Abstract
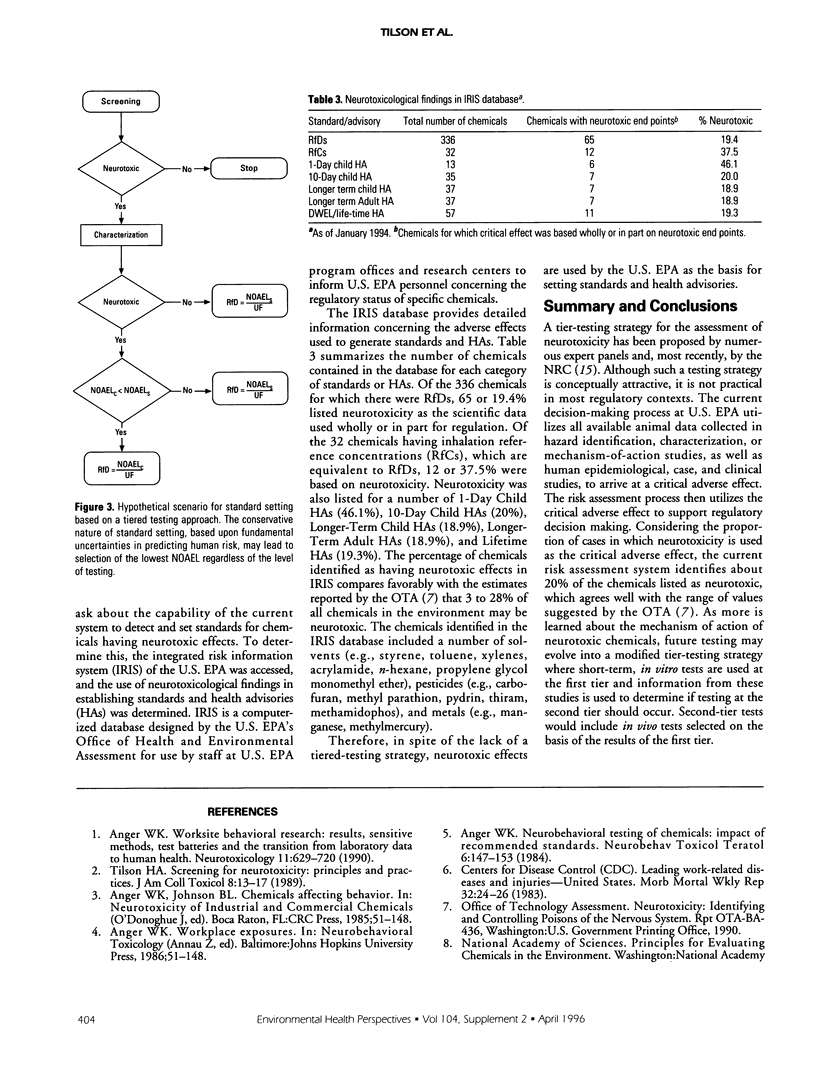
Increased emphasis on routine screening of chemicals for potential neurotoxicity has resulted in the development of testing guidelines and standardized procedures. A multiphased, tiered-testing strategy has been proposed by numerous expert panels to evaluate large numbers of chemicals. In a regulatory context, however, a formal tiered-testing approach is not used, mostly because of the constraints of differing regulatory authorities and the potential cost of such a testing strategy. Instead, current regulatory decision making utilizes all available animal and human data to identify a critical adverse effect which is then used for setting standards. Although the current decision-making process does not use a formal tiered-testing approach, it appears to identify chemicals with neurotoxic effects. An analysis of U.S. Environmental Protection Agency integrated risk information system (IRIS) indicates that about 20% of the chemicals having standards or health advisories are based on neurotoxicity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anger W. K. Neurobehavioral testing of chemicals: impact on recommended standards. Neurobehav Toxicol Teratol. 1984 Mar-Apr;6(2):147–153. [PubMed] [Google Scholar]
- Barnes D. G., Dourson M. Reference dose (RfD): description and use in health risk assessments. Regul Toxicol Pharmacol. 1988 Dec;8(4):471–486. doi: 10.1016/0273-2300(88)90047-5. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Francis E. Z., Farland W. H. Application of the preliminary developmental toxicity screen for chemical hazard identification under the Toxic Substances Control Act. Teratog Carcinog Mutagen. 1987;7(1):107–117. doi: 10.1002/tcm.1770070113. [DOI] [PubMed] [Google Scholar]
- Kimmel C. A. Current status of behavioral teratology: science and regulation. Crit Rev Toxicol. 1988;19(1):1–10. doi: 10.3109/10408448809040814. [DOI] [PubMed] [Google Scholar]
- Levine T. E., Butcher R. E. Workshop on the qualitative and quantitative comparability of human and animal developmental neurotoxicity, Work Group IV report: triggers for developmental neurotoxicity testing. Neurotoxicol Teratol. 1990 May-Jun;12(3):281–284. doi: 10.1016/0892-0362(90)90100-q. [DOI] [PubMed] [Google Scholar]
- Rees D. C., Francis E. Z., Kimmel C. A. Scientific and regulatory issues relevant to assessing risk for developmental neurotoxicity: an overview. Neurotoxicol Teratol. 1990 May-Jun;12(3):175–181. doi: 10.1016/0892-0362(90)90089-u. [DOI] [PubMed] [Google Scholar]
- Tilson H. A., Moser V. C. Comparison of screening approaches. Neurotoxicology. 1992 Spring;13(1):1–13. [PubMed] [Google Scholar]
- Tilson H. A. Neurotoxicology in the 1990s. Neurotoxicol Teratol. 1990 Jul-Aug;12(4):293–300. doi: 10.1016/0892-0362(90)90046-f. [DOI] [PubMed] [Google Scholar]


