephrine to evoke peripheral α-adrenoceptor-mediated vasoconstriction or sodium nitroprusside for peripheral vasodilatation for each mouse in day and night, respectively. For 3 of 6 wild-type mice and 3 of 5 Cry1−/−Cry2−/− mice, baroreflex sensitivity in the day was determined at 10:00–12:00 h or CT3–CT5 on the 3rd day after exposure to constant darkness, and then baroreflex sensitivity in the night was determined at 22:00–24:00 h or CT15–CT17 on the same day. For the remaining mice in each group, baroreflex sensitivity was determined at CT15–CT17 in the night on the 3rd day, and then in the day at CT3–CT5 the following day. An alternative order for the determination was adopted to exclude any effects of systemic errors on baroreflex sensitivity due to consecutive drug injections. No significant differences were observed between the sensitivities determined by the two orders.
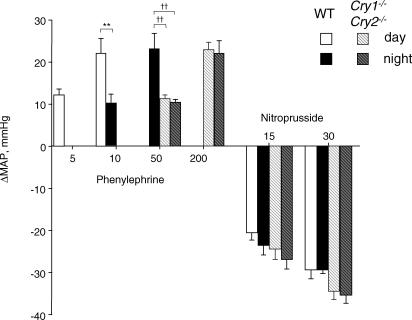
Before injecting the drugs, we confirmed that the animals were resting according to the range of MAP and HR in Table 1 and no activity was counted by locomotion sensors for at least 5 min. Then, 15 and 30 μg kg−1 of sodium nitroprusside, or 5 and 10 μg kg−1 of phenylephrine was injected using a 100 μl syringe (Hamilton, Reno, NV, USA) through the bifurcation of a Y-shaped tube (CMA/12; BAS, Tokyo) inserted into the catheter for arterial pressure measurement. Since MAP did not increase with 5 μg kg−1 of phenylephrine in the night for wild-type mice or with 10 μg kg−1 of phenylephrine in the day and night for Cry1−/−Cry2−/− mice, 10 μg kg−1 was initially injected in the night into wild-type mice, and 50 μg kg−1 in the day and night into Cry1−/−Cry2−/− mice. In addition, 50 μg kg−1 were injected in the night into wild-type mice and 200 μg kg−1 in the day and night into Cry1−/−Cry2−/− mice to increase MAP by ∼20 mmHg, as attained by 10 μg kg−1 in the day for wild-type mice. For drug-induced baroreflex analyses, we used the MAP response to phenylephrine seen at 10 μg kg−1 in the day and 50 μg kg−1 in the night for wild-type mice, and 200 μg kg−1 in the day and night for Cry1−/−Cry2−/− mice. See Fig. 4).
Figure 4. MAP response to 5, 10, 50 and 200 μg kg−1 of phenylephrine or 15 and 30 μg kg−1 of sodium nitroprusside.
Columns show means ± s.e.m. for 6 wild-type (WT) and 5 Cry1−/−Cry2−/− mice. **Significant difference from WT mice in the day at P < 0.01. ††Significant differences from WT mice in the night at P < 0.01.
Since the MAP responses to sodium nitroprusside did not change either in the night for wild-type or for Cry1−/−Cry2−/− mice, we measured the MAP response to 15 and 30 μg kg−1 of sodium nitroprusside in all conditions, and the response to 30 μg kg−1 was used for the baroreflex analyses. Each dose of sodium nitroprusside or phenylephrine was infused by one trial in each mouse during the day and night in a volume of < 10 μl. The responses were not measured until ∼1 s after the injection because one bifurcation of the Y-shaped tube connected to the pressure transducer was closed during injection into the other bifurcation. MAP and HR responses were recorded thereafter.
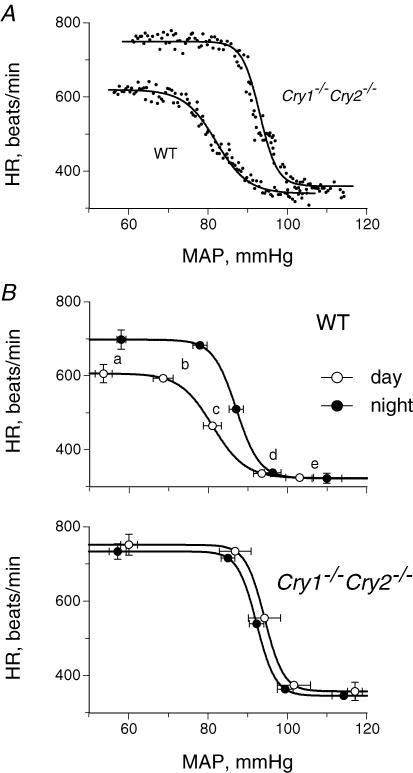
Drug-induced baroreflex curves, expressed as the relationship between MAP and HR, were analysed using a logistic sigmoid function according to the following equation (Kent et al. 1972; Merrill et al. 1996):
where α is the range between the upper and lower plateaux, e is an exponential function, β is a coefficient to calculate gain as a function of pressure, γ is MAP at the midpoint of the curve (midpoint), and δ is the lower plateau. Each parameter, the threshold pressure (lowest pressure that produces a significant decline in HR) and the saturation pressure (pressure necessary to achieve maximal inhibition of HR) were determined by fitting the equation to minimize the sum of the y distance between the experimental data (HR) and the y-value (HR) obtained by substituting the x-value (MAP) in the equation.
Statistics
Values are expressed as the means ± s.e.m. The differences in the means and variances of MAP, HR (Table 1), activity, spontaneous baroreflex sensitivity (Table 2), the number of data (Tables 1 and 2), MAP responses to phenylephrine or sodium nitroprusside (Fig. 4), and drug-induced baroreflex sensitivity (Table 3) were tested by a 2 (wild-type, Cry1−/−Cry2−/− mice) × 2 (day, night) ANOVA for repeated measures. Subsequent post hoc tests to determine significant differences in the various pair-wise comparisons were performed using Fisher's LSD. The null hypothesis was rejected at P < 0.05.
Table 3.
Parameters of drug-induced baroreflex sensitivity analyses
| Wild-type (n = 6) | Cry1−/−Cry2−/− (n = 5) | |||
|---|---|---|---|---|
| Day | Night | Day | Night | |
| Gain (beats min−1 mmHg−1) | −17.4 ± 2.0 | −32.1 ± 3.9** | −41.1 ± 3.2*** | −40.6 ± 1.4*** |
| Minimum (beats min−1) | 323 ± 10 | 323 ± 14 | 358 ± 25 | 346 ± 10 |
| Maximum (beats min−1) | 607 ± 25 | 698 ± 26*** | 752 ± 28** | 734 ± 21** |
| Saturation (mmHg) | 93.4 ± 2.1 | 96.2 ± 2.1 | 101.6 ± 4.2 | 99.4 ± 2.0 |
| Threshold (mmHg) | 68.6 ± 2.5 | 77.8 ± 1.8*** | 86.8 ± 4.0** | 85.0 ± 1.7*** |
| Midpoint (mmHg) | 81.0 ± 2.3 | 87.0 ± 1.8*** | 94.2 ± 4.0* | 92.2 ± 1.8** |
Values are means ± s.e.m. Significant differences from wild-type mice in the day:
P < 0.05
P < 0.01
P < 0.001.
Parameters that were used to develop the baroreflex curves in Fig. 3B are shown.
Results
Activity, MAP and HR
Activity in wild-type mice was 6769 ± 629 counts during the night, which was significantly higher than the 4205 ± 435 counts during the day (P = 0.018). On the other hand, these diurnal changes were abolished in Cry1−/−Cry2−/− mice: 4192 ± 885 counts during the day and 3700 ± 510 counts during the night (P = 0.40). The total activity for 24 h was 10 974 ± 785 counts in wild-type mice and 7892 ± 1348 counts in Cry1−/−Cry2−/− mice, with no significant differences between them (P = 0.069).
In both wild-type and Cry1−/−Cry2−/− mice, MAP and HR changed with activity throughout the day and night, increasing during movement and decreasing at rest (Fig. 1A). In the wild-type mouse, the baselines of MAP and HR were elevated during the night compared with the day (P < 0.007). In the Cry1−/−Cry2−/− mouse, these diurnal changes disappeared (P > 0.20) (Fig. 1A, Table 1), demonstrating that the circadian rhythms of MAP and HR were prominent in the wild-type mouse, but absent when CRY genes were absent.
In wild-type mice, HR variability at rest was twofold higher during the night than during the day (P = 0.0004), but there was no diurnal difference in MAP variability (P = 0.31) (Table 1), indicating that HR fluctuated more in response to spontaneous changes in MAP during the night. However, this diurnal difference in HR fluctuation disappeared in Cry1−/−Cry2−/− mice (P = 0.10), although HR in Cry1−/−Cry2−/− mice fluctuated more than in wild-type mice (P < 0.04) while MAP did not (P = 0.10) (Fig. 1B). Accordingly, we determined spontaneous baroreflex sensitivity by relating changes in HR (ΔHR) to spontaneous changes in MAP (ΔMAP).
Spontaneous baroreflex sensitivity
In wild-type mice, the number of data meeting the criteria for inclusion in spontaneous baroreflex analyses was significantly smaller during the night than during the day (P = 0.0009), but was not different in Cry1−/−Cry2−/− mice (P = 0.30) (Table 2). The total number of data meeting the criteria for inclusion in spontaneous baroreflex analyses during the whole day was not significantly different between wild-type and Cry1−/−Cry2−/− mice (P = 0.076). As in Fig. 2 and Table 2, spontaneous baroreflex sensitivity in wild-type mice was enhanced during the night compared with during the day (P = 0.0002), while this diurnal difference completely disappeared in Cry1−/−Cry2−/− mice (P = 0.15). Baroreflex sensitivity during the day and night in Cry1−/−Cry2−/− mice was remarkably enhanced compared with during the day for wild-type mice (P < 0.0005).
Drug-induced baroreflex sensitivity
These results were confirmed by the drug-induced baroreflex sensitivity determined from the sigmoid response of HR to changes in MAP after an intra-arterial injection of phenylephrine to evoke peripheral α-adrenoceptor-mediated vasoconstriction, or sodium nitroprusside for peripheral vasodilatation (Fig. 3). In wild-type mice, drug-induced baroreflex sensitivity (gain) in the night was enhanced twofold over that in the day with significantly higher values at the maximum, threshold, and midpoint (P< 0.0017) (Table 3). In addition, the HR range in the baroreflex curve (another index of baroreflex gain) for wild-type mice was 375 ± 28 beats min−1 in the night, which was significantly higher than the 283 ± 25 beats min−1 in the day (P = 0.0001). In contrast, for Cry1−/−Cry2−/− mice, there were no significant differences in the parameters between the day and night (P > 0.22). However, the sensitivity (gain) in the mice was significantly enhanced in the day and night compared with in the day for wild-type mice, with significantly higher values at the maximum, threshold, and midpoint (P < 0.015). In addition, the HR range in the baroreflex curve for Cry1−/−Cry2−/− mice was 394 ± 10 and 388 ± 12 beats min−1 in the day and night, respectively, significantly higher than in the day for wild-type mice (P = 0.0001).
Figure 3. The relationship between MAP and HR after an injection of phenylephrine or sodium nitroprusside.
A, typical examples of a wild-type (WT) and a Cry1−/−Cry2−/− mouse in the day. 111 measurements are presented for each mouse. The best-fit sigmoid baroreflex curve generated is also presented as described in the text. B, composite baroreflex curves and baroreflex parameters for WT and Cry1−/−Cry2−/− mice in the day and night are presented: a, maximum; b, threshold; c, midpoint; d, saturation; e, minimum. Points show means ± s.e.m. for 6 WT and 5 Cry1−/−Cry2−/− mice.
MAP responses to phenylephrine or sodium nitroprusside
In wild-type mice, the MAP increase as a result of the injection of 10 μg kg−1 of phenylephrine, an α1-adrenoceptor agonist, was smaller in the night (10 ± 2 mmHg at CT15–CT17) than in the day (22 ± 4 mmHg at CT3–CT5, P = 0.01) (Fig. 4). On the other hand, in Cry1−/−Cry2−/− mice, the injection of 10 μg kg−1 of phenylephrine did not increase MAP, and even 50 and 200 μg kg−1 of phenylephrine increased MAP by only 10–11 and 22–23 mmHg, respectively, in the day and night, with no diurnal differences in the response. In contrast, MAP responses to sodium nitroprusside in Cry1−/−Cry2−/− mice were almost identical to those in wild-type mice in the day and night (P > 0.09). Thus, the pressor responses to phenylephrine in wild-type mice were lower in the night than in the day, and were severely impaired in Cry1−/−Cry2−/− mice.
Discussion
The major findings in this study are (1) that MAP in wild-type mice was higher during the night than during the day, (2) the α-adrenoceptor-mediated pressor response to phenylephrine in the night for wild-type mice decreased to a half of that in the day, (3) the baroreflex sensitivities, determined by spontaneous or drug-induced methods, were both higher during the night for wild-type mice than those during the day, (4) these diurnal changes disappeared in Cry1−/−Cry2−/− mice, with a severely suppressed α-adrenoceptor-mediated pressor response to phenylephrine, and (5) baroreflex sensitivity was remarkably enhanced in Cry1−/−Cry2−/− mice.
In mammals, circadian rhythms are created by the hierarchical architecture of the central brain clock at the top and peripheral clocks in various organs at the bottom (Reppert & Weaver, 2002). In either the central or peripheral clocks, circadian clock genes are cyclically expressed by the circadian molecular core oscillator, which is composed of a set of common clock genes with an autoregulatory transcription–translation-based feedback loop (Balsalobre et al. 1998; Yagita et al. 2001). Nonaka et al. (2001) reported the circadian expression of clock genes in the aorta as well as the cultured vascular smooth muscle cells; however, there have been few studies demonstrating how these clock genes are involved in the circadian rhythm of arterial pressure regulation.
Reduced α-adrenoceptor responsiveness in Cry1−/−Cry2−/− mice
The extreme suppression of the α-adrenoceptor response in Cry1−/−Cry2−/− mice (Fig. 4) may be caused by an impaired intracellular pathway for α-adrenoceptor-mediated contraction of the vascular smooth muscle cells, including severely reduced expression of α-adrenoceptors. Indeed, clock genes are reportedly involved in the expression of various genes by their transcriptional mechanisms (Panda et al. 2002; Oishi et al. 2003). In addition, the expression of a set of peripheral clock genes in the liver was reportedly synchronized by electrical stimulation of the hepatic sympathetic nerve (Terazono et al. 2003). These results suggest that peripheral CRY genes contribute to circadian changes in arterial pressure regulation by primarily regulating α-adrenoceptor-mediated vasoconstriction in the peripheral vessels although it is unclear whether their expression is controlled by the central clock genes through sympathetic nervous input.
Another possible mechanism for the extreme suppression of the α-adrenoceptor response in Cry1−/−Cry2−/− mice is the down-regulation of α-adrenoceptors by sustained high sympathetic activity with enhanced baroreflex sensitivity. Experimentally, Grote et al. (2000) suggested that sustained higher sympathetic nerve activity and high plasma noradrenaline concentration causes the down-regulation of both α- and β-adrenoceptors. However, as shown in Tables 2 and 3, the HR response to spontaneous or drug-induced changes in MAP was much greater in Cry1−/−Cry2−/− mice than in wild-type mice during the day and night, indicating that the β-adrenoceptor response in the pacemaker cells of the heart was not reduced in Cry1−/−Cry2−/− mice, but was instead enhanced to compensate for attenuated peripheral α-adrenoceptor-mediated vasoconstriction to maintain MAP. These results suggest that the suppression of the α-adrenoceptor response in Cry1−/−Cry2−/− mice was caused by mechanisms specific for α-adrenoceptor-mediated vasoconstriction but not by non-specific mechanisms such as the low development of vascular smooth muscle. This idea might be supported by evidence of no significant differences in the vascular responses to sodium nitroprusside between wild-type mice and Cry1−/−Cry2−/− mice (Fig. 4).
These results suggest that a severely suppressed α-adrenoceptor response in Cry1−/−Cry2−/− mice is not caused by either the down-regulation of α-adrenoceptors due to sustained high sympathetic activity or other non-specific mechanisms in vascular smooth muscles, but is caused by local factors probably associated with the expression of CRY genes in the peripheral vessels.
Baroreflex sensitivities
This is the first study to determine baroreflex sensitivity from the HR response to spontaneous change in MAP every 4 s for 24 h in Cry1−/−Cry2−/− mice. Using this method, we successfully determined an average sensitivity that was strictly limited to that during the resting periods comprising ∼30% of the total measurement period, since baroreflex sensitivity is reportedly altered during movement (Burger et al. 1998). Experimentally, we previously reported that enhanced baroreflex sensitivity at rest fell by 50% during treadmill exercise in calponin knockout mice (Masuki et al. 2003c). In the present study, spontaneous baroreflex sensitivity in wild-type mice became less negative during locomotion as shown in Fig. 1A. Moreover, since the exogenous administration of phenylephrine or sodium nitroprusside to determine drug-induced baroreflex sensitivity is reported to directly affect HR or baroreflex sensitivity (Peveler et al. 1983; Williamson et al. 1994; Casadei & Paterson, 2000), we needed to confirm that drug-induced baroreflex sensitivity (Merrill et al. 1996) was identical to spontaneous baroreflex sensitivity (Masuki et al. 2003a). We found that both baroreflex sensitivities in wild-type mice were enhanced during the night compared with during the day, and that they were remarkably enhanced in Cry1−/−Cry2−/− mice regardless of time.
To clarify the mechanisms of these enhanced baroreflex sensitivities, we previously assessed baroreflex sensitivity in mice with targeted destruction of calponin, one of the mediators in the intracellular pathway for α-agonist-induced contraction of the vascular smooth muscle cells (Masuki et al. 2003a), but with normal clock genes, and suggested that the diurnal rhythm of MAP was flattened with the impairment of peripheral α-adrenoceptor responsiveness and the enhancement of baroreflex sensitivity (Masuki et al. 2003b; Fig. 5), as observed in Cry1−/−Cry2−/− mice in the present study. Indeed, the enhancement of baroreflex sensitivity during the night in wild-type mice, Cry1−/−Cry2−/− mice, and calponin-deficient mice was highly correlated with the reduction in pressor response to phenylephrine (Fig. 5). Consequently, MAP variability was well controlled in wild-type and Cry1−/−Cry2−/− mice (Table 1). In addition, Masuki et al. (2005) recently suggested that the baroreflex control of HR at a given time of day was enhanced in healthy young human subjects with attenuated α-adrenoceptor responsiveness so that arterial pressure was stabilized, consistent with the results in our animal studies. Thus, since baroreflex sensitivity was enhanced with the reduction in α-adrenoceptor responsiveness in both mice and humans with normal clock genes, this enhancement might be a compensatory adaptation to the reduced α-adrenoceptor-mediated vasoconstriction by other mechanisms not related to clock genes.
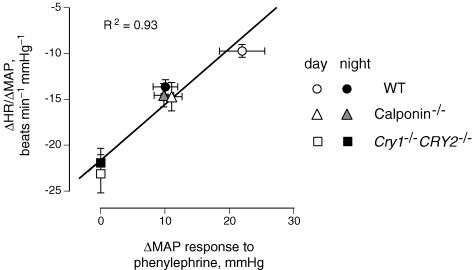
Figure 5. The relationship between the MAP response to 10 μg kg−1 of phenylephrine and the spontaneous baroreflex sensitivity in wild-type (WT), Cry1−/−Cry2−/− and calponin-deficient (calponin−/−) mice.
Means ± s.e.m. bars for 6 WT, 5 Cry1−/−Cry2−/− and 4 calponin-deficient mice. Measurements were performed under dark/dark conditions for WT and Cry1−/−Cry2−/− mice, and under light/dark conditions for calponin-deficient mice. The average period for which sensitivity was determined was ∼223 min (n = 134 000) during the day and night for each mouse. A significantly high correlation was observed between them (R2 = 0.93, P < 0.01), suggesting that spontaneous baroreflex sensitivity was enhanced when the α-adrenoceptor-mediated vasoconstrictor response was reduced. Thus, enhanced baroreflex sensitivity may be a compensatory mechanism for reduced α-adrenoceptor-mediated vasoconstriction to maintain arterial pressure, which is not directly related to the oscillation of central clock genes.
Baselines of MAP and HR
In this study, the baselines of MAP and HR at rest in wild-type mice were higher during the night than during the day, consistent with the previous results in mice (Li et al. 1999; Van Vliet et al. 2003) and rats (Zhang & Sannajust, 2000). Since the MAP increase during the night was ∼10% of that during the day, almost identical to the percentage increase in HR, the increase in MAP during the night could be explained by increased HR or cardiac output to supply a higher blood flow to the peripheral organs where the oxygen consumption rate and heat production were enhanced during the night (Nagashima et al. 2005).
On the other hand, in Cry1−/−Cry2−/− mice, diurnal changes in the baselines of HR and MAP disappeared and both increased to the same level as during the night in wild-type mice. We postulated that the oxygen consumption rate and body temperature during the day for Cry1−/−Cry2−/− mice would also increase to the same level as during the night in wild-type mice. However, Nagashima et al. (2005) measured the circadian rhythms of the core temperature and oxygen consumption rate for 24 h in Cry1−/−Cry2−/− mice under constant dark conditions and suggested that their circadian rhythms disappeared while the core temperature and oxygen consumption rate averaged for the whole day remained unchanged compared with wild-type mice. Thus, the upward shifts of MAP and HR during the day and the consequent disappearance of their diurnal rhythms in Cry1−/−Cry2−/− mice are not explained by either the elevated oxygen consumption rate or heat production during the day.
Detailed mechanisms of the well-maintained MAP level throughout the day and night in Cry1−/−Cry2−/− mice are not clear. Although it is partially explained by the enhanced HR response due to increased baroreflex function (Fig. 3, Table 3), it may not be sufficient for the reduced α-adrenoceptor responsiveness. Similar findings were also reported in recent knockout studies on the subtypes of α-adrenoceptors (α1A, α1B, α1D), suggesting that the level of MAP was relatively well maintained in these mice (Tanoue et al. 2002; Rokosh & Simpson, 2002; Hosoda et al. 2005). It was also suggested that plasma concentrations of noradrenaline, adrenaline, and angiotensin II did not increase in α1B-, α1D-, or α1BD-adrenoceptor-deficient mice (Hosoda et al. 2005) and that the vasoconstrictor response to either angiotensin II or vasopressin was not enhanced in α1D-adrenoceptor-deficient mice (Tanoue et al. 2002). Thus, although the maintenance of MAP in Cry1−/−Cry2−/− mice in this study was partially explained by the enhanced HR response, unknown adaptation mechanisms other than the systemic pressor hormones should be considered.
Limitations
In this study, since the α-adrenoceptor responsiveness was estimated from the increase in MAP after intra-arterial administration of the α-adrenoceptor agonist, reduced α-adrenoceptor responses in the night for wild-type mice and also in the day and night for Cry1−/−Cry2−/− mice might be overestimated by the enhanced decrease in HR due to enhanced baroreflex sensitivity (Jones et al. 2003). However, these differences in the MAP response were not observed after the administration of sodium nitroprusside, although baroreflex sensitivity was enhanced. These results suggest that HR changes after the administration of these drugs were caused by MAP changes through baroreflexes, and the effects of HR change on MAP were minor. In addition, the 10 μg kg−1 injection of phenylephrine was sufficient to increase MAP in wild-type mice but not in Cry1−/−Cry2−/− mice. These results indicate that α-adrenoceptor responsiveness was severely suppressed in Cry1−/−Cry2−/− mice.
In summary, the circadian rhythm of baroreflex sensitivity was absent in Cry1−/−Cry2−/− mice with severely suppressed α-adrenoceptor-mediated vasoconstrictor responses. Thus, CRY genes are involved in generating the circadian rhythm of baroreflex sensitivity, which is partially caused by regulating α-adrenoceptor responsiveness in the peripheral vessels.
Acknowledgments
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by the Ground-based Research Announcement for Space Utilization from the Japan Space Forum.
References
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Bonnefont X, Albus H, Meijer JH, van der Horst GT. Light signalling in cryptochrome-deficient mice. Novartis Found Symp. 2003;253:56–109. [PubMed] [Google Scholar]
- Brace RA. Fitting straight lines to experimental data. Am J Physiol. 1977;233:R94–R99. doi: 10.1152/ajpregu.1977.233.3.R94. [DOI] [PubMed] [Google Scholar]
- Burger HR, Chandler MP, Rodenbaugh DW, DiCarlo SE. Dynamic exercise shifts the operating point and reduces the gain of the arterial baroreflex in rats. Am J Physiol. 1998;275:R2043–R2048. doi: 10.1152/ajpregu.1998.275.6.R2043. [DOI] [PubMed] [Google Scholar]
- Casadei B, Paterson DJ. Should we still use nitrovasodilators to test baroreflex sensitivity? J Hypertens. 2000;18:3–6. doi: 10.1097/00004872-200018010-00002. [DOI] [PubMed] [Google Scholar]
- Di Rienzo M, Parati G, Castiglioni P, Tordi R, Mancia G, Pedotti A. Baroreflex effectiveness index: an additional measure of baroreflex control of heart rate in daily life. Am J Physiol. 2001;280:R744–R751. doi: 10.1152/ajpregu.2001.280.3.R744. [DOI] [PubMed] [Google Scholar]
- Gohar M, Daleau P, Atkinson J, Gargouil YM. Ultradian variations in sensitivity of rat aorta rings to noradrenaline. Eur J Pharmacol. 1992;229:69–73. doi: 10.1016/0014-2999(92)90287-e. [DOI] [PubMed] [Google Scholar]
- Grote L, Kraiczi H, Hedner J. Reduced alpha- and beta(2)-adrenergic vascular response in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:1480–1487. doi: 10.1164/ajrccm.162.4.9912028. [DOI] [PubMed] [Google Scholar]
- Hooper M, Hardy K, Handyside A, Hunter S, Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987;326:292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Hosoda C, Koshimizu TA, Tanoue A, Nasa Y, Oikawa R, Tomabechi T, et al. Two alpha1-adrenergic receptor subtypes regulating the vasopressor response have differential roles in blood pressure regulation. Mol Pharmacol. 2005;67:912–922. doi: 10.1124/mol.104.007500. [DOI] [PubMed] [Google Scholar]
- Jones PP, Christou DD, Jordan J, Seals DR. Baroreflex buffering is reduced with age in healthy men. Circulation. 2003;107:1770–1774. doi: 10.1161/01.CIR.0000057811.86187.88. [DOI] [PubMed] [Google Scholar]
- Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology. 1972;57:295–310. doi: 10.1159/000169528. [DOI] [PubMed] [Google Scholar]
- Keskil Z, Gorgun CZ, Hodoglugil U, Zengil H. Twenty-four-hour variations in the sensitivity of rat aorta to vasoactive agents. Chronobiol Int. 1996;13:465–475. doi: 10.3109/07420529609020917. [DOI] [PubMed] [Google Scholar]
- Li P, Sur SH, Mistlberger RE, Morris M. Circadian blood pressure and heart rate rhythms in mice. Am J Physiol. 1999;276:R500–R504. doi: 10.1152/ajpregu.1999.276.2.R500. [DOI] [PubMed] [Google Scholar]
- Maemura K, de la Monte SM, Chin MT, Layne MD, Hsieh CM, Yet SF, et al. CLIF, a novel cycle-like factor, regulates the circadian oscillation of plasminogen activator inhibitor-1 gene expression. J Biol Chem. 2000;275:36847–36851. doi: 10.1074/jbc.C000629200. [DOI] [PubMed] [Google Scholar]
- Makino M, Hayashi H, Takezawa H, Hirai M, Saito H, Ebihara S. Circadian rhythms of cardiovascular functions are modulated by the baroreflex and the autonomic nervous system in the rat. Circulation. 1997;96:1667–1674. doi: 10.1161/01.cir.96.5.1667. [DOI] [PubMed] [Google Scholar]
- Masubuchi S, Honma S, Abe H, Ishizaki K, Namihira M, Ikeda M, et al. Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. Eur J Neurosci. 2000;12:4206–4214. [PubMed] [Google Scholar]
- Masuki S, Eisenach JH, Dinenno FA, Joyner MJ. α-Adrenergic vasoconstrictor responsiveness and baroreflex control of heart rate in humans. FASEB J. 2005;19:A1300. [Google Scholar]
- Masuki S, Takeoka M, Taniguchi S, Nose H. Enhanced baroreflex sensitivity in free-moving calponin knockout mice. Am J Physiol. 2003a;284:H939–H946. doi: 10.1152/ajpheart.00610.2002. [DOI] [PubMed] [Google Scholar]
- Masuki S, Takeoka M, Taniguchi S, Nose H. Disappearance of diurnal activity rhythm in calponin knockout mice due to impaired arterial pressure regulation in the night. Jpn J Physiol. 2003b;53:S179. doi: 10.1113/jphysiol.2003.047803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuki S, Takeoka M, Taniguchi S, Yokoyama M, Nose H. Impaired arterial pressure regulation during exercise due to enhanced muscular vasodilatation in calponin knockout mice. J Physiol. 2003c;553:203–212. doi: 10.1113/jphysiol.2003.047803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill DC, Thompson MW, Carney CL, Granwehr BP, Schlager G, Robillard JE, et al. Chronic hypertension and altered baroreflex responses in transgenic mice containing the human renin and human angiotensinogen genes. J Clin Invest. 1996;97:1047–1055. doi: 10.1172/JCI118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountford P, Zevnik B, Duwel A, Nichols J, Li M, Dani C, et al. Dicistronic targeting constructs: reporters and modifiers of mammalian gene expression. Proc Natl Acad Sci U S A. 1994;91:4303–4307. doi: 10.1073/pnas.91.10.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K, Matsue K, Konishi M, Iidaka C, Miyazaki K, Ishida N, et al. The involvement of Cry1 and Cry2 genes in the regulation of the circadian body temperature rhythm in mice. Am J Physiol. 2005;288:R329–R335. doi: 10.1152/ajpregu.00395.2004. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Emoto N, Ikeda K, Fukuya H, Rohman MS, Raharjo SB, et al. Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation. 2001;104:1746–1748. doi: 10.1161/hc4001.098048. [DOI] [PubMed] [Google Scholar]
- Oishi K, Miyazaki K, Kadota K, Kikuno R, Nagase T, Atsumi G, et al. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J Biol Chem. 2003;278:41519–41527. doi: 10.1074/jbc.M304564200. [DOI] [PubMed] [Google Scholar]
- Okamura H, Miyake S, Sumi Y, Yamaguchi S, Yasui A, Muijtjens M, et al. Photic induction of mPer1 and mPer2 in cry-deficient mice lacking a biological clock. Science. 1999;286:2531–2534. doi: 10.1126/science.286.5449.2531. [DOI] [PubMed] [Google Scholar]
- Okamura H, Yamaguchi S, Yagita K. Molecular machinery of the circadian clock in mammals. Cell Tissue Res. 2002;309:47–56. doi: 10.1007/s00441-002-0572-5. [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Peveler RC, Bergel DH, Robinson JL, Sleight P. The effect of phenylephrine upon arterial pressure, carotid sinus radius and baroreflex sensitivity in the conscious greyhound. Clin Sci (Lond) 1983;64:455–461. doi: 10.1042/cs0640455. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Rokosh DG, Simpson PC. Knockout of the alpha 1A/C-adrenergic receptor subtype: the alpha 1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci U S A. 2002;99:9474–9479. doi: 10.1073/pnas.132552699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB, O'Leary DS, Kellogg DL., Jr . Integration of cardiovascular control systems in dynamic exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD, USA: American Physiological Society; 1996. pp. 770–838. [Google Scholar]
- Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, et al. The alpha(1D)-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest. 2002;109:765–775. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terazono H, Mutoh T, Yamaguchi S, Kobayashi M, Akiyama M, Udo R, et al. Adrenergic regulation of clock gene expression in mouse liver. Proc Natl Acad Sci U S A. 2003;100:6795–6800. doi: 10.1073/pnas.0936797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- Van Vliet BN, Chafe LL, Montani JP. Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. J Physiol. 2003;549:313–325. doi: 10.1113/jphysiol.2003.041897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci U S A. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson AP, Seifen E, Lindemann JP, Kennedy RH. Alpha 1a-adrenergic receptor mediated positive chronotropic effect in right atria isolated from rats. Can J Physiol Pharmacol. 1994;72:1574–1579. doi: 10.1139/y94-226. [DOI] [PubMed] [Google Scholar]
- Yagita K, Tamanini F, van der Horst GT, Okamura H. Molecular mechanisms of the biological clock in cultured fibroblasts. Science. 2001;292:278–281. doi: 10.1126/science.1059542. [DOI] [PubMed] [Google Scholar]
- Zhang B, Sannajust F. Diurnal rhythms of blood pressure, heart rate, and locomotor activity in adult and old male Wistar rats. Physiol Behav. 2000;70:375–380. doi: 10.1016/s0031-9384(00)00276-6. [DOI] [PubMed] [Google Scholar]