Abstract
PURPOSE Inhalational anthrax is an extremely rare infectious disease with nonspecific initial symptoms, thus making diagnosis on clinical grounds difficult. After a covert release of anthrax spores, primary care physicians will be among the first to evaluate cases. This study defines the primary care differential diagnosis of inhalational anthrax.
METHODS In May 2002, we mailed survey instruments consisting of 3 randomly chosen case vignettes describing patients with inhalational anthrax to a nationwide random sample of 665 family physicians. Nonrespondents received additional mailings. Physicians were asked to provide their most likely nonanthrax diagnosis for each case.
RESULTS The response rate was 36.9%. Diagnoses for inhalational anthrax were grouped into 35 diagnostic categories, with pneumonia (42%), influenza (10%), viral syndrome (9%), septicemia (8%), bronchitis (7%), central nervous system infection (6%), and gastroenteritis (4%) accounting for 86% of all diagnoses. Diagnoses differed significantly between cases that proved to be fatal and those that proved to be nonfatal.
CONCLUSIONS Inhalational anthrax resembles common diagnoses in primary care. Surveillance systems for early detection of bioterrorism events that rely only on diagnostic codes will be hampered by false-positive alerts. Consequently, educating front-line physicians to recognize and respond to bioterrorism is of the highest priority.
Keywords: Bioterrorism; primary health care; anthrax; surveillance; diagnosis, differential
INTRODUCTION
Covert release of anthrax (Bacillus anthracis) spores as an act of terrorism will result in the unknowing and unsuspected exposure of persons. Some of those exposed will become infected through inhalation of spores; many of these persons will manifest some of the constellation of symptoms that can be associated with inhalational anthrax.1 For frontline clinicians—typically physicians, physician assistants, and nurse-practitioners in primary care offices, urgent care centers, and emergency departments—early recognition of and response to inhalational anthrax is of paramount importance on both individual and populationwide levels.2
Inhalational anthrax is an extremely rare infectious disease. In 11 recent cases associated with bioterrorism, initial diagnoses were diverse, including gastroenteritis, bacterial meningitis, nonspecific viral syndrome, and bronchitis.3–7 A diagnosis of inhalational anthrax can be supported by imaging studies. Definitive diagnosis, however, depends on detection of B anthracis through culture, immunohistochemistry, or polymerase chain reaction.1,8 In past anthrax outbreaks, initial diagnoses were aided by contextual information, such as occupation.9 Covert bioterrorism involving a rare pathogen robs the clinician of most contextual diagnostic clues.
Early detection of bioterrorism events has become a national priority since September 11, 2001.10,11 Surveillance based on real-time evaluation of electronic medical data has been proposed as one approach providing the “extreme timeliness of detection” necessary for appropriate response.12 Electronic surveillance uses signal detection theory to identify patterns in diagnostic codes, reported symptoms, or combinations of codes.13,14 Implementation of electronic surveillance, however, depends on understanding the interface between patients and physicians in primary care settings. This venue is where initial diagnoses are made and where initial diagnostic codes are selected for billing purposes.15 Information regarding the primary care differential diagnosis of inhalational anthrax is scant and has not been evidence-based. Furthermore, it is not possible to construct adequately a differential diagnosis based on a review of diagnoses arrived at in past cases. The objective of this study was to assess the range of diagnostic conclusions reached by clinically active family physicians when confronted with the initial signs and symptoms of patients ultimately shown to have inhalational anthrax, thus creating a differential diagnosis.
METHODS
Physicians
A simple random sampling of the 33,365 clinically active members of the American Academy of Family Physicians (AAFP) produced a set of 665 physicians (2% of those eligible). We estimated the sample size to provide approximately 50 responses per case. Physician inclusion criteria included completion of residency training before 2001 and current involvement in any type of ambulatory care medicine, practice arrangement, and practice ownership arrangement. The University of Wisconsin Medical School Institutional Review Board approved the study, and we obtained appropriate informed consent according to the approved protocol.
Survey Instrument
We created 14 case vignettes from case descriptions of patients who sought care at various hospitals and clinics. Eleven of the vignettes were based on published, confirmed cases of terrorism-associated inhalational anthrax that occurred between October and November, 2001.3–7 Three other vignettes of cases of nonanthrax illnesses—diagnosed and managed by family physicians—served as comparisons. Specifically, we included 1 vignette describing a severely ill patient with community-acquired Legionella pneumonia who required medical intensive care unit support (case 2) and 2 vignettes describing influenza A managed successfully in the outpatient setting (cases 7 and 13).
The case vignettes included basic demographic data, medical history, patient’s symptoms, physical findings, and basic hematology values (hematocrit, white cell count, platelet count, and percentages of neutrophils, lymphocytes, and monocytes). The cases were modified to include only information that would be available in a primary care setting. Furthermore, results of chest radiographs, if obtained, were excluded.
Outcome Measures
The primary outcome measures were qualitative diagnoses. Although physicians were informed that some cases may have been inhalational anthrax, they were asked to give their most likely nonanthrax diagnosis for each of 3 case vignettes, based on the clinical description, history of present illness, symptoms, findings of physical examination, laboratory results, and their personal clinical experience. The physicians were asked to assume that each case occurred in a patient who visited their ambulatory practice site, and diagnostic studies were limited to those typically available at an outpatient, primary care clinic (eg, complete blood cell count, pulse oximetry, collection of specimens for blood culture, chest radiograph without radiologist interpretation).
Procedure
We mailed each physician an invitational letter describing the study and 3 of the 14 cases (Appendix 1, available online only at http://www.annfammed.org/cgi/content/full/2/5/438/DC1), along with a self-addressed, stamped response postcard. We selected cases at random and placed them in random order. The physician was informed that some of the case vignettes “may have originated in patients who were ultimately diagnosed with inhalational anthrax.” After reading each case, the physician was asked to provide the most likely nonanthrax diagnosis. This request was made because we wanted to determine what unsuspected cases of inhalational anthrax might look like to practicing family physicians, not because we were interested in the ability of family physicians to identify cases of anthrax—a rare and unlikely diagnosis. The original mailing was sent on May 6, 2002, and was followed by up to 2 repeated mailings to nonrespondents, occurring approximately 3 weeks apart.
Analysis
We checked for bias in response rates according to sex, regional location, specific case, and case type. The 5 regional locations were chosen based on the Centers for Disease Control and Prevention regional map.16 New England, Mid-Atlantic, and South Atlantic regions were combined into Eastern Seaboard; East North Central and East South Central regions were combined into East Central; and West North Central and West South Central regions were combined into West Central. Mountain and Pacific regions stayed the same. Chi-square analyses were performed to evaluate response bias.
We grouped specific diagnoses into general diagnostic categories based on their similarities. Where a respondent listed more than 1 diagnosis, the most serious of the illnesses was used to categorize the case. Data were recorded as unanswered if the diagnosis line was left blank or if the response could not be interpreted. We then grouped these data by case and into 5 case types by illness: total inhalational anthrax, nonfatal inhalational anthrax, fatal inhalational anthrax, influenza A, and Legionella pneumonia.
RESULTS
Response Rate and Bias
Three survey packets were returned because of a change in address. For the remainder, we obtained an overall response rate of 36.9% (244 of 662) after 3 mailings of the survey. Most respondents (67.2%) were male, reflecting the US family physician population (68.4%) (χ2 = 0.152, df = 1, P = not significant). There were between 40 and 61 responses for each of the 14 case vignettes (mean, 52.3 responses per vignette), resulting in 732 (244 × 3) case responses. Of these 732 diagnostic responses, 7 (0.96%) were left blank and an additional 9 (1.23%) inappropriately indicated anthrax as a diagnosis, thus reducing the sample size to 716 case diagnoses.
The regional distribution of respondents (Table 1 ▶) did not differ statistically from that of US family physicians (χ2 = 3.195, df = 4, P = not significant). We found no statistically significant differences among respondents and nonrespondents in terms of sex (χ2 = 1.408, df =1, P = .235), geographic region (χ2 = 8.057, df = 4, P = .090), specific case vignettes received (χ2 = 12.727, df = 13, P = .469), or type of case vignette received (χ2 = 0.787, df = 3, P = .853). Physicians in the Pacific region had the highest rate of response (45.5%), whereas those in the Mountain region had the lowest (28.3%).
Table 1.
Response Rates Based on Sex of Respondent and Location of Practice
| Characteristic | Respondents No. (%) | Nonrespondents No. (%) | Final Sample % | P Value* |
| Sex† | .235 | |||
| Male | 160 (35.7) | 286 (64.3) | 67.2 | |
| Female | 78 (40.8) | 113 (59.2) | 32.8 | |
| Location of practice‡ | .090 | |||
| Eastern Seaboard region | 71 (33.5) | 147 (66.5) | 30.6 | |
| East Central region | 60 (42.3) | 82 (57.7) | 24.8 | |
| West Central region | 51 (34.0) | 99 (66.0) | 21.1 | |
| Mountain region | 17 (28.3) | 43 (71.7) | 7.0 | |
| Pacific region | 40 (45.5) | 48 (54.5) | 16.5 |
* Values are for tests of differences between respondents and nonrespondents.
† Men and women comprise 68.4% and 31.6% of US family physicians, respectively.17
‡ Of US family physicians, 35.0% are located in the Eastern Seaboard region; 22.6%, in the East Central region; 19.4%, in the West Central region; 6.5%, in the Mountain region; and 16.7%, in the Pacific region.17
Differential Diagnosis
Family physicians provided an abundance of unique diagnoses for each case of inhalational anthrax (range, 13–27 diagnoses per case). Because of appreciable overlap of diagnoses, however, we used diagnostic categories to organize and simplify results. There were between 6 and 12 diagnostic categories per case (mean, 9.0 categories per case).
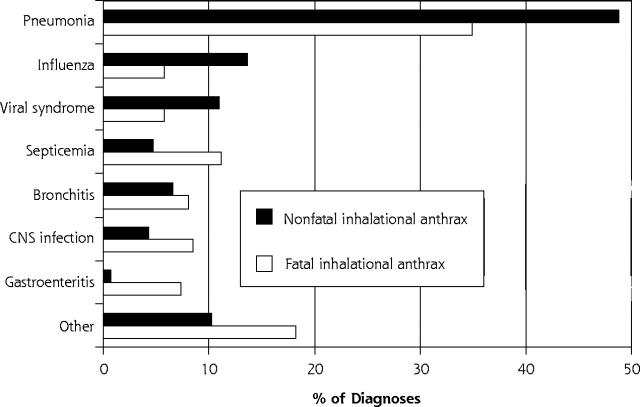
Although 35 distinct diagnostic categories were derived for inhalational anthrax (Table 2 ▶), 7 diagnostic categories—pneumonia, influenza, viral syndrome, septicemia, bronchitis, central nervous system (CNS) infection, and gastroenteritis—accounted for 86.1% of all diagnoses. The distributions of the top 7 diagnoses assigned to inhalational anthrax cases differed significantly between nonfatal and fatal cases (Figure 1 ▶; χ2 = 47.339, df = 6, P <.001). Pneumonia, influenza, bronchitis, and viral syndrome accounted for 80% of non-fatal anthrax diagnoses. Pneumonia, septicemia, CNS infection, bronchitis, and gastroenteritis accounted for 70.1% of fatal anthrax cases.
Table 2.
Primary Care Probabilistic Differential Diagnosis for Inhalation Anthrax
| Diagnoses (ICD-9 Code) | Responses No. (%) | Cumulative Percentage |
| 1. Pneumonia (480-486) | 237 (42.4) | 42.4 |
| 2. Influenza (487) | 56 (10.0) | 52.4 |
| 3. Viral syndrome (079.99) | 48 (8.6) | 61.0 |
| 4. Septicemia (038) | 43 (7.7) | 68.7 |
| 5. Bronchitis (466) | 41 (7.3) | 76.0 |
| 6. CNS infection (047-049, 320-323) | 35 (6.3) | 82.3 |
| 7. Gastroenteritis (008-009) | 21 (3.8) | 86.1 |
| 8. Upper respiratory tract infection (460, 465) | 17 (3.0) | 89.1 |
| 9. Nonspecific febrile illness (780.9) | 10 (1.8) | 90.9 |
| 10. Chronic obstructive pulmonary disease (496) | 8 (1.4) | 92.3 |
| 11. Congestive heart failure (428.0) | 7 (1.3) | 93.6 |
| 12. Pulmonary embolism (415.1) | 5 (0.9) | 94.5 |
| 13. Tuberculosis (011.0-011.9) | 4 (0.7) | 95.2 |
| 14. Sinusitis (461.0-461.9) | 2 (0.4) | 95.6 |
| 15. Dehydration (276.0-276.5) | 2 (0.4) | 95.9 |
| 16. Syncopal episode (780.2) | 2 (0.4) | 96.3 |
| 17. Hantavirus pulmonary syndrome (480.8) | 2 (0.4) | 96.6 |
| 18. Adult respiratory distress syndrome (518.5) | 2 (0.4) | 97.0 |
| 19. Dementia (294.1) | 1 (0.2) | 97.2 |
| 20. Acute multiple sclerosis (340) | 1 (0.2) | 97.4 |
| 21. Pyelonephritis (590.1) | 1 (0.2) | 97.6 |
| 22. Diabetic ketoacidosis (250.1) | 1 (0.2) | 97.7 |
| 23. Angina (413.0-413.9) | 1 (0.2) | 97.9 |
| 24. Coxsackie virus infection (074.0-074.8) | 1 (0.2) | 98.1 |
| 25. Pleurisy (511.0-511.9) | 1 (0.2) | 98.3 |
| 26. Lyme disease (088.81) | 1 (0.2) | 98.5 |
| 27. Leukocytosis (288.8) | 1 (0.2) | 98.7 |
| 28. Interstitial pneumonitis (515) | 1 (0.2) | 98.8 |
| 29. Mononucleosis (075) | 1 (0.2) | 99.0 |
| 30. Pericarditis (420.0-420.99) | 1 (0.2) | 99.2 |
| 31. Empyema (510) | 1 (0.2) | 99.4 |
| 32. Aortic dissection (441.0-441.9) | 1 (0.2) | 99.6 |
| 33. Plague (Yersinia pestis) (020.0-020.9) | 1 (0.2) | 99.7 |
| 34. Tularemia (021.0-021.8) | 1 (0.2) | 99.9 |
| 35. Intestinal perforation (569.83) | 1 (0.2) | 100.0 |
| Total | 559 (100) |
Note: Of the nonanthrax diagnoses, 559 were from inhalational anthrax cases, 52 from Legionella cases, and 105 from the influenza cases.
ICD-9 = International Classification of Diseases, 9th Revision; CNS = central nervous system.
Figure 1.
Percentage of diagnoses in each of 8 diagnostic categories assigned to cases of inhalational anthrax.
CNS = central nervous system.
Overall, pneumonia was the most common diagnosis assigned to 7 of the 11 inhalational anthrax case vignettes (Table 3 ▶). Influenza, CNS infection, gastro-enteritis, and viral syndrome were the most common diagnoses in each of the 4 other case vignettes. There was fairly good agreement between the diagnoses offered by the responding physicians and the initial diagnoses provided in case reports for each of the 11 inhalational anthrax cases.
Table 3.
Initial Diagnosis Provided for Cases of Fatal and Nonfatal Inhalational Anthrax,LegionellaPneumonia, and Influenza A, and Family Physicians’ Responses to Clinical Case Vignettes
| Family Physicians’ Responses | |||||
| Case No. | Type | Initial Diagnosis Reported3,7 | No. | Most Common Diagnosis | Second Most Common Diagnosis |
| Inhalational anthrax cases | |||||
| Fatal | |||||
| 1 | FIA | Meningitis | 56 | CNS infection | Sepsis |
| 6 | FIA | Viral syndrome | 52 | Pneumonia | Bronchitis |
| 8 | FIA | Gastroenteritis | 48 | Gastroenteritis | Viral syndrome |
| 12 | FIA | CHF | 60 | Pneumonia | CHF |
| 14 | FIA | Viral syndrome | 48 | Pneumonia | Influenza |
| Overall | FIA | . . . | 264 | Pneumonia, sepsis, and CNS infection | |
| Nonfatal | |||||
| 3 | NFIA | Pneumonia | 46 | Pneumonia | Septicemia |
| 4 | NFIA | Inhalational anthrax* | 61 | Pneumonia | Bronchitis |
| 5 | NFIA | Inhalational anthrax* | 45 | Pneumonia | Influenza |
| 9 | NFIA | Viral syndrome | 54 | Influenza | Pneumonia |
| 10 | NFIA | Inhalational anthrax* | 40 | Pneumonia | Influenza |
| 11 | NFIA | Bronchitis | 61 | Viral syndrome | Pneumonia |
| Overall | NFIA | . . . | 307 | Pneumonia, influenza, and viral syndrome | |
| Total† | . . . | 571 | Pneumonia, influenza, and viral syndrome | ||
| Other cases | |||||
| 2 | LEG | Pneumonia | 53 | Pneumonia | Sepsis |
| 7 | INF-A | Influenza | 50 | Pneumonia | Bronchitis |
| 13 | INF-A | Influenza | 58 | URI | Bronchitis |
Note: The first and second most common hypothetical diagnoses are provided. For summary categories, the total numbers of responses and top 3 hypothetical diagnoses are provided. The number of cases include 9 “anthrax” diagnoses and 7 cases for which the diagnosis was not listed.
FIA = fatal inhalational anthrax; CNS = central nervous system; CHF = congestive heart failure; NFIA = nonfatal inhalational anthrax; LEG = Legionella pneumonia; INF-A = influenza A; URI = upper respiratory infection.
* Initial diagnoses of inhalational anthrax were based on workplace exposure.
† All 11 fatal and nonfatal cases.
DISCUSSION
Inhalational anthrax is an extremely rare disorder with which physicians in the United States have virtually no experience. Events associated with the intentional release of anthrax spores in October 2001 have underscored the need to better define the differential diagnosis of inhalational anthrax and other potential agents of biological terrorism.10 It may be possible to enhance surveillance systems of disease syndromes by relying on the temporal-spatial clustering of diagnostic codes if the primary care differential diagnosis of inhalational anthrax is known.13,18
The syndrome defined by the initial symptoms of known terrorism-associated inhalational anthrax is nonspecific. Consequently, defining the differential diagnosis would require a large population of cases and the initial clinical diagnosis arrived at by a clinician for each case. It is not feasible to observe directly patients with inhalational anthrax seeking care from a representative sample of clinicians. As an alternative, we asked clinically active family physicians—whose scope of practice routinely involves making clinical diagnoses based on incomplete data19—to provide their most likely nonanthrax diagnosis to case vignettes. Clinical case vignettes are a commonly used tool in education and evaluation of physicians,20,21 and most physicians are familiar with reading and responding to such vignettes. Moreover, a recent study demonstrated the validity of the response to clinical vignettes compared with the response to standardized patients.20 Accordingly, a representative sample of 244 family physicians provided sets of diagnoses for each of 11 bioterrorism-associated cases of inhalational anthrax.
In this study, clinically active family physicians gave 35 distinct nonanthrax diagnostic categories for case vignettes based on patients with inhalational anthrax. A great majority of responses (86%), however, were encompassed by only 7 diagnostic categories: pneumonia, influenza, nonspecific viral syndrome, septicemia, acute bronchitis, CNS infections, and gastroenteritis. It was not surprising that pneumonia and influenza accounted for more than 52% of diagnoses, given the acute onset of respiratory tract symptoms, fever, and malaise in inhalational anthrax.
Diagnoses assigned to individual cases by respondents were reasonably similar to the reported initial diagnoses provided by the evaluating physicians. The diagnoses also agreed well with those reached by physicians in historical accounts involving occupational exposure to anthrax.22–24 The wide variety of responses undoubtedly reflected personal experience and regional trends. For example, a physician from New Mexico diagnosed hantavirus pulmonary syndrome, whereas a Wisconsin physician diagnosed Lyme disease.
Of note was the shift in the frequency of diagnoses between cases that proved to be fatal and those that did not. Patients dying of inhalational anthrax probably were initially seen later in the course of the disease and were manifesting more of the terminal symptoms associated with edema and lethal factors.1,25 Those who survived appear to have been evaluated in the early stages of anthrax.
This study has several limitations. First, study physicians were informed that the vignettes they received could include cases of inhalational anthrax. We made this decision because the primary goal was to establish a set of nonanthrax diagnoses for the cases, not to determine how sensitive family physicians are to the diagnosis of inhalational anthrax, and because some physicians might have had previous exposure to published case descriptions. This disclosure might have led physicians to make hypothetical diagnoses that were more serious than those they would have made in its absence. This disclosure did not, however, prevent them from assigning relatively benign diagnoses to influenza cases.
Second, the response rate was relatively low at 36.9%. We did not attempt to make adjustments for nonrespondents. Response was not biased, however, by sex, geographic location, specific case evaluated, or type of case evaluated. Moreover, survey respondents closely resembled US family physicians by sex and location (Table 1 ▶). Their differential diagnosis was arrived at through a qualitative, not quantitative, approach. For each case, the diagnostic impressions were rapidly saturated.
Third, physicians responded to written case vignettes that were inflexible to their personal evaluation styles. This approach allows the construction of a differential diagnosis and the evaluation of hypothetical management of an extremely rare disorder. Using clinical case vignettes has been validated for measuring quality of care20 and for assessing customary care in reference to malpractice.21
Fourth, we used published case reports as the basis of the case vignettes. Whether these cases were recognizable to physicians is not known. Information for 10 cases was obtained from a review in Emerging Infectious Diseases,3 a journal not commonly read by family physicians. Finally, all cases of inhalational anthrax used in this study resulted from a single strain of B anthracis.26 Whether other weapon-grade strains of anthrax would produce similar diagnostic profiles is not known.
Even with additional information on the differential diagnosis of inhalational anthrax, linking this information into realistic early detection of bioterrorism involving anthrax is tenuous. Pneumonia is a relatively common disorder in ambulatory practices across the United States, accounting for approximately 3 million patient visits per year.27 Visits for influenza, viral syndrome, bronchitis, and acute gastroenteritis are reported even more frequently. Because inhalational anthrax is an extremely rare condition, surveillance systems that use the scanning of electronic medical data for these diagnoses or clusters of diagnoses emerging from primary care are likely to produce high levels of false-positive signals.12 Their performance depends on large signals that may require simultaneous occurrences of several cases in a defined geographic area.
In the autumn 2001 release of anthrax, the 11 cases of inhalational anthrax were dispersed over great expanses of space and time. Cases that were fatal had substantially different diagnostic signals than nonfatal cases, as evidenced by the higher frequency of diagnoses of much less common syndromes, such as septicemia and CNS infection, among fatal cases. Surveillance systems tuned to these diagnoses may improve on specificity, but at the expense of extreme timeliness. Consequently, overreliance on the occurrence and detection of disease clusters may be a dangerous approach.
The 11 recent cases of bioterrorism-related inhalational anthrax were detected by astute physicians and laboratorians providing usual medical care to their patients. The consistent and appropriate medical care offered by the thin line of America’s frontline physicians should not be undervalued. Accordingly, efforts to provide timely information and improve the training of clinicians for bioterrorism recognition and response should receive the highest priority.
Acknowledgments
We would like to thank Marlon Mundt, University of Wisconsin Department of Family Medicine, for assistance with statistical analyses.
Conflicts of interest: none reported
Funding support: This work was supported through an Advanced Research Training Grant to Dr. Temte from the American Academy of Family Physicians. Mr. Zinkel was supported through the University of Wisconsin, Department of Family Medicine Summer Student Research and Clinical Assistantship program with stipend support from the Wisconsin Academy of Family Physicians.
REFERENCES
- 1.Inglesby TV, O’Toole T, Henderson DA, et al, for the Working Group on Civilian Biodefense. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA. 2002;287:2236–2252. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann AF, Meltzer MI, Schmid GP. The economic impact of a bioterrorist attack: are prevention and postattack intervention programs justified? Emerg Infect Dis. 1997;3:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jernigan JA, Stephens DS, Ashford DA, et al, and members of the Anthrax Bioterrorism Investigation Team. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis. 2001;7:933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush LM, Abrams BH, Beall A, Johnson CC. Index case of fatal inhalational anthrax due to bioterrorism in the United States. N Engl J Med. 2001;345:1607–1610. [DOI] [PubMed] [Google Scholar]
- 5.Borio L, Frank D, Mani V, et al. Death due to bioterrorism-related inhalational anthrax: report of 2 patients. JAMA. 2001;286:2554–2559. [DOI] [PubMed] [Google Scholar]
- 6.Mina B, Dym JP, Kuepper F, et al. Fatal inhalational anthrax with unknown source of exposure in a 61-year-old woman in New York City. JAMA. 2002;287:858–862. [DOI] [PubMed] [Google Scholar]
- 7.Barakat LA, Quentzel HL, Jernigan JA, et al, for the Anthrax Bioterrorism Investigation Team. Fatal inhalational anthrax in a 94-year-old Connecticut woman. JAMA. 2002;287:863–868. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. Update: investigation of bioterrorism-related anthrax and interim guidelines for clinical evaluation of persons with possible anthrax. MMWR. 2001;50:941–948. [PubMed] [Google Scholar]
- 9.Brachman PS, Plotkin SA, Bumford FH, Atchison MM. An epidemic of inhalation anthrax: the first in the twentieth century. II. Epidemiology. Am J Hyg. 1960;72:6–23. [DOI] [PubMed] [Google Scholar]
- 10.Gerberding JL, Hughes JM, Koplan JP. Bioterrorism preparedness and response: clinicians and public health agencies as essential partners. JAMA. 2002;287:989–900. [DOI] [PubMed] [Google Scholar]
- 11.Lober WB, Karras BT, Wagner MM, et al. Roundtable of bioterrorism detection. Information system-based surveillance. J Am Med Informatics Assoc. 2002;9:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner MM, Tsui F-C, Espino JU, et al. The emerging science of very early detection of disease outbreaks. J Pub Health Management Pract. 2001;7:51–59. [DOI] [PubMed] [Google Scholar]
- 13.Lazarus R, Kleinman K, Dashevsky I, et al. Use of automated ambulatory-care encounter records for detection of acute illness clusters, including potential bioterrorism events. Emerg Infect Dis. 2002;8:753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barthell EN, Cordell WH, Moorhead JC, et al. The Frontlines of Medicine project: a proposal for the standardized communication of emergency department data for public health uses including syndromic surveillance for biological and chemical terrorism. Ann Emerg Med. 2002:39:422–429. [DOI] [PubMed] [Google Scholar]
- 15.Green LA, Fryer GE Jr, Yawn BP, Lanier D, Dovey SM. The ecology of medical care revisited. N Engl J Med. 2001;344:2021–2025. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. US map for regional bar charts. Available at: http://www.ncid.cdc.gov/FLU/RegionCharts/usmap.htm. Accessed July 9, 2002.
- 17.American Academy of Family Physicians. 2003 FACTS about family practice, Tables 3 and 7. Available at: http://www.aafp.org/x530.xml#x529. Accessed December 29, 2003.
- 18.Teich JM, Wagner MM, Mackenzie CF, Schafer KO. The informatics response in disaster, terrorism, and war. J Am Med Informatics Assoc. 2002;9:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okkes IM, Oskam SK, Lamberts H. The probability of specific diagnoses for patient presenting with common symptoms to Dutch family physicians. J Fam Pract. 2002;51:31–36. [PubMed] [Google Scholar]
- 20.Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction. A prospective validation study of 3 methods for measuring quality. JAMA. 2000;283:1715–1722. [DOI] [PubMed] [Google Scholar]
- 21.Hartz A, Lucas J, Cramm T, et al. Physician surveys to assess customary care in medical malpractice cases. J Gen Int Med. 2002;17:546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brachman PS. Inhalation anthrax. Ann NY Acad Sci. 1980;353:83–93. [DOI] [PubMed] [Google Scholar]
- 23.Plotkin SA, Brachman PS, Utell M, Bumford FH, Atchison MM. An epidemic of inhalation anthrax, the first in the twentieth century. I. Clinical features. Am J Med. 1960;29:992–1001. [DOI] [PubMed] [Google Scholar]
- 24.Gold H. Anthrax: a report of one hundred seventeen cases. Arch Intern Med. 1955;96:387–396. [DOI] [PubMed] [Google Scholar]
- 25.Brachman PS, Kaufmann AF. Anthrax. In: Evans AS, Brachman PS, eds. Bacterial Infections of Humans. 3rd ed. New York, NY: Plenum Publishing Corp; 1998:95–107.
- 26.Hoffmaster AR, Fitzgerald CC, Ribot E, Mayer LW, Popovic T. Molecular subtyping of Bacillus anthracis and the 2001 bioterrorism-associate anthrax outbreak, United States. Emerg Infect Dis. 2002;8:1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schappert SM, Nelson C. National Ambulatory Medical Care Survey: 1995–96 summary. Vital & Health Statistics- Series 13: Data from the National Health Survey. 1999;142:1–122. [PubMed] [Google Scholar]