Abstract
The activity of individual afferent neurones in the mammalian cochlea can be driven by neurotransmitter released from a single synaptic ribbon in a single inner hair cell. Thus, a ribbon synapse must be able to transmit all the information on sound frequency, intensity and timing carried centrally. This task is made still more demanding by the process of binaural sound localization that utilizes separate computations of time and intensity, with temporal resolution as fine as 10 μs in central nuclei. These computations may rely in part on the fact that the response phase (at the characteristic frequency) of individual afferent neurones is invariant with intensity. Somehow, the ribbon synapse can provide stronger synaptic drive to signal varying intensity, without accompanying changes in transmission time that ordinarily occur during chemical neurotransmission. Recent ultrastructural and functional studies suggest features of the ribbon that may underlie these capabilities.
Introduction
Cochlear hair cells transduce vibrations of sound into analogous voltage waveforms. These receptor potentials cause transmitter release onto associated spiral ganglion neurones (SGNs), whose axons project to the central nervous system. Remarkably, individual SGNs usually make a single synaptic contact, receiving transmitter released by a single presynaptic specialization, the dense body (or ribbon, by analogy to related structures in retinal cells), of one inner hair cell (Liberman, 1980; Kiang et al. 1982; Spoendlin, 1985). Each inner hair possesses 10–30 such ribbons, thereby driving an equivalent number of SGNs that serve as labelled lines for the acoustic characteristic frequency of that cochlear position. Thus, each synaptic ribbon fulfils a demanding signalling task. This includes driving spontaneous activity in afferent nerve fibres at rates that can exceed 100 Hz (Rose et al. 1967; Liberman, 1978; Guth et al. 1991). Then, during the presentation of sound, the synapse accelerates afferent activity, settling at average steady-state rates of 200–300 Hz after initially higher transients (Geisler et al. 1974). In addition to encoding sound intensity in afferent firing rate, the synapse also provides critical information on the timing of the stimulus. At frequencies below about 5 kHz, the action potentials of individual afferents can phase-lock, occurring at a preferred time during the wave cycle. Both time and intensity coding are essential to the binaural computations that underlie the process of sound localization (Moore, 1991).
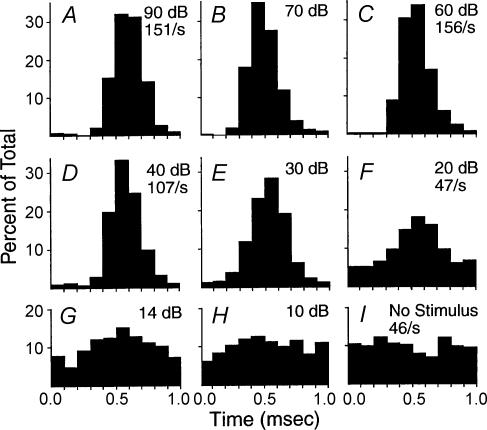
Further inspection of afferent activity reveals additional noteworthy features. In particular, the response phase at the fibre's most sensitive (best or characteristic) frequency does not vary with intensity (Rose et al. 1967). That is, the relative timing of action potentials remains constant throughout the afferent's intensity range, even as synaptic efficacy rises to produce more action potentials per tone cycle (Fig. 1). This observation presents a conundrum since stronger synaptic drive would ordinarily be expected to reduce synaptic delay as at other chemical synapses (Bollmann et al. 2000; Schneggenburger & Neher, 2000), thus producing a phase advance. Recent studies on the ultrastructure and function of ribbon synapses suggest how vesicular release might be configured to solve this conundrum.
Figure 1. Intensity invariant response phase.
Histograms showing timing of action potentials in a single auditory afferent neurone during presentation of a tone at the characteristic frequency (1000 Hz). The time of peak firing remains constant as intensity changes from 10 to 90 dB. (From Rose et al. 1967; with permission.)
Structure of ribbons in hair cells
Viewed in electron micrographs, the synaptic ribbons of mammalian cochlear hair cells are spherical or ellipsoidal electron-dense bodies 100–200 nm in diameter (Merchan-Perez & Liberman, 1996). Clear-core, 35 nm diameter vesicles are attached to the ribbon through 20 nm long tethers (Fig. 2). Serial reconstructions of ribbons in frog hair cells have provided more thorough quantification (Lenzi et al. 1999). These larger ribbons (nearly 500 nm in diameter) were found to tether ∼400 vesicles and to present an average of 32 vesicles to the underlying plasma membrane (with a maximum of 138 at close-packing). Assuming proportionality, the much smaller ribbons of mammalian hair cells would provide correspondingly fewer vesicles, ∼60 on tethers, and an average of five vesicles at the plasma membrane. Such numbers immediately raise a question regarding the spontaneous and driven activity of afferent neurones that can range up to several hundred hertz. To support such activity the ribbon not only must drive vesicles rapidly to docking sites on the plasma membrane, but also efficiently resupply from as yet unidentified cytoplasmic sources. Even during sustained maximal depolarization of frog saccular hair cells, the ribbons maintain a fraction of tethered vesicles, although the number docked at the plasma membrane drops sharply (Lenzi et al. 2002). Observations on retinal bipolar cells suggest that free diffusion of vesicles may suffice to resupply the ribbons (Holt et al. 2004), and similarly large numbers of vesicles are found in the cytoplasm of saccular hair cells (Lenzi et al. 1999, 2002). Recent observations using fluorescent dye (FM1-43) to label synaptic vesicles suggest that rapid resupply from a cytoplasmic pool of preformed vesicles also may occur in mammalian cochlear hair cells (Griesinger & Ashmore, 2005). Overall, ultrastructural studies confirm that ribbons are indeed capable of rapid and sustained vesicular release, as required by eighth nerve activity patterns. Measurements of membrane capacitance have been used to further support this conclusion.
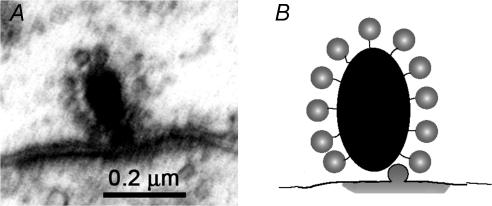
Figure 2. The ribbon synapse.
A, transmission electron micrograph of a synaptic ribbon of an inner (cochlear) hair cell in a 2-month-old rat. The electron-dense ribbon is surrounded by a halo of small vesicles (∼30 nm diameter). The plasma membranes are thickened beneath the ribbon, that of the postsynaptic afferent neurone more obviously so. (Micrograph by T. Pongstaphone, unpublished.) B, ribbon schematic showing vesicles tethered to the dense body (ribbon), with one vesicle having fused to plasma membrane to release its contents (grey ‘cloud’ flattened by adjoining postsynaptic membrane).
Capacitance measurements
Cellular recordings of membrane capacitance can report the gain and/or loss of surface area, corresponding to vesicular release and re-uptake in synaptic studies. Capacitance measurements from hair cells (Parsons et al. 1994; Beutner & Moser, 2001; Beutner et al. 2001; Eisen et al. 2004) have shown that hair cells are capable of both transient and sustained membrane fusion (see reviews in Fuchs et al. 2003; Parsons & Sterling, 2003). With high levels of added calcium buffer, membrane depolarization caused a rapid and saturating increase in capacitance that would correspond to the fusion of five to eight vesicles at each of the two dozen ribbons of mammalian inner hair cells (Moser & Beutner, 2000). With lower levels of exogenous calcium buffer, or relying on the hair cell's intrinsic buffering, slower processes of fusion are observed that imply the existence of many thousands of releasable vesicles (Parsons et al. 1994; Moser & Beutner, 2000; Eisen et al. 2004). This latter pool is severalfold larger than the total number of vesicles tethered to ribbons in hair cells, but may result from release at non-ribbon locations (Khimich et al. 2005). Likewise, the rapidly releasable pool of five to eight vesicles per ribbon corresponds with the average number of docked vesicles estimated from ultrastructural analysis. One additional capacitance measurement is of particular interest. When neonatal mouse cochlear hair cells were voltage clamped with an action-potential-like waveform (broad calcium action potentials occur in these immature hair cells prior to the onset of hearing; Kros et al. 1998; Marcotti et al. 2003), they produced an average capacitance increase that would correspond to the release of approximately 1000 vesicles (Beutner & Moser, 2001), implying that each of the one to two dozen ribbons found in these hair cells (Griesinger et al. 2005; Khimich et al. 2005) would be capable of releasing 40–80 vesicles during a slow calcium action potential.
Intracellular recording from cochlear afferents
Postsynaptic activity in hair cell afferents has been recorded largely from non-mammalian or non-auditory end-organs where each afferent is postsynaptic to multiple presynaptic ribbons and/or hair cells (Furukawa et al. 1978; Crawford & Fettiplace, 1980; Art et al. 1984; Starr & Sewell, 1991; Locke et al. 1999), in contrast to the single hair cell ribbon that drives most mammalian cochlear afferents. A series of recordings made with sharp electrodes in guinea pig cochlear afferents revealed what appeared to be quantal fluctuations in synaptic amplitude (Siegel, 1992), with most of these events leading to the generation of action potentials. Recently, using an ex vivo preparation of the rat organ of Corti, it proved possible to make voltage-clamp recordings from afferent boutons at their point of contact with the inner hair cell (Glowatzki & Fuchs, 2002). These recordings provide a very high resolution view of the synaptic output of single hair cell ribbons. Since the presynaptic hair cell was not stimulated directly, only ongoing, spontaneous transmitter release was observed. However, it was possible to depolarize the hair cells with high potassium saline, and in this way greatly accelerate ongoing release. As will be discussed, other than frequency, the characteristics of release were not altered by this procedure.
The majority of excitatory postsynaptic currents (EPSCs) recorded at –90 mV had brief waveforms, with decay time constants of 1 ms at room temperature (Fig. 3A). The EPSCs reversed in sign near 0 mV, and were sensitive to glutamatergic ligands, consistent with previous studies suggesting that cochlear afferent transmission is mediated by AMPA-type glutamate receptors (Ruel et al. 1999). What was unexpected, however, was that some of the EPSCs were quite large, reaching an equivalent conductance increase of nearly 10 nS. More generally, there was a wide variation in EPSC amplitude, with a greater than 20-fold range observed in most cells. Histogram analysis of EPSC amplitude revealed highly asymmetric distributions with modes near an equivalent conductance of 0.40 nS, means of ∼2.0 nS, and with coefficients of variation ranging between 77 and 95%.
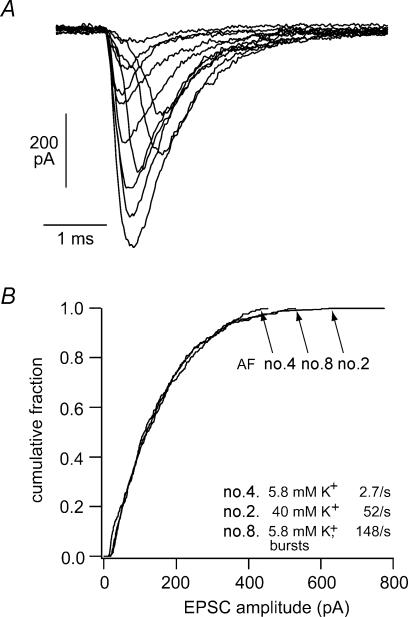
Figure 3. EPSCs in cochlear afferents.
A, excitatory postsynaptic currents recorded from afferent boutons at their point of contact with the inner hair cell. Membrane potential –94 mV. B, cumulative EPSC amplitude plots constructed from 3 different afferent fibres with different mean rates of release. Fibre no. 4 was measured in ‘normal’ conditions where membrane potentials are –60 mV on average. Fibre no. 2 was bathed in 40 mm K+ saline, which depolarized hair cells to ∼–35 mV. Fibre no. 8 had ‘bursting’ EPSCs whose timing suggested that they were produced by calcium action potentials in the neonatal hair cell. These exemplar recordings illustrate the fact that release probability did not correlate with EPSC amplitude distribution. (From Glowatzki & Fuchs, 2002; with permission; ©Nature Publishing Group, http:///www.nature.com).
Various considerations suggested that the modal value of the amplitude histogram might correspond to the release of a single vesicle from the presynaptic ribbon. For example, the equivalent conductance (0.4 nS) lies very near to that established for glutaminergic ‘minis’ in the central nervous system (Sahara & Takahashi, 2001). If correct, this assumption leads to the further conclusions that the average EPSC was composed of three to six vesicles, while the largest events resulted from the simultaneous release of 20 or more (assuming linear summation). It is noteworthy how well these values approximate those derived from ultrastructural and capacitance studies. From all three of these approaches it appears that a ribbon in mammalian cochlear hair cells keeps approximately five vesicles ready for release at the plasma membrane. From the previous ultrastructural analysis of vesicle placement with close-packing we can also estimate that a single mammalian ribbon could maximally position 29 vesicles for simultaneous release, approximating the largest EPSCs observed in these records. Finally, we can return to the foregoing capacitance experiments in which action potential waveforms were assumed to cause the release of 40–80 vesicles from individual ribbons in mouse inner hair cells. For comparison, bursts of EPSCs were occasionally found in the afferent bouton records, with timing and duration similar to those of hair cell action potentials, and so thought to be produced by them. Again assuming linear summation, these bursts included 47 vesicles on average in three different afferents.
Thus, the proposed view of hair cell synaptic function, based on ultrastructure, capacitance measurements and postsynaptic recording, is that individual ribbons are capable of releasing from one to many synaptic vesicles simultaneously. Similar proposals of multivesicular release have been advanced for retinal photoreceptor and bipolar cell ribbons (Maple et al. 1994; Singer et al. 2004). Multivesicular release from hair cells occurs spontaneously, in the absence of explicit changes in membrane potential. This last point is consistent with many studies showing that spontaneous activity in VIIIth nerve cochlear afferent fibres results from stochastic, calcium-dependent transmitter release from inner hair cells (IHCs) (Robertson & Paki, 2002; Sueta et al. 2004).
The afferent bouton recordings proved still more revealing when the hair cells were depolarized from rest to approximately −30 mV with elevated external potassium saline. After an initial period of bursting release, EPSC frequency stabilized at an average frequency of 27 Hz. Still higher rates of release ( > 100 Hz) were observed in circumstances thought to reflect spontaneous action potentials in the neonatal IHCs (peak amplitudes near 0 mV). The amplitude distributions of three exemplar cases are shown as cumulative fraction plots in Fig. 3B. These display the relative proportions of the sample population as a function of EPSC amplitude. The three distributions shown here arose at average frequencies of 2.7, 52 and 148 per second in three different fibres at rest, in high potassium, and during action potential-induced bursts, respectively. As is evident from the figure, the EPSC amplitude distribution was identical in all three conditions. That is, although release probability (frequency) varied more than 50-fold, the average EPSC amplitude, and the proportions of small and large EPSCs was the same in each case. In other words, the average number of vesicles released per EPSC did not increase, even though calcium channel gating must have increased to produce more frequent EPSCs. This lack of correlation implies that the quantum content, or number of vesicles making up each release event, is somehow calcium independent at the ribbon synapse.
This final point is reminiscent of the observation from capacitance measurements that the most rapid phase of vesicular fusion (1–2 vesicles per millisecond per ribbon) is independent of the level of internal calcium buffering (Moser & Beutner, 2000). It must be emphasized that these considerations do not imply that release per se is calcium independent, as the frequency of EPSCs does indeed rise with depolarization and so calcium influx; i.e. vesicular fusion and transmitter release are triggered by calcium. However, some process distinct from calcium-dependent vesicular fusion must mediate the loading of vesicles into the release-ready pool. It seems reasonable to propose that this is an as-yet-undefined mechanism of the synaptic ribbon.
Conclusion
These observations on transmitter release are provocative and beg the question of what mechanisms serve to provide the widely varying distribution of readily releasable vesicles. Recent findings from retina (Holt et al. 2004) suggest that free diffusion can deliver vesicles rapidly enough to sustain fully loaded ribbons while 70% of docked vesicles are released during 1 min of depolarization. (Further, the rate of vesicular diffusion was not dependent on calcium.) Thus, if the ribbon functions to ‘stockpile’ vesicles near to fusion sites, this process occurs relatively rapidly. Indeed, recent observations found that dye-loaded vesicles reappeared almost as rapidly as they were released by ribbons of cochlear hair cells, approximately 1–3 vesicles ms−1 (Griesinger et al. 2005). Ultrastructural studies in frog show that tethers on ribbons remain fully loaded with vesicles while those docked at the membrane are reduced (Lenzi et al. 2002). Thus the rate-limiting step would appear to be the binding of vesicles to docking sites on the plasma membrane. The amplitude distributions of EPSCs (Glowatzki & Fuchs, 2002) suggest that single vesicles were released most commonly; however, the average EPSC was caused by three to six vesicles, and rarely, as many as 20 or more vesicles could be released at once. One hypothesis to achieve such distributions would posit that a relatively large number of vesicles are always available to fill some 20 or so docking sites beneath the ribbon, but that the vesicle–docking site interactions are brief, random and not dependent on calcium. These might correspond to calcium-independent, partially zippered states of the SNARE complex (Sorensen, 2004).
Does the multivesicular release hypothesis help to understand the coding of timing and intensity by cochlear afferent neurones? Perhaps. Consider the observed phase-constancy with varying sound intensity illustrated in Fig. 1. As stated earlier, the difficulty is that ordinary chemical synapses ought to exhibit a phase advance with increasing intensity. Two factors should be pre-eminent determinants of this effect. First, increasing depolarization causes larger presynaptic calcium influx to drive the process of transmitter release faster. Second, if increased release also raised quantum content, then increasingly larger EPSPs would drive the postsynaptic membrane potential to threshold more quickly. How might the hair cell ribbon synapse avoid these interdependencies and so code time and intensity separately? The invariant amplitude distributions shown in Fig. 3 demonstrate that at least the latter, postsynaptic effect may not occur at the ribbon. Mean EPSC amplitude and overall distribution were similar across a wide range of release rates. Indeed, EPSC amplitude was essentially randomized with respect to release probability, thereby avoiding a postsynaptic correlation between time to threshold and the extent of presynaptic depolarization (‘intensity’).
What about the presynaptic process? Clearly there is greater calcium influx as the hair cell depolarizes, since EPSC frequency goes up steeply, increasing 100-fold on average for an ∼50 mV change in membrane potential. Increasing calcium influx should accelerate vesicle–membrane fusion reactions. How does release by the ribbon maintain a constant delay as depolarization (‘intensity’) progresses? Perhaps the absolute EPSC rates suggest a partial solution. At the hair cell resting potential EPSCs occurred approximately once per second on average. We will assume that each release event results from the infrequent opening of calcium channels near the foot of the voltage activation curve. Whole-cell voltage clamp recordings from inner hair cells indicate that each ribbon possesses a functional pool of 10–100 voltage-gated calcium channels (Platzer et al. 2000; Beutner & Moser, 2001; Marcotti et al. 2003). Binomial statistics then suggest that as many as 10 channels must open simultaneously to explain the low resting rates of release. The implication is that the fusion process has a relatively low affinity, but steep concentration dependence for calcium. If a large fraction of the available channels is required to initiate release, little concentration difference will be found between ‘threshold’ and maximal calcium signals, and consequently little change in fusion kinetics.
Finally, these considerations of time and intensity coding lead to a suggestion for ribbon function. If calcium must sum from multiple channels for a release ‘event’, and a release event can involve multiple vesicles (as above), the causal entity would appear to act globally at the active zone. Perhaps this is what the ribbon does, serving as a calcium-triggered, microscopic stamp press to force loaded vesicles against the plasma membrane.
It is important to note that the present observations arise largely from immature (prehearing) hair cells. Release efficiency improves after the onset of hearing (Beutner & Moser, 2001; Johnson et al. 2005), as does spontaneous firing rate in afferent nerve fibres (Romand, 1984). The characteristics of release from mature ribbon synapses therefore, will be of considerable interest.
Why do ribbons work as they do? What is presented here is only a heuristic argument – that multivesicular release exists to de-convolve time and intensity. Other considerations could apply as well. The ribbon synapse may have evolved to ensure that a supply of vesicles is always available for ongoing, tonic release. Multivesicular release would be a useful side product of this design that requires a large pool of ready-to-dock vesicles. Certainly, the study of vesicular release from cochlear hair cell ribbons will provide future insights into the underlying molecular mechanisms and will have broader implications for neural function more generally.
Acknowledgments
With thanks to Drs E. Glowatzki and E. Yi for helpful discussion. Related work in the author's laboratory was supported by grants NIDCD R01 DC00276 and NIDCD P30 DC05211.
References
- Art JJ, Fettiplace R, Fuchs PA. Synaptic hyperpolarization and inhibition of turtle cochlear hair cells. J Physiol. 1984;356:525–550. doi: 10.1113/jphysiol.1984.sp015481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Moser T. The presynaptic function of mouse cochlear inner hair cells during development of hearing. J Neurosci. 2001;21:4593–4599. doi: 10.1523/JNEUROSCI.21-13-04593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B, Borst JG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- Crawford AC, Fettiplace R. The frequency selectivity of auditory nerve fibres and hair cells in the cochlea of the turtle. J Physiol. 1980;306:79–125. doi: 10.1113/jphysiol.1980.sp013387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MD, Spassova M, Parsons TD. Large releasable pool of synaptic vesicles in chick cochlear hair cells. J Neurophysiol. 2004;91:2422–2428. doi: 10.1152/jn.01130.2003. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Glowatzki E, Moser T. The afferent synapse of cochlear hair cells. Curr Opin Neurobiol. 2003;13:452–458. doi: 10.1016/s0959-4388(03)00098-9. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Hayashida Y, Matsuura S. Quantal analysis of the size of excitatory post-synaptic potentials at synapses between hair cells and afferent nerve fibres in goldfish. J Physiol. 1978;276:211–226. doi: 10.1113/jphysiol.1978.sp012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler CD, Rhode WS, Kennedy DT. Responses to tonal stimuli of single auditory nerve fibers and their relationship to basilar membrane motion in the squirrel monkey. J Neurophysiol. 1974;37:1156–1172. doi: 10.1152/jn.1974.37.6.1156. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5:147–154. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Griesinger CB, Richards CD, Ashmore JF. Fast vesicle replenishment allows indefatigable signalling at the first auditory synapse. Nature. 2005 doi: 10.1038/nature03567. (in press) [DOI] [PubMed] [Google Scholar]
- Guth PS, Aubert A, Ricci AJ, Norris CH. Differential modulation of spontaneous and evoked neurotransmitter release from hair cells: some novel hypotheses. Hear Res. 1991;56:69–78. doi: 10.1016/0378-5955(91)90155-3. [DOI] [PubMed] [Google Scholar]
- Holt M, Cooke A, Neef A, Lagnado L. High mobility of vesicles supports continuous exocytosis at a ribbon synapse. Curr Biol. 2004;14:173–183. doi: 10.1016/j.cub.2003.12.053. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W, Kros CJ. Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. J Physiol. 2005;563:177–191. doi: 10.1113/jphysiol.2004.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khimich D, Nouvain R, Pujol R, tom Dieck S, Egner A, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Kiang NY, Rho JM, Northrop CC, Liberman MC, Ryugo DK. Hair-cell innervation by spiral ganglion cells in adult cats. Science. 1982;217:175–177. doi: 10.1126/science.7089553. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rusch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–284. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Crum J, Ellisman MH, Roberts WM. Depolarization redistributes synaptic membrane and creates a gradient of vesicles on the synaptic body at a ribbon synapse. Neuron. 2002;36:649–659. doi: 10.1016/s0896-6273(02)01025-5. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Runyeon JW, Crum J, Ellisman MH, Roberts WM. Synaptic vesicle populations in saccular hair cells reconstructed by electron tomography. J Neurosci. 1999;19:119–132. doi: 10.1523/JNEUROSCI.19-01-00119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am. 1978;63:442–455. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Morphological differences among radial afferent fibers in the cat cochlea: an electron-microscopic study of serial sections. Hear Res. 1980;3:45–63. doi: 10.1016/0378-5955(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Locke R, Vautrin J, Highstein S. Miniature EPSPs and sensory encoding in the primary afferents of the vestibular lagena of the toadfish. Opsanus tau. Ann N Y Acad Sci. 1999;871:35–50. doi: 10.1111/j.1749-6632.1999.tb09174.x. [DOI] [PubMed] [Google Scholar]
- Maple BR, Werblin FS, Wu SM. Miniature excitatory postsynaptic currents in bipolar cells of the tiger salamander retina. Vision Res. 1994;34:2357–2362. doi: 10.1016/0042-6989(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Rusch A, Kros CJ. Sodium and calcium currents shape action potentials in immature mouse inner hair cells. J Physiol. 2003;552:743–761. doi: 10.1113/jphysiol.2003.043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchan-Perez A, Liberman MC. Ultrastructural differences among afferent synapses on cochlear hair cells: correlations with spontaneous discharge rate. J Comp Neurol. 1996;371:208–221. doi: 10.1002/(SICI)1096-9861(19960722)371:2<208::AID-CNE2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Moore DR. Anatomy and physiology of binaural hearing. Audiology. 1991;30:125–134. doi: 10.3109/00206099109072878. [DOI] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci U S A. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Lenzi D, Almers W, Roberts WM. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell: capacitance measurements in saccular hair cells. Neuron. 1994;13:875–883. doi: 10.1016/0896-6273(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Sterling P. Synaptic ribbon. Conveyor belt or safety belt? Neuron. 2003;37:379–382. doi: 10.1016/s0896-6273(03)00062-x. [DOI] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Robertson D, Paki B. Role of L-type Ca2+ channels in transmitter release from mammalian inner hair cells. II. Single-neuron activity. J Neurophysiol. 2002;87:2734–2740. doi: 10.1152/jn.2002.87.6.2734. [DOI] [PubMed] [Google Scholar]
- Romand R. Functional properties of auditory-nerve fibers during postnatal development in the kitten. Exp Brain Res. 1984;56:395–402. doi: 10.1007/BF00237980. [DOI] [PubMed] [Google Scholar]
- Rose JE, Brugge JF, Anderson DJ, Hind JE. Phase-locked response to low-frequency tones in single auditory nerve fibers of the squirrel monkey. J Neurophysiol. 1967;30:769–793. doi: 10.1152/jn.1967.30.4.769. [DOI] [PubMed] [Google Scholar]
- Ruel J, Chen C, Pujol R, Bobbin RP, Puel JL. AMPA-preferring glutamate receptors in cochlear physiology of adult guinea-pig. J Physiol. 1999;518:667–680. doi: 10.1111/j.1469-7793.1999.0667p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara Y, Takahashi T. Quantal components of the excitatory postsynaptic currents at a rat central auditory synapse. J Physiol. 2001;536:189–197. doi: 10.1111/j.1469-7793.2001.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Siegel JH. Spontaneous synaptic potentials from afferent terminals in the guinea pig cochlea. Hear Res. 1992;59:85–92. doi: 10.1016/0378-5955(92)90105-v. [DOI] [PubMed] [Google Scholar]
- Singer JH, Lassova L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci. 2004;7:826–833. doi: 10.1038/nn1280. [DOI] [PubMed] [Google Scholar]
- Sorensen JB. Formation, stabilisation and fusion of the readily releasable pool of secretory vesicles. Pflugers Arch. 2004;448:347–362. doi: 10.1007/s00424-004-1247-8. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Anatomy of cochlear innervation. Am J Otolaryngol. 1985;6:453–467. doi: 10.1016/s0196-0709(85)80026-0. [DOI] [PubMed] [Google Scholar]
- Starr PA, Sewell WF. Neurotransmitter release from hair cells and its blockade by glutamate-receptor antagonists. Hear Res. 1991;52:23–41. doi: 10.1016/0378-5955(91)90185-c. [DOI] [PubMed] [Google Scholar]
- Sueta T, Zhang SY, Sellick PM, Patuzzi R, Robertson D. Effects of a calcium channel blocker on spontaneous neural noise and gross action potential waveforms in the guinea pig cochlea. Hear Res. 2004;188:117–125. doi: 10.1016/S0378-5955(03)00374-5. [DOI] [PubMed] [Google Scholar]