Abstract
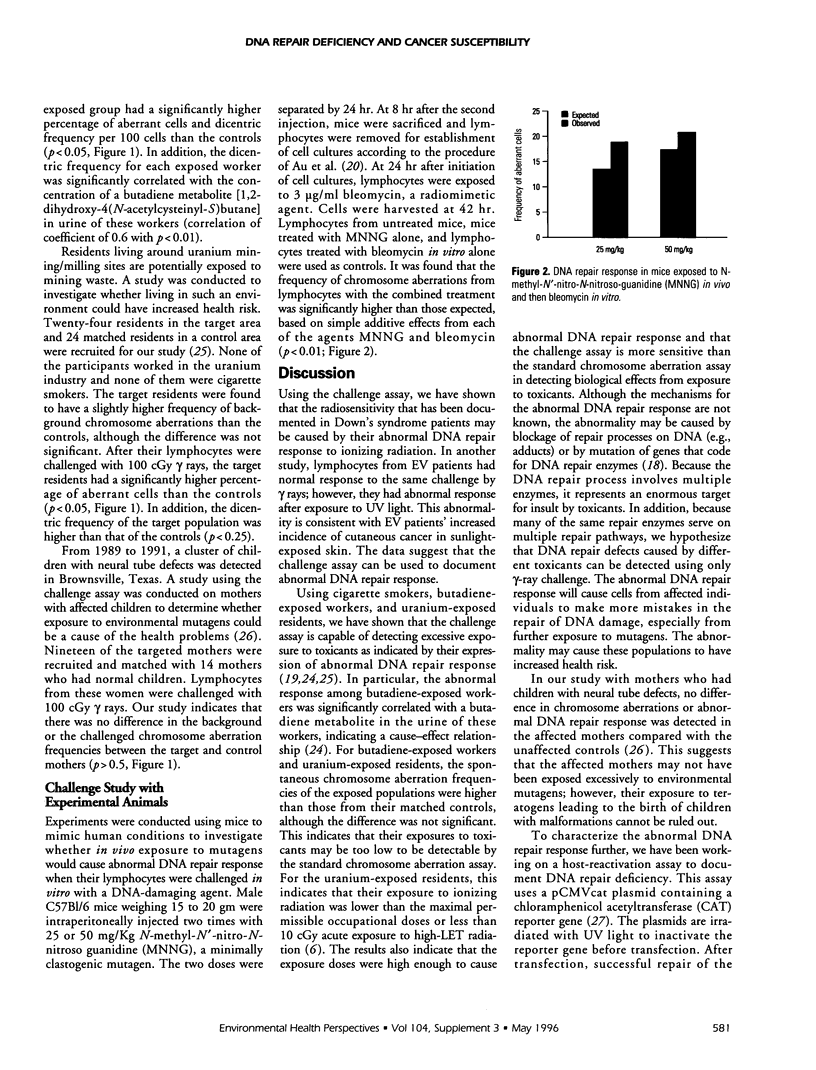
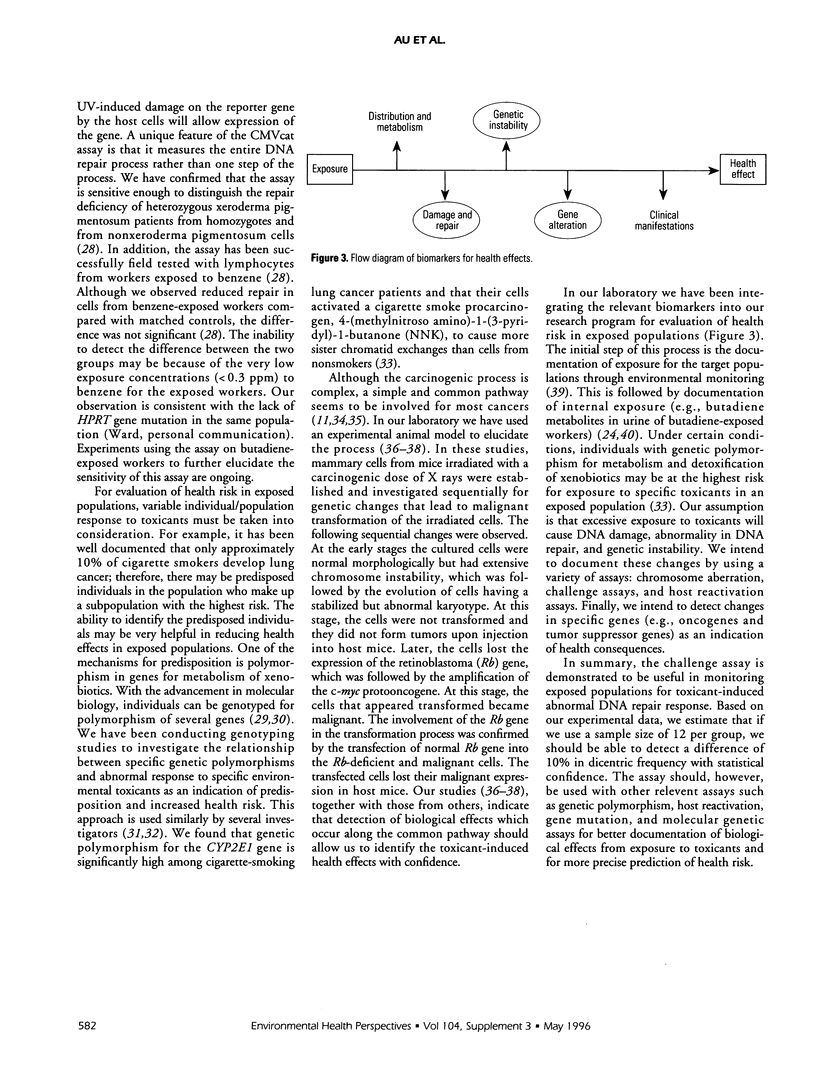
The induction of a mutator phenotype has been hypothesized to cause the accumulation of multiple mutations in the development of cancer. Recent evidence suggests that the mutator phenotype is associated with DNA repair deficiencies. We have been using a challenge assay to study exposed populations to test our hypothesis that exposure to environmental toxicants induce DNA repair deficiency in somatic cells. In this assay, lymphocytes were irradiated in vitro to challenge cells to repair the radiation-induction DNA strand breaks. An increase of chromosome aberrations in the challenged cells from toxicant-exposed populations compared to nonexposed populations is used to indicate abnormal DNA repair response. From studies of cigarette smokers, butadiene-exposed workers, and uranium-exposed residents, the assay showed that these exposed populations had mutagen-induced abnormal DNA repair response. The phenomenon was also demonstrated using experimental animals. Mice were exposed in vivo to two different doses of N-methyl-N'-nitro-N-nitroso-guanidine (MNNG) and their lymphocytes were challenged with one dose of a radiomimetic chemical, bleomycin, in vitro. These challenged lymphocytes showed an MNNG dose-dependent increase of abnormal DNA repair response. In a population that was potentially exposed to teratogens--mothers having children with neural tube defects--lymphocytes from these mothers did not have the abnormal response in our assay. In studies with patients, we reported that lymphocytes from Down's syndrome patients have the abnormal DNA repair response. Lymphocytes from skin cancer-prone patients (epidermodysplasia verruciformis) have normal response to gamma-ray challenge but abnormal response to UV-light challenge. These patient studies also indicate that the challenge assay is useful in documenting the radiosensitivity of Down's syndrome and the UV sensitivity in EV patients. In most cases, the challenge assay is more sensitive in detecting biological effects than the standard chromosome aberration assay. Our series of studies indicates that the challenge assay can be used to document biological effects from exposure to mutagens and that the effect is an abnormal DNA repair response. This abnormality can increase the risk for development of cancer. The repair deficiency is currently being validated using a plasmid transfection (host-reactivation) assay. The need to integrate chromosome aberration and the challenge assays with other relevant assays for better documentation of biological effects and for more precise prediction of health risk will be presented. Our experience in using genetic polymorphism and host-reactivation assays will be discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Athas W. F., Hedayati M. A., Matanoski G. M., Farmer E. R., Grossman L. Development and field-test validation of an assay for DNA repair in circulating human lymphocytes. Cancer Res. 1991 Nov 1;51(21):5786–5793. [PubMed] [Google Scholar]
- Au W. W. Abnormal chromosome repair and risk of developing cancer. Environ Health Perspect. 1993 Oct;101 (Suppl 3):303–308. doi: 10.1289/ehp.93101s3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au W. W., Bechtold W. E., Whorton E. B., Jr, Legator M. S. Chromosome aberrations and response to gamma-ray challenge in lymphocytes of workers exposed to 1,3-butadiene. Mutat Res. 1995 Apr;334(2):125–130. doi: 10.1016/0165-1161(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Au W. W., Lane R. G., Legator M. S., Whorton E. B., Wilkinson G. S., Gabehart G. J. Biomarker monitoring of a population residing near uranium mining activities. Environ Health Perspect. 1995 May;103(5):466–470. doi: 10.1289/ehp.95103466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au W. W., Walker D. M., Ward J. B., Jr, Whorton E., Legator M. S., Singh V. Factors contributing to chromosome damage in lymphocytes of cigarette smokers. Mutat Res. 1991 Jun;260(2):137–144. doi: 10.1016/0165-1218(91)90001-3. [DOI] [PubMed] [Google Scholar]
- Au W. W., Ward J. B., Jr, Ramanujam V. M., Harper B. L., Moslen M. T., Legator M. S. Genotoxic effects of a sub-acute low-level inhalation exposure to a mixture of carcinogenic chemicals. Mutat Res. 1988 Apr;203(2):103–115. doi: 10.1016/0165-1161(88)90025-8. [DOI] [PubMed] [Google Scholar]
- Awa A. A., Honda T., Sofuni T., Neriishi S., Yoshida M. C., Matsui T. Chromosome-aberration frequency in cultured blood-cells in relation to radiation dose of A-bomb survivors. Lancet. 1971 Oct 23;2(7730):903–905. doi: 10.1016/s0140-6736(71)92505-0. [DOI] [PubMed] [Google Scholar]
- Bender M. A., Awa A. A., Brooks A. L., Evans H. J., Groer P. G., Littlefield L. G., Pereira C., Preston R. J., Wachholz B. W. Current status of cytogenetic procedures to detect and quantify previous exposures to radiation. Mutat Res. 1988 Sep;196(2):103–159. doi: 10.1016/0165-1110(88)90017-6. [DOI] [PubMed] [Google Scholar]
- Celotti L., Furlan D., Ferraro P., Levis A. G. DNA repair and replication in lymphocytes from smokers exposed in vitro to UV light. Mutagenesis. 1989 Mar;4(2):82–86. doi: 10.1093/mutage/4.2.82. [DOI] [PubMed] [Google Scholar]
- Greenblatt M. S., Bennett W. P., Hollstein M., Harris C. C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- Hagmar L., Brøgger A., Hansteen I. L., Heim S., Högstedt B., Knudsen L., Lambert B., Linnainmaa K., Mitelman F., Nordenson I. Cancer risk in humans predicted by increased levels of chromosomal aberrations in lymphocytes: Nordic study group on the health risk of chromosome damage. Cancer Res. 1994 Jun 1;54(11):2919–2922. [PubMed] [Google Scholar]
- Hallberg L. M., el Zein R., Grossman L., Au W. W. Measurement of DNA repair deficiency in workers exposed to benzene. Environ Health Perspect. 1996 May;104 (Suppl 3):529–534. doi: 10.1289/ehp.96104s3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idle J. R., Daly A. K. New opportunities in cancer risk evaluation using PCR-based DNA analysis for CYP2D6. Environ Health Perspect. 1993 Oct;101 (Suppl 3):117–120. doi: 10.1289/ehp.93101s3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlubar F. F., Butler M. A., Kaderlik K. R., Chou H. C., Lang N. P. Polymorphisms for aromatic amine metabolism in humans: relevance for human carcinogenesis. Environ Health Perspect. 1992 Nov;98:69–74. doi: 10.1289/ehp.929869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A. Microsatellite instability: marker of a mutator phenotype in cancer. Cancer Res. 1994 Oct 1;54(19):5059–5063. [PubMed] [Google Scholar]
- Loeb L. A. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991 Jun 15;51(12):3075–3079. [PubMed] [Google Scholar]
- Lucas J. N., Awa A., Straume T., Poggensee M., Kodama Y., Nakano M., Ohtaki K., Weier H. U., Pinkel D., Gray J. Rapid translocation frequency analysis in humans decades after exposure to ionizing radiation. Int J Radiat Biol. 1992 Jul;62(1):53–63. doi: 10.1080/09553009214551821. [DOI] [PubMed] [Google Scholar]
- Madden J. J., Falek A., Shafer D. A., Glick J. H. Effects of opiates and demographic factors on DNA repair synthesis in human leukocytes. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5769–5773. doi: 10.1073/pnas.76.11.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer J., Warburton D., Jeffrey A. M., Pero R., Walles S., Andrews L., Toor M., Latriano L., Wazneh L., Tang D. Biologic markers in ethylene oxide-exposed workers and controls. Mutat Res. 1991 May;248(1):163–176. doi: 10.1016/0027-5107(91)90098-9. [DOI] [PubMed] [Google Scholar]
- Modrich P. Mismatch repair, genetic stability, and cancer. Science. 1994 Dec 23;266(5193):1959–1960. doi: 10.1126/science.7801122. [DOI] [PubMed] [Google Scholar]
- Oesch F., Klein S. Relevance of environmental alkylating agents to repair protein O6-alkylguanine-DNA alkyltransferase: determination of individual and collective repair capacities of O6-methylguanine. Cancer Res. 1992 Apr 1;52(7):1801–1803. [PubMed] [Google Scholar]
- Rupa D. S., Hasegawa L., Eastmond D. A. Detection of chromosomal breakage in the 1cen-1q12 region of interphase human lymphocytes using multicolor fluorescence in situ hybridization with tandem DNA probes. Cancer Res. 1995 Feb 1;55(3):640–645. [PubMed] [Google Scholar]
- Sagher D., Karrison T., Schwartz J. L., Larson R. A., Strauss B. Heterogeneity of O6-alkylguanine-DNA alkyltransferase activity in peripheral blood lymphocytes: relationship between this activity in lymphocytes and in lymphoblastoid lines from normal controls and from patients with Hodgkin's disease or non-Hodgkin's lymphoma. Cancer Res. 1989 Oct 1;49(19):5339–5344. [PubMed] [Google Scholar]
- Shafik H. M., Au W. W., Legator M. S. Chromosomal radiosensitivity of Down syndrome lymphocytes at different stages of the cell cycle. Hum Genet. 1988 Jan;78(1):71–75. doi: 10.1007/BF00291238. [DOI] [PubMed] [Google Scholar]
- Shafik H. M., Au W. W., Whorton E. B., Jr, Legator M. S. Distribution of X-ray-induced chromosome breakpoints in Down syndrome lymphocytes. Am J Med Genet Suppl. 1990;7:195–200. doi: 10.1002/ajmg.1320370740. [DOI] [PubMed] [Google Scholar]
- Sorsa M., Ojajärvi A., Salomaa S. Cytogenetic surveillance of workers exposed to genotoxic chemicals: preliminary experiences from a prospective cancer study in a cytogenetic cohort. Teratog Carcinog Mutagen. 1990;10(3):215–221. doi: 10.1002/tcm.1770100304. [DOI] [PubMed] [Google Scholar]
- Tucker J. D., Ramsey M. J., Lee D. A., Minkler J. L. Validation of chromosome painting as a biodosimeter in human peripheral lymphocytes following acute exposure to ionizing radiation in vitro. Int J Radiat Biol. 1993 Jul;64(1):27–37. doi: 10.1080/09553009314551081. [DOI] [PubMed] [Google Scholar]
- Uusküla M., Järventaus H., Hirvonen A., Sorsa M., Norppa H. Influence of GSTM1 genotype on sister chromatid exchange induction by styrene-7,8-oxide and 1,2-epoxy-3-butene in cultured human lymphocytes. Carcinogenesis. 1995 Apr;16(4):947–950. doi: 10.1093/carcin/16.4.947. [DOI] [PubMed] [Google Scholar]
- Ward J. B., Jr, Ammenheuser M. M., Bechtold W. E., Whorton E. B., Jr, Legator M. S. hprt mutant lymphocyte frequencies in workers at a 1,3-butadiene production plant. Environ Health Perspect. 1994 Nov;102 (Suppl 9):79–85. doi: 10.1289/ehp.94102s979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiencke J. K., Kelsey K. T., Lamela R. A., Toscano W. A., Jr Human glutathione S-transferase deficiency as a marker of susceptibility to epoxide-induced cytogenetic damage. Cancer Res. 1990 Mar 1;50(5):1585–1590. [PubMed] [Google Scholar]


