Abstract
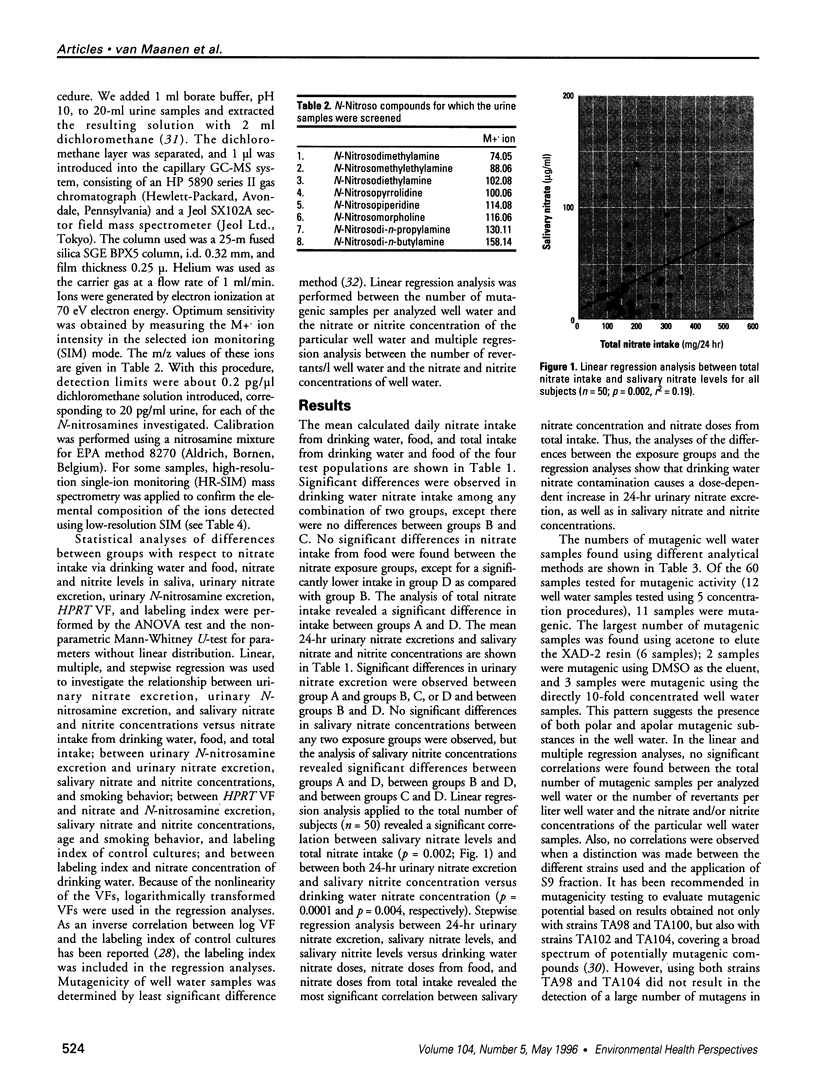
We studied peripheral lymphocyte HPRT variant frequency and endogenous nitrosation in human populations exposed to various nitrate levels in their drinking water. Four test populations of women volunteers were compared. Low and medium tap water nitrate exposure groups (14 and 21 subjects) were using public water supplies with nitrate levels of 0.02 and 17.5 mg/l, respectively. Medium and high well water nitrate exposure groups (6 and 9 subjects) were using private water wells with mean nitrate levels of 25 and 135 mg/l, respectively. Higher nitrate intake by drinking water consumption resulted in a dose-dependent increase in 24-hr urinary nitrate excretion and in increased salivary nitrate and nitrite levels. The mean log variant frequency of peripheral lymphocytes was significantly higher in the medium well water exposure group than in the low and medium tap water exposure groups. An inverse correlation between peripheral lymphocyte labeling index and nitrate concentration of drinking water was observed. Analysis of N-nitrosamine in the urine of 22 subjects by gas chromatography-mass spectrometry revealed the presence of N-nitrosopyrrolidine in 18 subjects. Analysis of the mutagenicity of well water samples showed that a small number of the well water samples were mutagenic in the Ames Salmonella typhimurium test after concentration over XAD-2 resin. In conclusion, consumption of drinking water, especially well water, with high nitrate levels can imply a genotoxic risk for humans as indicated by increased HPRT variant frequencies and by endogenous formation of carcinogenic N-nitroso compounds from nitrate-derived nitrite.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeing H. Epidemiological research in stomach cancer: progress over the last ten years. J Cancer Res Clin Oncol. 1991;117(2):133–143. doi: 10.1007/BF01613137. [DOI] [PubMed] [Google Scholar]
- Boeing H., Frentzel-Beyme R., Berger M., Berndt V., Göres W., Körner M., Lohmeier R., Menarcher A., Männl H. F., Meinhardt M. Case-control study on stomach cancer in Germany. Int J Cancer. 1991 Apr 1;47(6):858–864. doi: 10.1002/ijc.2910470612. [DOI] [PubMed] [Google Scholar]
- Buiatti E., Palli D., Decarli A., Amadori D., Avellini C., Bianchi S., Bonaguri C., Cipriani F., Cocco P., Giacosa A. A case-control study of gastric cancer and diet in Italy: II. Association with nutrients. Int J Cancer. 1990 May 15;45(5):896–901. doi: 10.1002/ijc.2910450520. [DOI] [PubMed] [Google Scholar]
- Chen C. S. [Investigation on nitrosamine in drinking water in Huang Shi--a high-risk area of stomach cancer in Fujian province]. Zhonghua Liu Xing Bing Xue Za Zhi. 1989 Dec;10(6):359–362. [PubMed] [Google Scholar]
- Cuello C., Correa P., Haenszel W., Gordillo G., Brown C., Archer M., Tannenbaum S. Gastric cancer in Colombia. I. Cancer risk and suspect environmental agents. J Natl Cancer Inst. 1976 Nov;57(5):1015–1020. doi: 10.1093/jnci/57.5.1015. [DOI] [PubMed] [Google Scholar]
- Gangolli S. D., van den Brandt P. A., Feron V. J., Janzowsky C., Koeman J. H., Speijers G. J., Spiegelhalder B., Walker R., Wisnok J. S. Nitrate, nitrite and N-nitroso compounds. Eur J Pharmacol. 1994 Nov 1;292(1):1–38. doi: 10.1016/0926-6917(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Gao Z. Q. [Study on the effect of micronucleus with drinking water in the high risk area of stomach cancer]. Zhonghua Yu Fang Yi Xue Za Zhi. 1989 Sep;23(5):308–310. [PubMed] [Google Scholar]
- Gao Z. Q. [Unscheduled DNA synthesis (UDS) in human lymphocytes induced by drinking water in high risk areas of stomach cancer]. Zhonghua Bing Li Xue Za Zhi. 1989 Sep;18(3):171–174. [PubMed] [Google Scholar]
- Garland W. A., Kuenzig W., Rubio F., Kornychuk H., Norkus E. P., Conney A. H. Urinary excretion of nitrosodimethylamine and nitrosoproline in humans: interindividual and intraindividual differences and the effect of administered ascorbic acid and alpha-tocopherol. Cancer Res. 1986 Oct;46(10):5392–5400. [PubMed] [Google Scholar]
- Garland W. A., Kuenzig W., Rubio F., Kornychuk H., Norkus E. P., Conney A. H. Urinary excretion of nitrosodimethylamine and nitrosoproline in humans: interindividual and intraindividual differences and the effect of administered ascorbic acid and alpha-tocopherol. Cancer Res. 1986 Oct;46(10):5392–5400. [PubMed] [Google Scholar]
- Gilli G., Corrao G., Favilli S. Concentrations of nitrates in drinking water and incidence of gastric carcinomas: first descriptive study of the Piemonte Region, Italy. Sci Total Environ. 1984 Mar 1;34(1-2):35–48. doi: 10.1016/0048-9697(84)90039-1. [DOI] [PubMed] [Google Scholar]
- Hageman G., Kikken R., Ten Hoor F., Kleinjans J. Assessment of mutagenic activity of repeatedly used deep-frying fats. Mutat Res. 1988 Apr;204(4):593–604. doi: 10.1016/0165-1218(88)90062-6. [DOI] [PubMed] [Google Scholar]
- Hageman G., Welle I., Stierum R., Albering H., Kleinjans J. Detection of 6-thioguanine-resistant human peripheral blood lymphocytes using 5-bromodeoxyuridine labeling in combination with immunocytochemical staining. Mutagenesis. 1993 Nov;8(6):495–501. doi: 10.1093/mutage/8.6.495. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Yokokura T., Kawai Y., Mutai M. Dimethylnitrosamine formation in the gastro-intestinal tract of rats. Food Cosmet Toxicol. 1976 Dec;14(6):553–556. doi: 10.1016/s0015-6264(76)80007-7. [DOI] [PubMed] [Google Scholar]
- Jansen J. G., Mohn G. R., Vrieling H., van Teijlingen C. M., Lohman P. H., van Zeeland A. A. Molecular analysis of hprt gene mutations in skin fibroblasts of rats exposed in vivo to N-methyl-N-nitrosourea or N-ethyl-N-nitrosourea. Cancer Res. 1994 May 1;54(9):2478–2485. [PubMed] [Google Scholar]
- Kleinjans J. C., Albering H. J., Marx A., van Maanen J. M., van Agen B., ten Hoor F., Swaen G. M., Mertens P. L. Nitrate contamination of drinking water: evaluation of genotoxic risk in human populations. Environ Health Perspect. 1991 Aug;94:189–193. doi: 10.1289/ehp.94-1567968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligerman A. D., Chapin R. E., Erexson G. L., Germolec D. R., Kwanyuen P., Yang R. S. Analyses of cytogenetic damage in rodents following exposure to simulated groundwater contaminated with pesticides and a fertilizer. Mutat Res. 1993 Jul;300(2):125–134. doi: 10.1016/0165-1218(93)90130-6. [DOI] [PubMed] [Google Scholar]
- Knight T., Pirastu R., Palli D., Cocco P., Leach S., Packer P., Iannarilli R., Manca P., Møller H., Forman D. Nitrate and N-nitrosoproline excretion in two Italian regions with contrasting rates of gastric cancer: the role of nitrate and other factors in endogenous nitrosation. Int J Cancer. 1992 Mar 12;50(5):736–739. doi: 10.1002/ijc.2910500512. [DOI] [PubMed] [Google Scholar]
- Kool H. J., van Kreyl C. F. Mutagenic activity in drinking water prepared from groundwater: a survey of ten cities in The Netherlands. Sci Total Environ. 1988 Nov 1;77(1):51–60. doi: 10.1016/0048-9697(88)90314-2. [DOI] [PubMed] [Google Scholar]
- Lu S. H., Ohshima H., Fu H. M., Tian Y., Li F. M., Blettner M., Wahrendorf J., Bartsch H. Urinary excretion of N-nitrosamino acids and nitrate by inhabitants of high- and low-risk areas for esophageal cancer in Northern China: endogenous formation of nitrosoproline and its inhibition by vitamin C. Cancer Res. 1986 Mar;46(3):1485–1491. [PubMed] [Google Scholar]
- Luster M. I., Blank J. A., Dean J. H. Molecular and cellular basis of chemically induced immunotoxicity. Annu Rev Pharmacol Toxicol. 1987;27:23–49. doi: 10.1146/annurev.pa.27.040187.000323. [DOI] [PubMed] [Google Scholar]
- Magee P. N. The experimental basis for the role of nitroso compounds in human cancer. Cancer Surv. 1989;8(2):207–239. [PubMed] [Google Scholar]
- Meier J. R. Genotoxic activity of organic chemicals in drinking water. Mutat Res. 1988 Nov;196(3):211–245. doi: 10.1016/0165-1110(88)90008-5. [DOI] [PubMed] [Google Scholar]
- Mirvish S. S. The etiology of gastric cancer. Intragastric nitrosamide formation and other theories. J Natl Cancer Inst. 1983 Sep;71(3):629–647. [PubMed] [Google Scholar]
- Møller H., Landt J., Pedersen E., Jensen P., Autrup H., Jensen O. M. Endogenous nitrosation in relation to nitrate exposure from drinking water and diet in a Danish rural population. Cancer Res. 1989 Jun 1;49(11):3117–3121. [PubMed] [Google Scholar]
- Ohshima H., Bartsch H. Quantitative estimation of endogenous nitrosation in humans by monitoring N-nitrosoproline excreted in the urine. Cancer Res. 1981 Sep;41(9 Pt 1):3658–3662. [PubMed] [Google Scholar]
- Risch H. A., Jain M., Choi N. W., Fodor J. G., Pfeiffer C. J., Howe G. R., Harrison L. W., Craib K. J., Miller A. B. Dietary factors and the incidence of cancer of the stomach. Am J Epidemiol. 1985 Dec;122(6):947–959. doi: 10.1093/oxfordjournals.aje.a114199. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder B., Preussmann R. In vivo nitrosation of amidopyrine in humans: use of 'ethanol effect' for biological monitoring of N-nitrosodimethylamine in urine. Carcinogenesis. 1985 Apr;6(4):545–548. doi: 10.1093/carcin/6.4.545. [DOI] [PubMed] [Google Scholar]
- Tricker A. R., Mostafa M. H., Spiegelhalder B., Preussmann R. Urinary excretion of nitrate, nitrite and N-nitroso compounds in Schistosomiasis and bilharzia bladder cancer patients. Carcinogenesis. 1989 Mar;10(3):547–552. doi: 10.1093/carcin/10.3.547. [DOI] [PubMed] [Google Scholar]
- Tricker A. R., Stickler D. J., Chawla J. C., Preussmann R. Increased urinary nitrosamine excretion in paraplegic patients. Carcinogenesis. 1991 May;12(5):943–946. doi: 10.1093/carcin/12.5.943. [DOI] [PubMed] [Google Scholar]
- Tsugane S., Tsuda M., Gey F., Watanabe S. Cross-sectional study with multiple measurements of biological markers for assessing stomach cancer risks at the population level. Environ Health Perspect. 1992 Nov;98:207–210. doi: 10.1289/ehp.9298207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Chen J., Ohshima H., Pignatelli B., Boreham J., Li J., Campbell T. C., Peto R., Bartsch H. Geographic association between urinary excretion of N-nitroso compounds and oesophageal cancer mortality in China. Int J Cancer. 1993 Jul 9;54(5):713–719. doi: 10.1002/ijc.2910540502. [DOI] [PubMed] [Google Scholar]
- Xu G., Song P., Reed P. I. The relationship between gastric mucosal changes and nitrate intake via drinking water in a high-risk population for gastric cancer in Moping county, China. Eur J Cancer Prev. 1992 Oct;1(6):437–443. doi: 10.1097/00008469-199210000-00007. [DOI] [PubMed] [Google Scholar]
- Zatonski W., Ohshima H., Przewozniak K., Drosik K., Mierzwinska J., Krygier M., Chmielarczyk W., Bartsch H. Urinary excretion of N-nitrosamino acids and nitrate by inhabitants of high- and low-risk areas for stomach cancer in Poland. Int J Cancer. 1989 Nov 15;44(5):823–827. doi: 10.1002/ijc.2910440513. [DOI] [PubMed] [Google Scholar]
- Zeng Y., Ohshima H., Bouvier G., Roy P., Zhong J., Li B., Brouet I., de Thé G., Bartsch H. Urinary excretion of nitrosamino acids and nitrate by inhabitants of high- and low-risk areas for nasopharyngeal carcinoma in southern China. Cancer Epidemiol Biomarkers Prev. 1993 May-Jun;2(3):195–200. [PubMed] [Google Scholar]
- van Maanen J. M., van Dijk A., Mulder K., de Baets M. H., Menheere P. C., van der Heide D., Mertens P. L., Kleinjans J. C. Consumption of drinking water with high nitrate levels causes hypertrophy of the thyroid. Toxicol Lett. 1994 Jun;72(1-3):365–374. doi: 10.1016/0378-4274(94)90050-7. [DOI] [PubMed] [Google Scholar]