Abstract
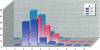
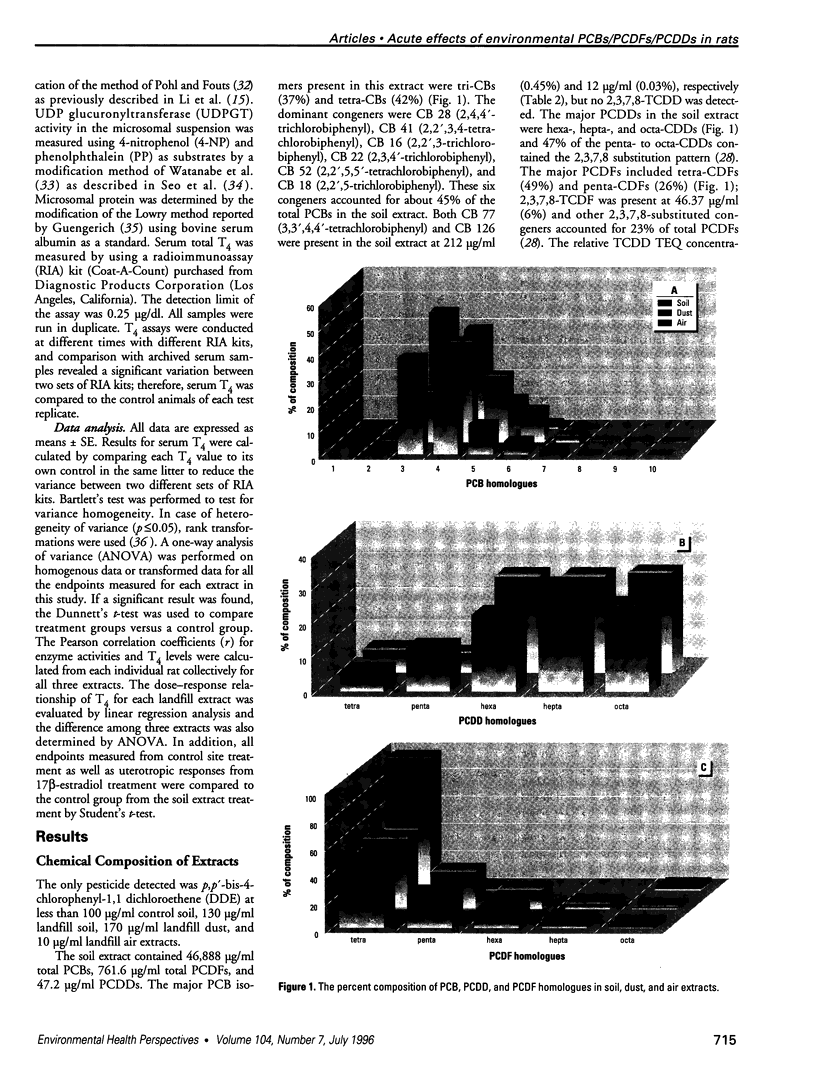
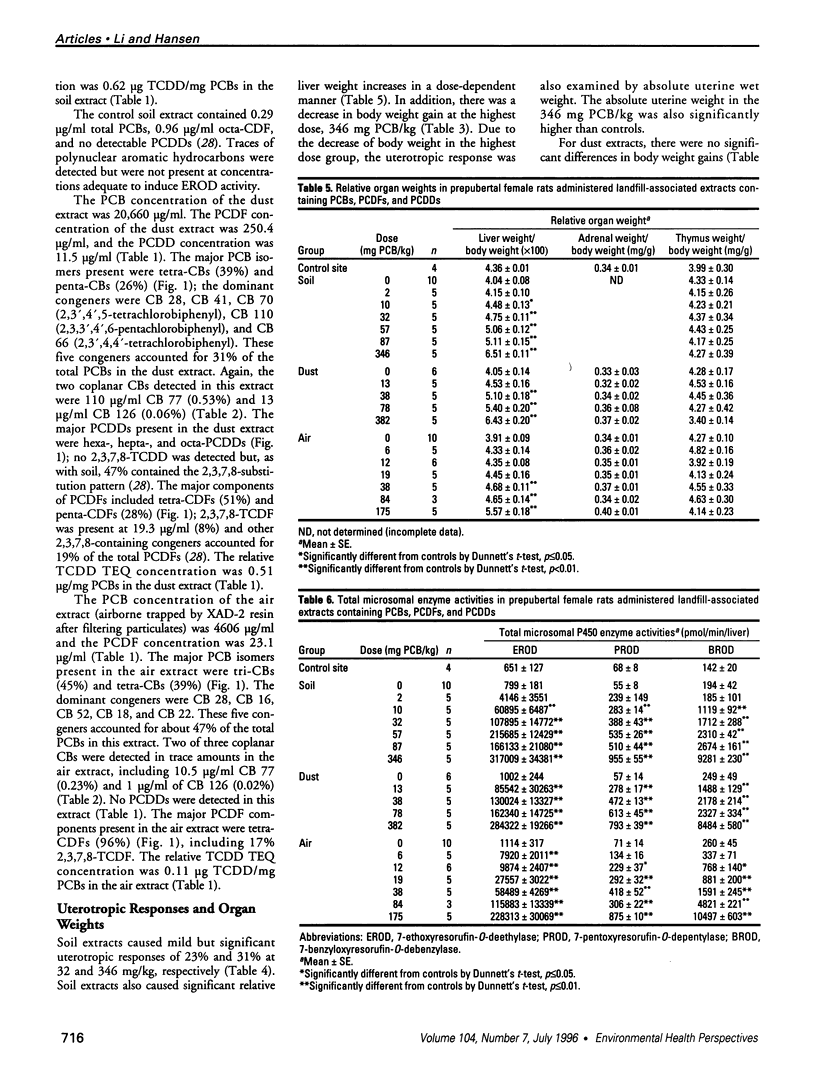
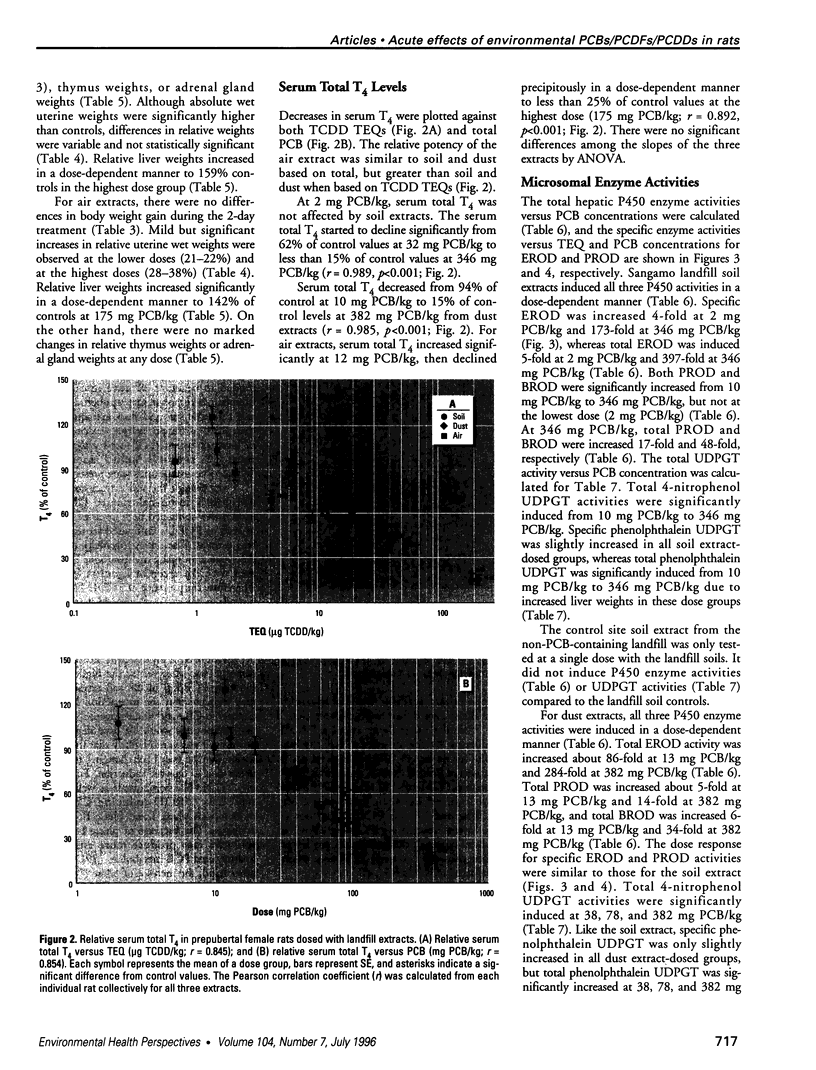
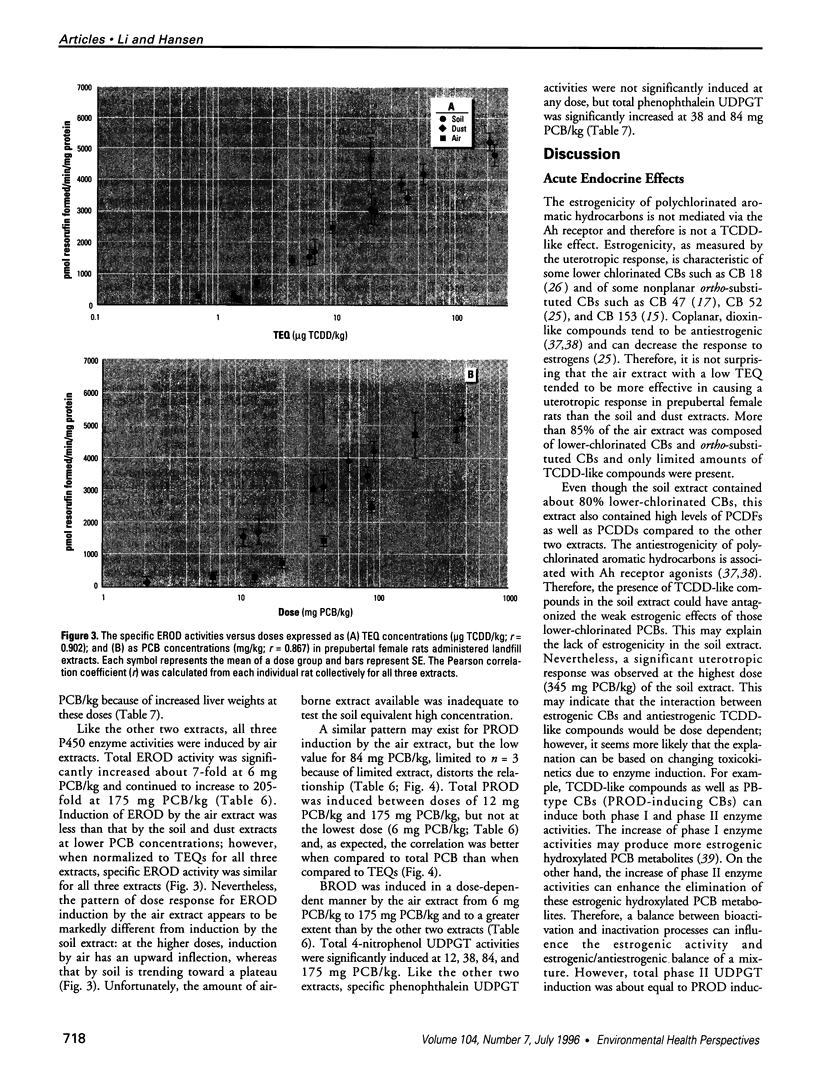
Air, subsurface soil, and superficial dust from a National Priorities List landfill located in southern Illinois were sampled to determine their potential toxicities. The major components of these landfill extracts were polychlorinated biphenyls (PCBs), with significant amounts of polychlorinated dibenzofurans (PCDFs) and small amounts of polychlorinated dibenzodioxins (PCDDs). The 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) toxic equivalency factor approach has been proposed to estimate the toxic potency of complex mixtures of chlorinated aromatics for environmental risk assessment. However, most components of environmental residues are nonplanar and do not act as aryl hydrocarbon (Ah) receptor agonists, so there is a great risk of not identifying adverse responses that are not dioxinlike. We used a 2-day prepubertal female rat bioassay to examine multiple biological responses, including both dioxinlike and nondioxinlike effects from these landfill extracts. As expected, both types of effects were detected. The soil and dust extracts produced similar dose-response relationships for 7-ethoxyresorufin O-deethylase, 7-pentoxyresorufin O-depentylase, 7-benzyloxyresorufin O-debenzylase, and 4-nitrophenol UDP-glucuronyltransferase induction; the dose response for the air extract deviated from the other two extracts. Soil, dust, and air extracts effectively reduced serum total thyroxine (T4) with similar dose-response relationships, despite the significantly different TCDD toxic equivalent (TEQ) values of these three extracts. Both soil (346 mg PCB/kg) and air (175 mg PCB/kg) extracts caused a greater than 30% increase in uterine wet weight. This study suggests that a more comprehensive approach is required to improve current risk assessment of environmental mixtures. TCDD TEQs reflect only a portion of effects and may especially underpredict effects on T4.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastomsky C. H. Effects of a polychlorinated biphenyl mixture (aroclor 1254) and DDT on biliary thyroxine excretion in rats. Endocrinology. 1974 Oct;95(4):1150–1155. doi: 10.1210/endo-95-4-1150. [DOI] [PubMed] [Google Scholar]
- Bastomsky C. H., Murthy P. V., Banovac K. Alterations in thyroxine metabolism produced by cutaneous application of microscope immersion oil: effects due to polychlorinated biphenyls. Endocrinology. 1976 May;98(5):1309–1314. doi: 10.1210/endo-98-5-1309. [DOI] [PubMed] [Google Scholar]
- Bastomsky C. H., Murthy P. V. Enchanced in vitro hepatic glucuronidation of thyroxine in rats following cutaneous application or ingestion of polychlorinated biphenyls. Can J Physiol Pharmacol. 1976 Feb;54(1):23–26. doi: 10.1139/y76-004. [DOI] [PubMed] [Google Scholar]
- Brouwer A. Inhibition of thyroid hormone transport in plasma of rats by polychlorinated biphenyls. Arch Toxicol Suppl. 1989;13:440–445. doi: 10.1007/978-3-642-74117-3_87. [DOI] [PubMed] [Google Scholar]
- Byrne J. J., Carbone J. P., Hanson E. A. Hypothyroidism and abnormalities in the kinetics of thyroid hormone metabolism in rats treated chronically with polychlorinated biphenyl and polybrominated biphenyl. Endocrinology. 1987 Aug;121(2):520–527. doi: 10.1210/endo-121-2-520. [DOI] [PubMed] [Google Scholar]
- Chen Z. Y., Eaton D. L. Differential regulation of cytochrome(s) P450 2B1/2 by phenobarbital in hepatic hyperplastic nodules induced by aflatoxin B1 or diethylnitrosamine plus 2-acetylaminofluorene in male F344 rats. Toxicol Appl Pharmacol. 1991 Oct;111(1):132–144. doi: 10.1016/0041-008x(91)90142-2. [DOI] [PubMed] [Google Scholar]
- De Vito M. J., Maier W. E., Diliberto J. J., Birnbaum L. S. Comparative ability of various PCBs, PCDFs, and TCDD to induce cytochrome P450 1A1 and 1A2 activity following 4 weeks of treatment. Fundam Appl Toxicol. 1993 Jan;20(1):125–130. [PubMed] [Google Scholar]
- Dickerson R., Keller L. H., Safe S. Alkyl polychlorinated dibenzofurans and related compounds as antiestrogens in the female rat uterus: structure-activity studies. Toxicol Appl Pharmacol. 1995 Dec;135(2):287–298. doi: 10.1006/taap.1995.1235. [DOI] [PubMed] [Google Scholar]
- Fensterheim R. J. Documenting temporal trends of polychlorinated biphenyls in the environment. Regul Toxicol Pharmacol. 1993 Oct;18(2):181–201. doi: 10.1006/rtph.1993.1052. [DOI] [PubMed] [Google Scholar]
- Hansen L. G., Li M. H., Saeed A., Bush B. Environmental polychlorinated biphenyls: acute toxicity of landfill soil extract to female prepubertal rats. Arch Environ Contam Toxicol. 1995 Oct;29(3):334–343. doi: 10.1007/BF00212498. [DOI] [PubMed] [Google Scholar]
- Harrad S. J., Sewart A. P., Alcock R., Boumphrey R., Burnett V., Duarte-Davidson R., Halsall C., Sanders G., Waterhouse K., Wild S. R. Polychlorinated biphenyls (PCBs) in the British environment: sinks, sources and temporal trends. Environ Pollut. 1994;85(2):131–146. doi: 10.1016/0269-7491(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Harris M., Zacharewski T., Safe S. Comparative potencies of Aroclors 1232, 1242, 1248, 1254, and 1260 in male Wistar rats--assessment of the toxic equivalency factor (TEF) approach for polychlorinated biphenyls (PCBs). Fundam Appl Toxicol. 1993 May;20(4):456–463. doi: 10.1006/faat.1993.1056. [DOI] [PubMed] [Google Scholar]
- Jansen H. T., Cooke P. S., Porcelli J., Liu T. C., Hansen L. G. Estrogenic and antiestrogenic actions of PCBs in the female rat: in vitro and in vivo studies. Reprod Toxicol. 1993 May-Jun;7(3):237–248. doi: 10.1016/0890-6238(93)90230-5. [DOI] [PubMed] [Google Scholar]
- Kennedy S. W., Lorenzen A., James C. A., Collins B. T. Ethoxyresorufin-O-deethylase and porphyrin analysis in chicken embryo hepatocyte cultures with a fluorescence multiwell plate reader. Anal Biochem. 1993 May 15;211(1):102–112. doi: 10.1006/abio.1993.1239. [DOI] [PubMed] [Google Scholar]
- Kohn M. C., Sewall C. H., Lucier G. W., Portier C. J. A mechanistic model of effects of dioxin on thyroid hormones in the rat. Toxicol Appl Pharmacol. 1996 Jan;136(1):29–48. doi: 10.1006/taap.1996.0004. [DOI] [PubMed] [Google Scholar]
- Kopponen P., Törrönen R., Mäki-Paakkanen J., von Wright A., Kärenlampi S. Comparison of CYP1A1 induction and genotoxicity in vitro as indicators of potentially harmful effects of environmental samples. Arch Toxicol. 1994;68(3):167–173. doi: 10.1007/s002040050050. [DOI] [PubMed] [Google Scholar]
- Korach K. S., Sarver P., Chae K., McLachlan J. A., McKinney J. D. Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls: conformationally restricted structural probes. Mol Pharmacol. 1988 Jan;33(1):120–126. [PubMed] [Google Scholar]
- Krishnan V., Safe S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), and dibenzofurans (PCDFs) as antiestrogens in MCF-7 human breast cancer cells: quantitative structure-activity relationships. Toxicol Appl Pharmacol. 1993 May;120(1):55–61. doi: 10.1006/taap.1993.1086. [DOI] [PubMed] [Google Scholar]
- Lans M. C., Klasson-Wehler E., Willemsen M., Meussen E., Safe S., Brouwer A. Structure-dependent, competitive interaction of hydroxy-polychlorobiphenyls, -dibenzo-p-dioxins and -dibenzofurans with human transthyretin. Chem Biol Interact. 1993 Jul;88(1):7–21. doi: 10.1016/0009-2797(93)90081-9. [DOI] [PubMed] [Google Scholar]
- Li M. H., Hansen L. G. Uterotropic and enzyme induction effects of 2,2',5-trichlorobiphenyl. Bull Environ Contam Toxicol. 1995 Apr;54(4):494–500. doi: 10.1007/BF00192590. [DOI] [PubMed] [Google Scholar]
- Li M. H., Zhao Y. D., Hansen L. G. Multiple dose toxicokinetic influence on the estrogenicity of 2,2',4,4',5,5'-hexachlorobiphenyl. Bull Environ Contam Toxicol. 1994 Oct;53(4):583–590. doi: 10.1007/BF00199030. [DOI] [PubMed] [Google Scholar]
- Mason G., Farrell K., Keys B., Piskorska-Pliszczynska J., Safe L., Safe S. Polychlorinated dibenzo-p-dioxins: quantitative in vitro and in vivo structure-activity relationships. Toxicology. 1986 Oct;41(1):21–31. doi: 10.1016/0300-483x(86)90101-0. [DOI] [PubMed] [Google Scholar]
- Mason G., Sawyer T., Keys B., Bandiera S., Romkes M., Piskorska-Pliszczynska J., Zmudzka B., Safe S. Polychlorinated dibenzofurans (PCDFs): correlation between in vivo and in vitro structure-activity relationships. Toxicology. 1985 Oct;37(1-2):1–12. doi: 10.1016/0300-483x(85)90108-8. [DOI] [PubMed] [Google Scholar]
- McKinney J. D., Waller C. L. Polychlorinated biphenyls as hormonally active structural analogues. Environ Health Perspect. 1994 Mar;102(3):290–297. doi: 10.1289/ehp.94102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. C., Groen D., Veerman M., van Amerongen C. J., Koëter H. B., Smits van Prooije A. E., Visser T. J., Koeman J. H., Brouwer A. Interference of polychlorinated biphenyls in hepatic and brain thyroid hormone metabolism in fetal and neonatal rats. Toxicol Appl Pharmacol. 1993 Sep;122(1):27–33. doi: 10.1006/taap.1993.1168. [DOI] [PubMed] [Google Scholar]
- Namkung M. J., Yang H. L., Hulla J. E., Juchau M. R. On the substrate specificity of cytochrome P450IIIA1. Mol Pharmacol. 1988 Nov;34(5):628–637. [PubMed] [Google Scholar]
- Ness D. K., Schantz S. L., Moshtaghian J., Hansen L. G. Effects of perinatal exposure to specific PCB congeners on thyroid hormone concentrations and thyroid histology in the rat. Toxicol Lett. 1993 Jun;68(3):311–323. doi: 10.1016/0378-4274(93)90023-q. [DOI] [PubMed] [Google Scholar]
- Pohl R. J., Fouts J. R. A rapid method for assaying the metabolism of 7-ethoxyresorufin by microsomal subcellular fractions. Anal Biochem. 1980 Sep 1;107(1):150–155. doi: 10.1016/0003-2697(80)90505-9. [DOI] [PubMed] [Google Scholar]
- Safe S. H. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 1994;24(2):87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Safe S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs). Crit Rev Toxicol. 1990;21(1):51–88. doi: 10.3109/10408449009089873. [DOI] [PubMed] [Google Scholar]
- Schuetz E. G., Wrighton S. A., Barwick J. L., Guzelian P. S. Induction of cytochrome P-450 by glucocorticoids in rat liver. I. Evidence that glucocorticoids and pregnenolone 16 alpha-carbonitrile regulate de novo synthesis of a common form of cytochrome P-450 in cultures of adult rat hepatocytes and in the liver in vivo. J Biol Chem. 1984 Feb 10;259(3):1999–2006. [PubMed] [Google Scholar]
- Schuetz E. G., Wrighton S. A., Safe S. H., Guzelian P. S. Regulation of cytochrome P-450p by phenobarbital and phenobarbital-like inducers in adult rat hepatocytes in primary monolayer culture and in vivo. Biochemistry. 1986 Mar 11;25(5):1124–1133. doi: 10.1021/bi00353a027. [DOI] [PubMed] [Google Scholar]
- Seo B. W., Li M. H., Hansen L. G., Moore R. W., Peterson R. E., Schantz S. L. Effects of gestational and lactational exposure to coplanar polychlorinated biphenyl (PCB) congeners or 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on thyroid hormone concentrations in weanling rats. Toxicol Lett. 1995 Aug;78(3):253–262. doi: 10.1016/0378-4274(95)03329-j. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., McQuiddy P., Kasper C. B. Induction of the hepatic mixed-function oxidase system by synthetic glucocorticoids. Transcriptional and post-transcriptional regulation. J Biol Chem. 1987 Jan 5;262(1):326–332. [PubMed] [Google Scholar]
- Soontornchat S, Li MH, Cooke PS, Hansen LG. Toxicokinetic and Toxicodynamic Influences on Endocrine Disruption by Polychlorinated Biphenyls. Environ Health Perspect. 1994 Jun;102(6-7):568–571. doi: 10.1289/ehp.94102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H. K., Hoskins B., Ho I. K. Selective inhibitory effect of organophosphates on UDP-glucuronyl transferase activities in rat liver microsomes. Biochem Pharmacol. 1986 Feb 1;35(3):455–460. doi: 10.1016/0006-2952(86)90219-4. [DOI] [PubMed] [Google Scholar]