Abstract
The goal of researchers working in the area of developmental toxicology is to prevent adverse reproductive outcomes (early pregnancy loss, birth defects, reduced birth weight, and altered functional development) in humans due to exposures to environmental contaminants, therapeutic drugs, and other factors. To best achieve that goal, it is important that relevant information be gathered and assimilated in the risk assessment process. One of the major challenges of improved risk assessment is to better use all pertinent biological and mechanistic information. This may be done qualitatively (e.g., demonstrating that the experimental model is not appropriate for extrapolation purposes); semiquantitatively (using information to reduce the degree of uncertainty present under default extrapolation procedures), or quantitatively (formally describing the relationships between exposure and adverse outcome in mathematical forms, including components that directly reflect individual steps in the overall progression of toxicity). In this paper we review the recent advances in the risk assessment process for developmental toxicants and hypothesize on future directions that may revolutionize our thinking in this area. The road to these changes sometimes appears to be a well-mapped course on a relatively smooth surface; at other times the path is bumpy and obscure, while at still other times it is only a wish in the eye of the engineer to cross an uncharted and rugged environment.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkema M. J., van der Lugt N. M., Bobeldijk R. C., Berns A., van Lohuizen M. Transformation of axial skeleton due to overexpression of bmi-1 in transgenic mice. Nature. 1995 Apr 20;374(6524):724–727. doi: 10.1038/374724a0. [DOI] [PubMed] [Google Scholar]
- Allen B. C., Kavlock R. J., Kimmel C. A., Faustman E. M. Dose-response assessment for developmental toxicity. II. Comparison of generic benchmark dose estimates with no observed adverse effect levels. Fundam Appl Toxicol. 1994 Nov;23(4):487–495. doi: 10.1006/faat.1994.1133. [DOI] [PubMed] [Google Scholar]
- Allen B. C., Kavlock R. J., Kimmel C. A., Faustman E. M. Dose-response assessment for developmental toxicity. III. Statistical models. Fundam Appl Toxicol. 1994 Nov;23(4):496–509. doi: 10.1006/faat.1994.1134. [DOI] [PubMed] [Google Scholar]
- Barnes D. G., Daston G. P., Evans J. S., Jarabek A. M., Kavlock R. J., Kimmel C. A., Park C., Spitzer H. L. Benchmark Dose Workshop: criteria for use of a benchmark dose to estimate a reference dose. Regul Toxicol Pharmacol. 1995 Apr;21(2):296–306. doi: 10.1006/rtph.1995.1043. [DOI] [PubMed] [Google Scholar]
- Barnes D. G., Dourson M. Reference dose (RfD): description and use in health risk assessments. Regul Toxicol Pharmacol. 1988 Dec;8(4):471–486. doi: 10.1016/0273-2300(88)90047-5. [DOI] [PubMed] [Google Scholar]
- Boike G. M., Deppe G., Young J. D., Malone J. M., Jr, Malviya V. K., Sokol R. J. Chemotherapy in a pregnant rat model. 2.5-fluorouracil: nonlinear kinetics and placental transfer. Gynecol Oncol. 1989 Aug;34(2):191–194. doi: 10.1016/0090-8258(89)90140-6. [DOI] [PubMed] [Google Scholar]
- Bryant S. V., Gardiner D. M. Retinoic acid, local cell-cell interactions, and pattern formation in vertebrate limbs. Dev Biol. 1992 Jul;152(1):1–25. doi: 10.1016/0012-1606(92)90152-7. [DOI] [PubMed] [Google Scholar]
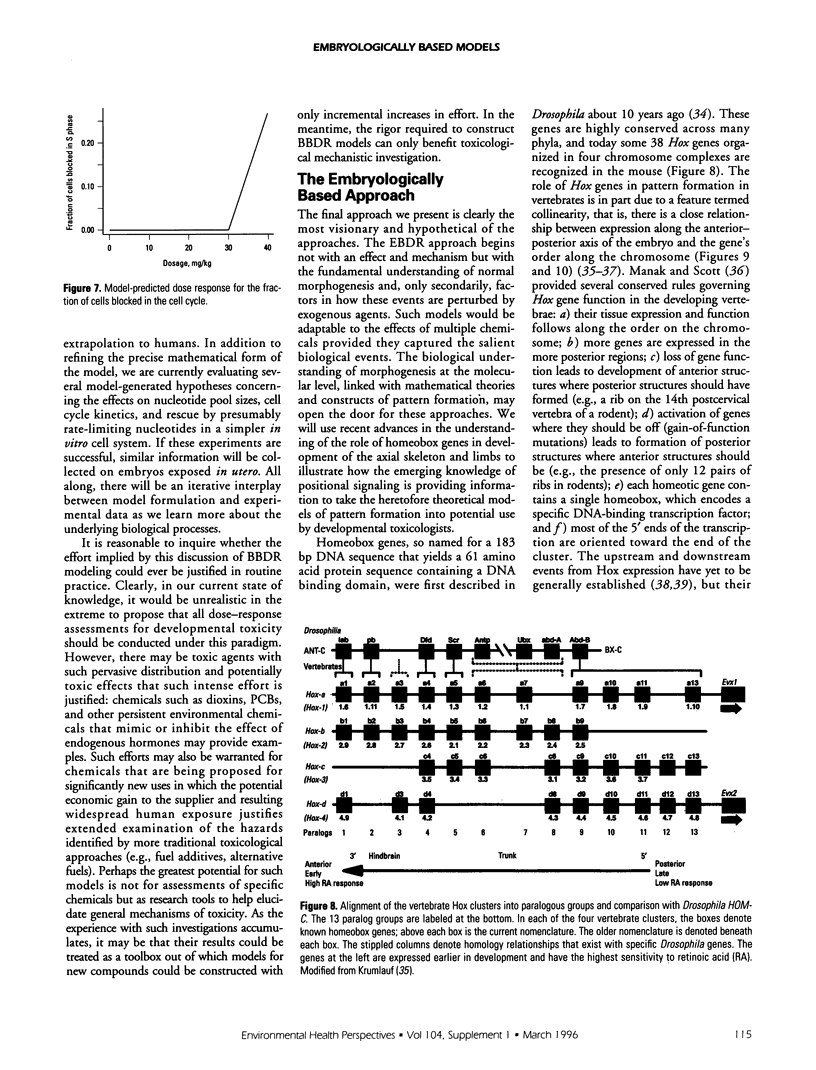
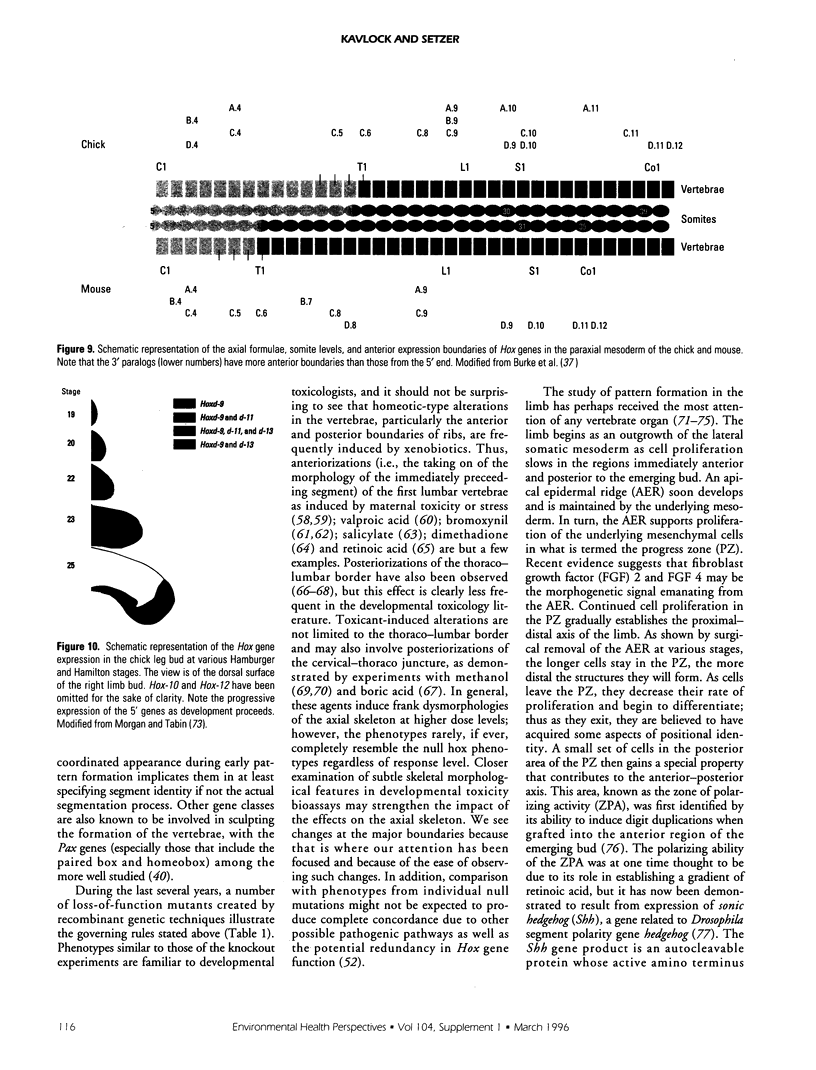
- Burke A. C., Nelson C. E., Morgan B. A., Tabin C. Hox genes and the evolution of vertebrate axial morphology. Development. 1995 Feb;121(2):333–346. doi: 10.1242/dev.121.2.333. [DOI] [PubMed] [Google Scholar]
- Catalano P. J., Scharfstein D. O., Ryan L. M., Kimmel C. A., Kimmel G. L. Statistical model for fetal death, fetal weight, and malformation in developmental toxicity studies. Teratology. 1993 Apr;47(4):281–290. doi: 10.1002/tera.1420470405. [DOI] [PubMed] [Google Scholar]
- Chernoff N., Miller D. B., Rosen M. B., Mattscheck C. L. Developmental effects of maternal stress in the CD-1 mouse induced by restraint on single days during the period of major organogenesis. Toxicology. 1988 Sep;51(1):57–65. doi: 10.1016/0300-483x(88)90080-7. [DOI] [PubMed] [Google Scholar]
- Chernoff N., Rogers J. M., Turner C. I., Francis B. M. Significance of supernumerary ribs in rodent developmental toxicity studies: postnatal persistence in rats and mice. Fundam Appl Toxicol. 1991 Oct;17(3):448–453. doi: 10.1016/0272-0590(91)90196-b. [DOI] [PubMed] [Google Scholar]
- Chisaka O., Capecchi M. R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991 Apr 11;350(6318):473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- Clark R. L., Robertson R. T., Minsker D. H., Cohen S. M., Tocco D. J., Allen H. L., James M. L., Bokelman D. L. Diflunisal-induced maternal anemia as a cause of teratogenicity in rabbits. Teratology. 1984 Dec;30(3):319–332. doi: 10.1002/tera.1420300304. [DOI] [PubMed] [Google Scholar]
- Clark R. L., Robertson R. T., Peter C. P., Bland J. A., Nolan T. E., Oppenheimer L., Bokelman D. L. Association between adverse maternal and embryo-fetal effects in norfloxacin-treated and food-deprived rabbits. Fundam Appl Toxicol. 1986 Aug;7(2):272–286. doi: 10.1016/0272-0590(86)90157-0. [DOI] [PubMed] [Google Scholar]
- Collins J. M., Dedrick R. L., King F. G., Speyer J. L., Myers C. E. Nonlinear pharmacokinetic models for 5-fluorouracil in man: intravenous and intraperitoneal routes. Clin Pharmacol Ther. 1980 Aug;28(2):235–246. doi: 10.1038/clpt.1980.156. [DOI] [PubMed] [Google Scholar]
- Condie B. G., Capecchi M. R. Mice homozygous for a targeted disruption of Hoxd-3 (Hox-4.1) exhibit anterior transformations of the first and second cervical vertebrae, the atlas and the axis. Development. 1993 Nov;119(3):579–595. doi: 10.1242/dev.119.3.579. [DOI] [PubMed] [Google Scholar]
- Condie B. G., Capecchi M. R. Mice with targeted disruptions in the paralogous genes hoxa-3 and hoxd-3 reveal synergistic interactions. Nature. 1994 Jul 28;370(6487):304–307. doi: 10.1038/370304a0. [DOI] [PubMed] [Google Scholar]
- Conolly R. B., Andersen M. E. Biologically based pharmacodynamic models: tools for toxicological research and risk assessment. Annu Rev Pharmacol Toxicol. 1991;31:503–523. doi: 10.1146/annurev.pa.31.040191.002443. [DOI] [PubMed] [Google Scholar]
- Crump K. S. A new method for determining allowable daily intakes. Fundam Appl Toxicol. 1984 Oct;4(5):854–871. doi: 10.1016/0272-0590(84)90107-6. [DOI] [PubMed] [Google Scholar]
- Davis A. P., Capecchi M. R. Axial homeosis and appendicular skeleton defects in mice with a targeted disruption of hoxd-11. Development. 1994 Aug;120(8):2187–2198. doi: 10.1242/dev.120.8.2187. [DOI] [PubMed] [Google Scholar]
- Dietrich S., Schubert F. R., Gruss P. Altered Pax gene expression in murine notochord mutants: the notochord is required to initiate and maintain ventral identity in the somite. Mech Dev. 1993 Dec;44(2-3):189–207. doi: 10.1016/0925-4773(93)90067-8. [DOI] [PubMed] [Google Scholar]
- Dollé P., Dierich A., LeMeur M., Schimmang T., Schuhbaur B., Chambon P., Duboule D. Disruption of the Hoxd-13 gene induces localized heterochrony leading to mice with neotenic limbs. Cell. 1993 Nov 5;75(3):431–441. doi: 10.1016/0092-8674(93)90378-4. [DOI] [PubMed] [Google Scholar]
- Faustman E. M., Allen B. C., Kavlock R. J., Kimmel C. A. Dose-response assessment for developmental toxicity. I. Characterization of database and determination of no observed adverse effect levels. Fundam Appl Toxicol. 1994 Nov;23(4):478–486. doi: 10.1006/faat.1994.1132. [DOI] [PubMed] [Google Scholar]
- Frederick C. B. Limiting the uncertainty in risk assessment by the development of physiologically based pharmacokinetic and pharmacodynamic models. Toxicol Lett. 1993 May;68(1-2):159–175. doi: 10.1016/0378-4274(93)90128-k. [DOI] [PubMed] [Google Scholar]
- Gaylor D. W. Incidence of developmental defects at the no observed adverse effect level (NOAEL). Regul Toxicol Pharmacol. 1992 Apr;15(2 Pt 1):151–160. doi: 10.1016/0273-2300(92)90046-c. [DOI] [PubMed] [Google Scholar]
- Gaylor D. W., Razzaghi M. Process of building biologically based dose-response models for developmental defects. Teratology. 1992 Dec;46(6):573–581. doi: 10.1002/tera.1420460607. [DOI] [PubMed] [Google Scholar]
- Gendron-Maguire M., Mallo M., Zhang M., Gridley T. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell. 1993 Dec 31;75(7):1317–1331. doi: 10.1016/0092-8674(93)90619-2. [DOI] [PubMed] [Google Scholar]
- Heindel J. J., Price C. J., Field E. A., Marr M. C., Myers C. B., Morrissey R. E., Schwetz B. A. Developmental toxicity of boric acid in mice and rats. Fundam Appl Toxicol. 1992 Feb;18(2):266–277. doi: 10.1016/0272-0590(92)90055-m. [DOI] [PubMed] [Google Scholar]
- Horan G. S., Kovàcs E. N., Behringer R. R., Featherstone M. S. Mutations in paralogous Hox genes result in overlapping homeotic transformations of the axial skeleton: evidence for unique and redundant function. Dev Biol. 1995 May;169(1):359–372. doi: 10.1006/dbio.1995.1150. [DOI] [PubMed] [Google Scholar]
- Horan G. S., Wu K., Wolgemuth D. J., Behringer R. R. Homeotic transformation of cervical vertebrae in Hoxa-4 mutant mice. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12644–12648. doi: 10.1073/pnas.91.26.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. C. A kinetic model of regulation of the deoxyribonucleoside triphosphate pool composition. Pharmacol Ther. 1984;24(2):279–301. doi: 10.1016/0163-7258(84)90038-x. [DOI] [PubMed] [Google Scholar]
- Jeannotte L., Lemieux M., Charron J., Poirier F., Robertson E. J. Specification of axial identity in the mouse: role of the Hoxa-5 (Hox1.3) gene. Genes Dev. 1993 Nov;7(11):2085–2096. doi: 10.1101/gad.7.11.2085. [DOI] [PubMed] [Google Scholar]
- Kavlock R. J., Chernoff N., Rogers E. H. The effect of acute maternal toxicity on fetal development in the mouse. Teratog Carcinog Mutagen. 1985;5(1):3–13. doi: 10.1002/tcm.1770050103. [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991 Oct 4;67(1):89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- Kimmel C. A., Wilson J. G. Skeletal deviations in rats: malformations or variations? Teratology. 1973 Dec;8(3):309–315. doi: 10.1002/tera.1420080311. [DOI] [PubMed] [Google Scholar]
- Kostic D., Capecchi M. R. Targeted disruptions of the murine Hoxa-4 and Hoxa-6 genes result in homeotic transformations of components of the vertebral column. Mech Dev. 1994 Jun;46(3):231–247. doi: 10.1016/0925-4773(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994 Jul 29;78(2):191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Le Mouellic H., Lallemand Y., Brûlet P. Homeosis in the mouse induced by a null mutation in the Hox-3.1 gene. Cell. 1992 Apr 17;69(2):251–264. doi: 10.1016/0092-8674(92)90406-3. [DOI] [PubMed] [Google Scholar]
- Leisenring W., Ryan L. Statistical properties of the NOAEL. Regul Toxicol Pharmacol. 1992 Apr;15(2 Pt 1):161–171. doi: 10.1016/0273-2300(92)90047-d. [DOI] [PubMed] [Google Scholar]
- Levin M. A Julia set model of field-directed morphogenesis: developmental biology and artificial life. Comput Appl Biosci. 1994 Apr;10(2):85–103. doi: 10.1093/bioinformatics/10.2.85. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Dierich A., LeMeur M., Mark M., Chambon P. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell. 1991 Sep 20;66(6):1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Mattison D. R., Sandler J. D. Summary of the workshop on issues in risk assessment: quantitative methods for developmental toxicology. Risk Anal. 1994 Aug;14(4):595–604. doi: 10.1111/j.1539-6924.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Garber R. L., Wirz J., Kuroiwa A., Gehring W. J. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell. 1984 Jun;37(2):403–408. doi: 10.1016/0092-8674(84)90370-2. [DOI] [PubMed] [Google Scholar]
- Moore J. A. An assessment of lithium using the IEHR Evaluative Process for Assessing Human Developmental and Reproductive Toxicity of Agents. IEHR Expert Scientific Committee. Reprod Toxicol. 1995 Mar-Apr;9(2):175–210. doi: 10.1016/0890-6238(94)00069-7. [DOI] [PubMed] [Google Scholar]
- Moore J. A., Daston G. P., Faustman E., Golub M. S., Hart W. L., Hughes C., Jr, Kimmel C. A., Lamb J. C., 4th, Schwetz B. A., Scialli A. R. An evaluative process for assessing human reproductive and developmental toxicity of agents. Reprod Toxicol. 1995 Jan-Feb;9(1):61–95. doi: 10.1016/0890-6238(94)00057-4. [DOI] [PubMed] [Google Scholar]
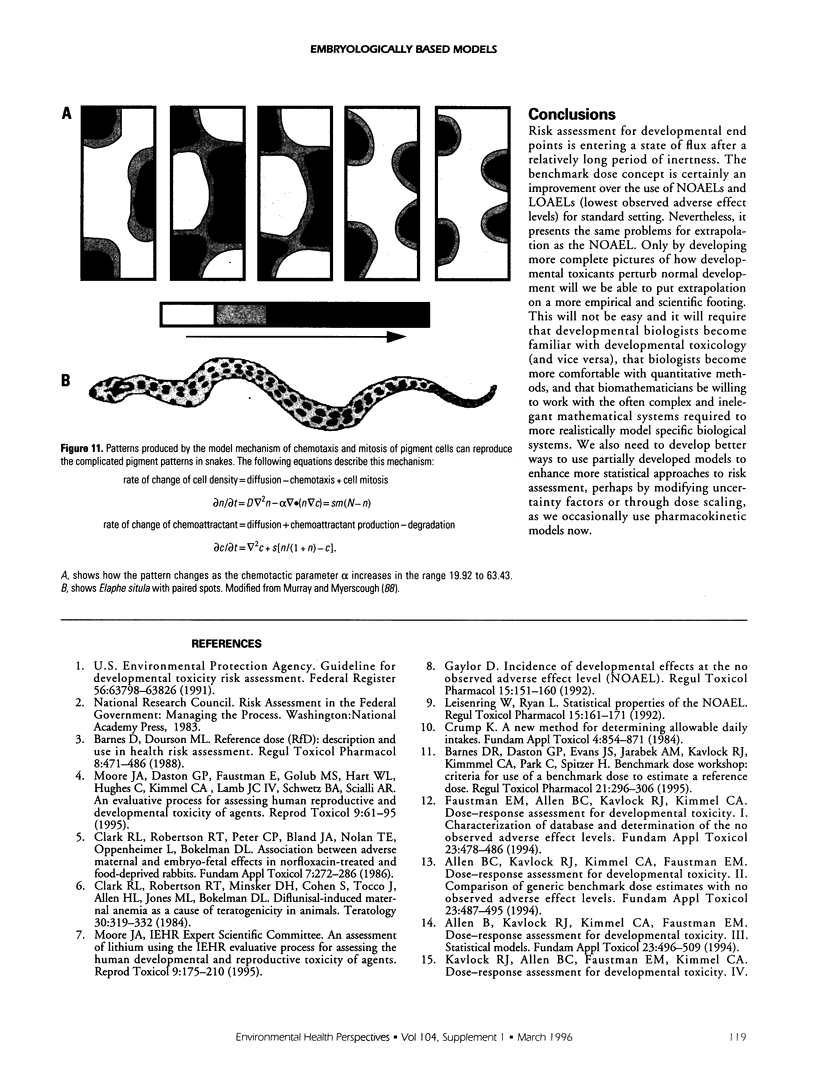
- Murray J. D., Myerscough M. R. Pigmentation pattern formation on snakes. J Theor Biol. 1991 Apr 7;149(3):339–360. doi: 10.1016/s0022-5193(05)80310-8. [DOI] [PubMed] [Google Scholar]
- Nagarajan M., Johnson L. F. Regulation of thymidylate synthase gene expression in mouse fibroblasts synchronized by mitotic selection. Exp Cell Res. 1989 Mar;181(1):289–297. doi: 10.1016/0014-4827(89)90203-6. [DOI] [PubMed] [Google Scholar]
- Narotsky M. G., Francis E. Z., Kavlock R. J. Developmental toxicity and structure-activity relationships of aliphatic acids, including dose-response assessment of valproic acid in mice and rats. Fundam Appl Toxicol. 1994 Feb;22(2):251–265. doi: 10.1006/faat.1994.1029. [DOI] [PubMed] [Google Scholar]
- O'Flaherty E. J., Scott W., Schreiner C., Beliles R. P. A physiologically based kinetic model of rat and mouse gestation: disposition of a weak acid. Toxicol Appl Pharmacol. 1992 Feb;112(2):245–256. doi: 10.1016/0041-008x(92)90194-w. [DOI] [PubMed] [Google Scholar]
- Oster G. F., Murray J. D. Pattern formation models and developmental constraints. J Exp Zool. 1989 Aug;251(2):186–202. doi: 10.1002/jez.1402510207. [DOI] [PubMed] [Google Scholar]
- Parr B. A., McMahon A. P. Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature. 1995 Mar 23;374(6520):350–353. doi: 10.1038/374350a0. [DOI] [PubMed] [Google Scholar]
- Ramírez-Solis R., Zheng H., Whiting J., Krumlauf R., Bradley A. Hoxb-4 (Hox-2.6) mutant mice show homeotic transformation of a cervical vertebra and defects in the closure of the sternal rudiments. Cell. 1993 Apr 23;73(2):279–294. doi: 10.1016/0092-8674(93)90229-j. [DOI] [PubMed] [Google Scholar]
- Rancourt D. E., Tsuzuki T., Capecchi M. R. Genetic interaction between hoxb-5 and hoxb-6 is revealed by nonallelic noncomplementation. Genes Dev. 1995 Jan 1;9(1):108–122. doi: 10.1101/gad.9.1.108. [DOI] [PubMed] [Google Scholar]
- Riddle R. D., Johnson R. L., Laufer E., Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993 Dec 31;75(7):1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Rijli F. M., Mark M., Lakkaraju S., Dierich A., Dollé P., Chambon P. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993 Dec 31;75(7):1333–1349. doi: 10.1016/0092-8674(93)90620-6. [DOI] [PubMed] [Google Scholar]
- Rogers J. M., Francis B. M., Barbee B. D., Chernoff N. Developmental toxicity of bromoxynil in mice and rats. Fundam Appl Toxicol. 1991 Oct;17(3):442–447. doi: 10.1016/0272-0590(91)90195-a. [DOI] [PubMed] [Google Scholar]
- Rogers J. M., Mole M. L., Chernoff N., Barbee B. D., Turner C. I., Logsdon T. R., Kavlock R. J. The developmental toxicity of inhaled methanol in the CD-1 mouse, with quantitative dose-response modeling for estimation of benchmark doses. Teratology. 1993 Mar;47(3):175–188. doi: 10.1002/tera.1420470302. [DOI] [PubMed] [Google Scholar]
- Schneider-Maunoury S., Topilko P., Seitandou T., Levi G., Cohen-Tannoudji M., Pournin S., Babinet C., Charnay P. Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell. 1993 Dec 17;75(6):1199–1214. doi: 10.1016/0092-8674(93)90329-o. [DOI] [PubMed] [Google Scholar]
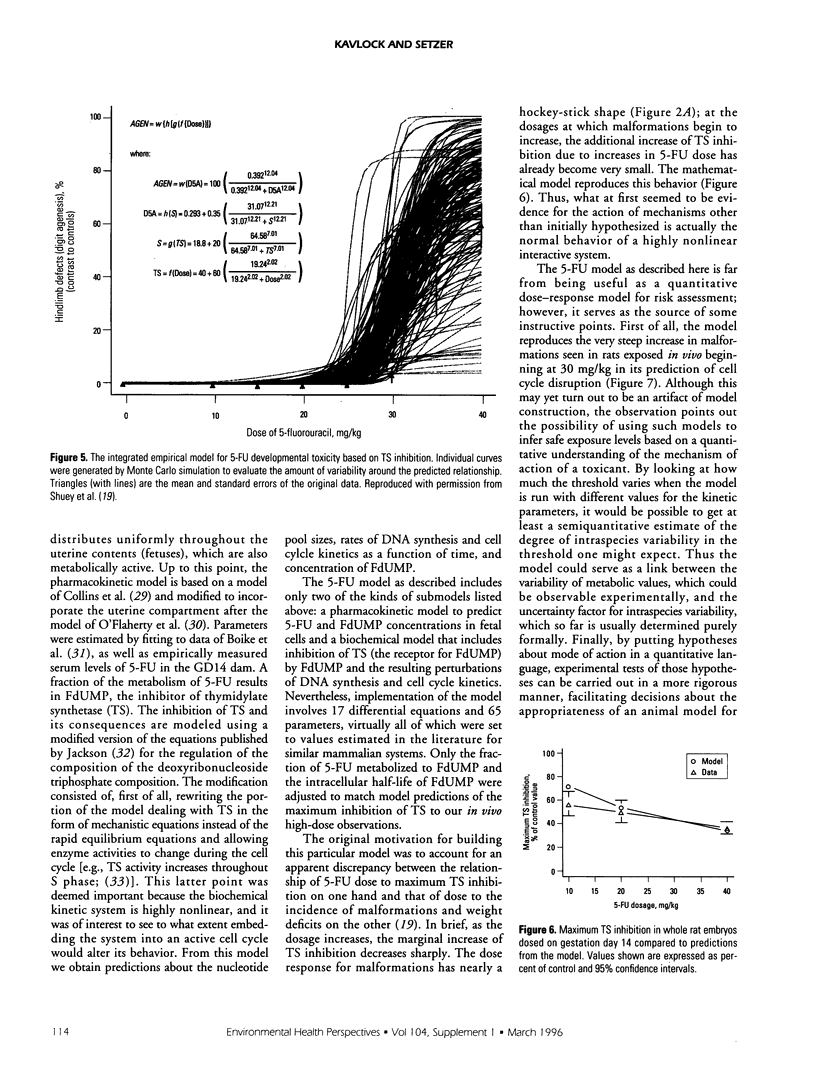
- Shuey D. L., Lau C., Logsdon T. R., Zucker R. M., Elstein K. H., Narotsky M. G., Setzer R. W., Kavlock R. J., Rogers J. M. Biologically based dose-response modeling in developmental toxicology: biochemical and cellular sequelae of 5-fluorouracil exposure in the developing rat. Toxicol Appl Pharmacol. 1994 May;126(1):129–144. doi: 10.1006/taap.1994.1099. [DOI] [PubMed] [Google Scholar]
- Small K. M., Potter S. S. Homeotic transformations and limb defects in Hox A11 mutant mice. Genes Dev. 1993 Dec;7(12A):2318–2328. doi: 10.1101/gad.7.12a.2318. [DOI] [PubMed] [Google Scholar]
- Suemori H., Takahashi N., Noguchi S. Hoxc-9 mutant mice show anterior transformation of the vertebrae and malformation of the sternum and ribs. Mech Dev. 1995 Jun;51(2-3):265–273. doi: 10.1016/0925-4773(95)00371-1. [DOI] [PubMed] [Google Scholar]
- Tabin C. J. Retinoids, homeoboxes, and growth factors: toward molecular models for limb development. Cell. 1991 Jul 26;66(2):199–217. doi: 10.1016/0092-8674(91)90612-3. [DOI] [PubMed] [Google Scholar]
- Tabin C. J. Why we have (only) five fingers per hand: hox genes and the evolution of paired limbs. Development. 1992 Oct;116(2):289–296. doi: 10.1242/dev.116.2.289. [DOI] [PubMed] [Google Scholar]
- Tickle C., Eichele G. Vertebrate limb development. Annu Rev Cell Biol. 1994;10:121–152. doi: 10.1146/annurev.cb.10.110194.001005. [DOI] [PubMed] [Google Scholar]
- Tukey J. W., Ciminera J. L., Heyse J. F. Testing the statistical certainty of a response to increasing doses of a drug. Biometrics. 1985 Mar;41(1):295–301. [PubMed] [Google Scholar]
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969 Oct;25(1):1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information revisited. Development. 1989;107 (Suppl):3–12. doi: 10.1242/dev.107.Supplement.3. [DOI] [PubMed] [Google Scholar]