Abstract
The utility of biomarkers for evaluating the genotoxicity of environmental exposures is well documented. Biomarkers of both exposure and effect provide bases for assessing human-genotoxicant interactions and may be indicative of future disease risk. At present, there is little information on the predictive value of these assays for either a population or the individuals tested. This paper describes some aspects of biomarker assays, the possible use of susceptibility measures in biomonitoring protocols, and the need for evaluation of disease relevance. A population study involving epidemiologists, geneticists, toxicologists, statisticians, and physicians is proposed to determine the disease relevance of these biomarkers.
Full text
PDF

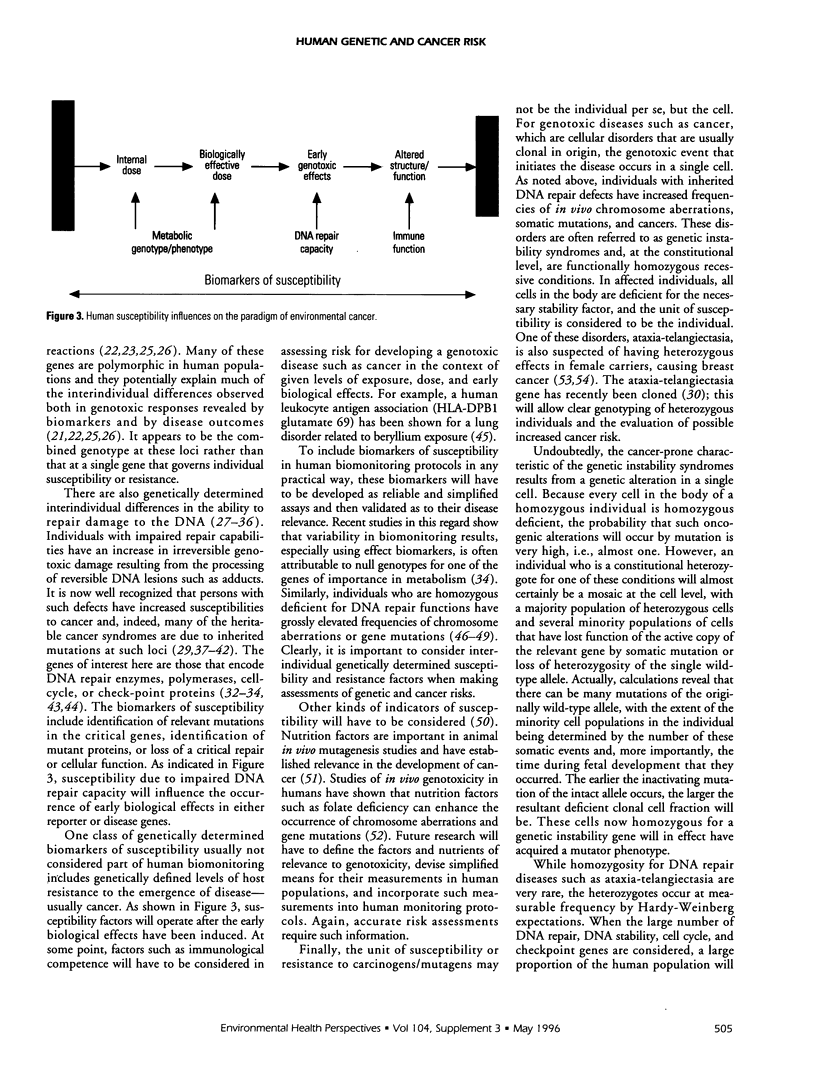
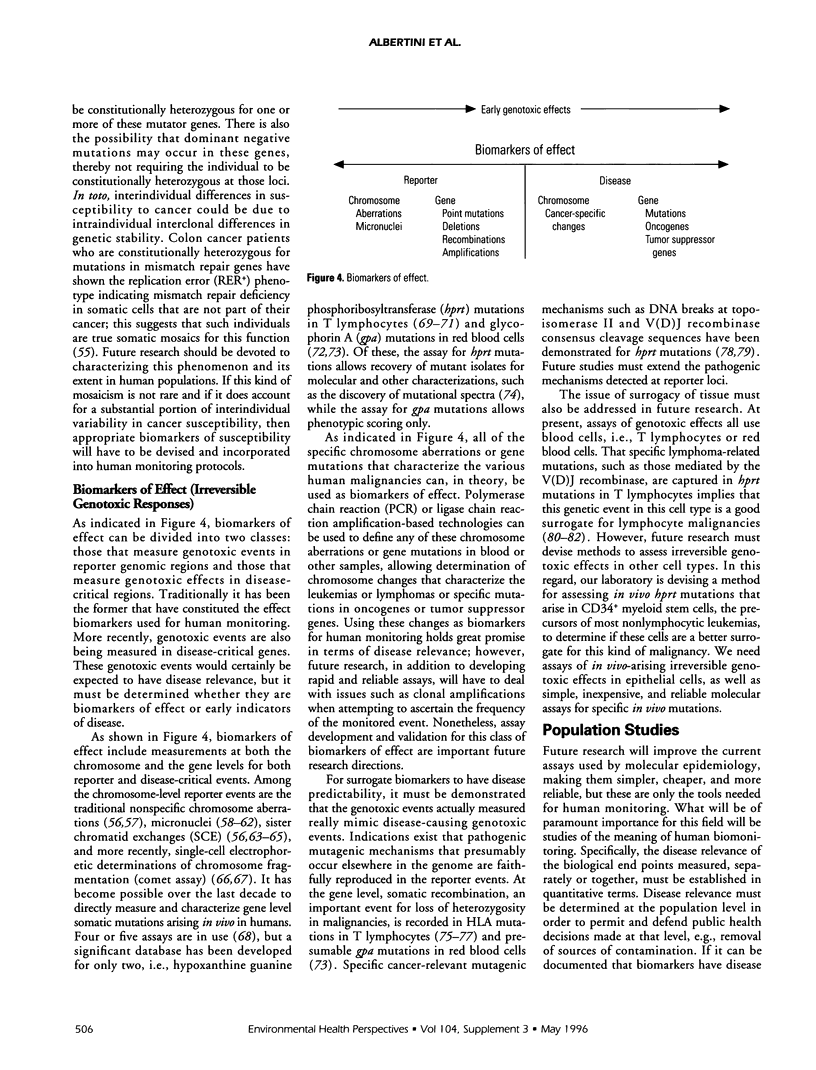




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aidoo A., Lyn-Cook L. E., Lensing S., Wamer W. Ascorbic acid (vitamin C) modulates the mutagenic effects produced by an alkylating agent in vivo. Environ Mol Mutagen. 1994;24(3):220–228. doi: 10.1002/em.2850240311. [DOI] [PubMed] [Google Scholar]
- Albertini R. J., Castle K. L., Borcherding W. R. T-cell cloning to detect the mutant 6-thioguanine-resistant lymphocytes present in human peripheral blood. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6617–6621. doi: 10.1073/pnas.79.21.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini R. J., Nicklas J. A., Fuscoe J. C., Skopek T. R., Branda R. F., O'Neill J. P. In vivo mutations in human blood cells: biomarkers for molecular epidemiology. Environ Health Perspect. 1993 Mar;99:135–141. doi: 10.1289/ehp.9399135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar W. A. Monitoring of human populations at risk by different cytogenetic end points. Environ Health Perspect. 1994 Oct;102 (Suppl 4):131–134. doi: 10.1289/ehp.94102s4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigbee W. L., Langlois R. G., Swift M., Jensen R. H. Evidence for an elevated frequency of in vivo somatic cell mutations in ataxia telangiectasia. Am J Hum Genet. 1989 Mar;44(3):402–408. [PMC free article] [PubMed] [Google Scholar]
- Boehm T., Rabbitts T. H. The human T cell receptor genes are targets for chromosomal abnormalities in T cell tumors. FASEB J. 1989 Oct;3(12):2344–2359. doi: 10.1096/fasebj.3.12.2676678. [DOI] [PubMed] [Google Scholar]
- Bonassi S., Abbondandolo A., Camurri L., Dal Prá L., De Ferrari M., Degrassi F., Forni A., Lamberti L., Lando C., Padovani P. Are chromosome aberrations in circulating lymphocytes predictive of future cancer onset in humans? Preliminary results of an Italian cohort study. Cancer Genet Cytogenet. 1995 Feb;79(2):133–135. doi: 10.1016/0165-4608(94)00131-t. [DOI] [PubMed] [Google Scholar]
- Branda R. F., O'Neill J. P., Sullivan L. M., Albertini R. J. Factors influencing mutation at the hprt locus in T-lymphocytes: women treated for breast cancer. Cancer Res. 1991 Dec 15;51(24):6603–6607. [PubMed] [Google Scholar]
- Breit T. M., Mol E. J., Wolvers-Tettero I. L., Ludwig W. D., van Wering E. R., van Dongen J. J. Site-specific deletions involving the tal-1 and sil genes are restricted to cells of the T cell receptor alpha/beta lineage: T cell receptor delta gene deletion mechanism affects multiple genes. J Exp Med. 1993 Apr 1;177(4):965–977. doi: 10.1084/jem.177.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L., Cheng J. T., Chen Q., Siciliano M. J., Crist W., Buchanan G., Baer R. Site-specific recombination of the tal-1 gene is a common occurrence in human T cell leukemia. EMBO J. 1990 Oct;9(10):3343–3351. doi: 10.1002/j.1460-2075.1990.tb07535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhart J. G. Perspectives on molecular assays for measuring mutation in humans and rodents. Environ Mol Mutagen. 1995;25 (Suppl 26):88–101. doi: 10.1002/em.2850250613. [DOI] [PubMed] [Google Scholar]
- Cariello N. F., Skopek T. R. Analysis of mutations occurring at the human hprt locus. J Mol Biol. 1993 May 5;231(1):41–57. doi: 10.1006/jmbi.1993.1255. [DOI] [PubMed] [Google Scholar]
- Cole J., Arlett C. F., Norris P. G., Stephens G., Waugh A. P., Beare D. M., Green M. H. Elevated hprt mutant frequency in circulating T-lymphocytes of xeroderma pigmentosum patients. Mutat Res. 1992 Mar;273(2):171–178. doi: 10.1016/0921-8777(92)90078-h. [DOI] [PubMed] [Google Scholar]
- Collins A., Duthie S., Ross M. Micronutrients and oxidative stress in the aetiology of cancer. Proc Nutr Soc. 1994 Mar;53(1):67–75. doi: 10.1079/pns19940011. [DOI] [PubMed] [Google Scholar]
- Dipple A. DNA adducts of chemical carcinogens. Carcinogenesis. 1995 Mar;16(3):437–441. doi: 10.1093/carcin/16.3.437. [DOI] [PubMed] [Google Scholar]
- EVANS H. J., NEARY G. J., WILLIAMSON F. S. The relative biological efficiency of single doses of fast neutrons and gamma-rays on Vicia faba roots and the effect of oxygen. Part II. Chromosone damage: the production of micronuclei. Int J Radiat Biol Relat Stud Phys Chem Med. 1959 Jul;1:216–229. doi: 10.1080/09553005914550311. [DOI] [PubMed] [Google Scholar]
- Evans H. J. Mutation cytogenetics: past, present and future. Mutat Res. 1988 Mar;204(3):355–363. doi: 10.1016/0165-1218(88)90034-1. [DOI] [PubMed] [Google Scholar]
- Fairbairn D. W., Olive P. L., O'Neill K. L. The comet assay: a comprehensive review. Mutat Res. 1995 Feb;339(1):37–59. doi: 10.1016/0165-1110(94)00013-3. [DOI] [PubMed] [Google Scholar]
- Fenech M., Morley A. A. Cytokinesis-block micronucleus method in human lymphocytes: effect of in vivo ageing and low dose X-irradiation. Mutat Res. 1986 Jul;161(2):193–198. doi: 10.1016/0027-5107(86)90010-2. [DOI] [PubMed] [Google Scholar]
- Fenech M. The cytokinesis-block micronucleus technique and its application to genotoxicity studies in human populations. Environ Health Perspect. 1993 Oct;101 (Suppl 3):101–107. doi: 10.1289/ehp.93101s3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finette B. A., Sullivan L. M., O'Neill J. P., Nicklas J. A., Vacek P. M., Albertini R. J. Determination of hprt mutant frequencies in T-lymphocytes from a healthy pediatric population: statistical comparison between newborn, children and adult mutant frequencies, cloning efficiency and age. Mutat Res. 1994 Jul 16;308(2):223–231. doi: 10.1016/0027-5107(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993 Dec 3;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Fuscoe J. C., Zimmerman L. J., Lippert M. J., Nicklas J. A., O'Neill J. P., Albertini R. J. V(D)J recombinase-like activity mediates hprt gene deletion in human fetal T-lymphocytes. Cancer Res. 1991 Nov 1;51(21):6001–6005. [PubMed] [Google Scholar]
- Grant S. G., Bigbee W. L. In vivo somatic mutation and segregation at the human glycophorin A (GPA) locus: phenotypic variation encompassing both gene-specific and chromosomal mechanisms. Mutat Res. 1993 Jul;288(1):163–172. doi: 10.1016/0027-5107(93)90217-4. [DOI] [PubMed] [Google Scholar]
- Grist S. A., McCarron M., Kutlaca A., Turner D. R., Morley A. A. In vivo human somatic mutation: frequency and spectrum with age. Mutat Res. 1992 Apr;266(2):189–196. doi: 10.1016/0027-5107(92)90186-6. [DOI] [PubMed] [Google Scholar]
- Groopman J. D., Donahue P. R., Zhu J. Q., Chen J. S., Wogan G. N. Aflatoxin metabolism in humans: detection of metabolites and nucleic acid adducts in urine by affinity chromatography. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6492–6496. doi: 10.1073/pnas.82.19.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmar L., Brøgger A., Hansteen I. L., Heim S., Högstedt B., Knudsen L., Lambert B., Linnainmaa K., Mitelman F., Nordenson I. Cancer risk in humans predicted by increased levels of chromosomal aberrations in lymphocytes: Nordic study group on the health risk of chromosome damage. Cancer Res. 1994 Jun 1;54(11):2919–2922. [PubMed] [Google Scholar]
- Harris C. C. Chemical and physical carcinogenesis: advances and perspectives for the 1990s. Cancer Res. 1991 Sep 15;51(18 Suppl):5023s–5044s. [PubMed] [Google Scholar]
- Harris C. C., Vahakangas K., Newman M. J., Trivers G. E., Shamsuddin A., Sinopoli N., Mann D. L., Wright W. E. Detection of benzo[a]pyrene diol epoxide-DNA adducts in peripheral blood lymphocytes and antibodies to the adducts in serum from coke oven workers. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6672–6676. doi: 10.1073/pnas.82.19.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen A., Becher G., Benestad C., Vahakangas K., Trivers G. E., Newman M. J., Harris C. C. Determination of polycyclic aromatic hydrocarbons in the urine, benzo(a)pyrene diol epoxide-DNA adducts in lymphocyte DNA, and antibodies to the adducts in sera from coke oven workers exposed to measured amounts of polycyclic aromatic hydrocarbons in the work atmosphere. Cancer Res. 1986 Aug;46(8):4178–4183. [PubMed] [Google Scholar]
- Heddle J. A., Carrano A. V. The DNA content of micronuclei induced in mouse bone marrow by gamma-irradiation: evidence that micronuclei arise from acentric chromosomal fragments. Mutat Res. 1977 Jul;44(1):63–69. doi: 10.1016/0027-5107(77)90115-4. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H. Human nucleotide excision repair syndromes: molecular clues to unexpected intricacies. Eur J Cancer. 1994;30A(13):1912–1921. doi: 10.1016/0959-8049(94)00381-e. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M., Johansson I., Persson I., Oscarson M., Hu Y., Bertilsson L., Dahl M. L., Sjöqvist F. Genetic polymorphism of cytochrome P450. Functional consequences and possible relationship to disease and alcohol toxicity. EXS. 1994;71:197–207. doi: 10.1007/978-3-0348-7330-7_20. [DOI] [PubMed] [Google Scholar]
- Janatipour M., Trainor K. J., Kutlaca R., Bennett G., Hay J., Turner D. R., Morley A. A. Mutations in human lymphocytes studied by an HLA selection system. Mutat Res. 1988 Mar;198(1):221–226. doi: 10.1016/0027-5107(88)90058-9. [DOI] [PubMed] [Google Scholar]
- Kadlubar F. F. Biochemical individuality and its implications for drug and carcinogen metabolism: recent insights from acetyltransferase and cytochrome P4501A2 phenotyping and genotyping in humans. Drug Metab Rev. 1994;26(1-2):37–46. doi: 10.3109/03602539409029783. [DOI] [PubMed] [Google Scholar]
- Keith G., Dirheimer G. Postlabeling: a sensitive method for studying DNA adducts and their role in carcinogenesis. Curr Opin Biotechnol. 1995 Feb;6(1):3–11. doi: 10.1016/0958-1669(95)80002-6. [DOI] [PubMed] [Google Scholar]
- Kleinerman R. A., Littlefield L. G., Tarone R. E., Sayer A. M., Cookfair D. L., Wactawski-Wende J., Inskip P. D., Block A., Ramesh K. H., Boice J. D., Jr Chromosome aberrations in lymphocytes from women irradiated for benign and malignant gynecological disease. Radiat Res. 1994 Jul;139(1):40–46. [PubMed] [Google Scholar]
- Kraemer K. H., Levy D. D., Parris C. N., Gozukara E. M., Moriwaki S., Adelberg S., Seidman M. M. Xeroderma pigmentosum and related disorders: examining the linkage between defective DNA repair and cancer. J Invest Dermatol. 1994 Nov;103(5 Suppl):96S–101S. doi: 10.1111/1523-1747.ep12399329. [DOI] [PubMed] [Google Scholar]
- Kroemer H. K., Eichelbaum M. "It's the genes, stupid". Molecular bases and clinical consequences of genetic cytochrome P450 2D6 polymorphism. Life Sci. 1995;56(26):2285–2298. doi: 10.1016/0024-3205(95)00223-s. [DOI] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Jensen R. H., German J. Evidence for increased in vivo mutation and somatic recombination in Bloom's syndrome. Proc Natl Acad Sci U S A. 1989 Jan;86(2):670–674. doi: 10.1073/pnas.86.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Jensen R. H. Measurements of the frequency of human erythrocytes with gene expression loss phenotypes at the glycophorin A locus. Hum Genet. 1986 Dec;74(4):353–362. doi: 10.1007/BF00280485. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Sister chromatid exchanges, indices of human chromosome damage and repair: detection by fluorescence and induction by mitomycin C. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3162–3166. doi: 10.1073/pnas.71.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. M., Strickland P. T. Antibodies to carcinogen-DNA adducts in mice chronically exposed to polycyclic aromatic hydrocarbons. Immunol Lett. 1993 May;36(2):117–123. doi: 10.1016/0165-2478(93)90042-z. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Norris P. G. DNA repair and cancer: speculations based on studies with xeroderma pigmentosum, Cockayne's syndrome and trichothiodystrophy. Carcinogenesis. 1989 Aug;10(8):1353–1356. doi: 10.1093/carcin/10.8.1353. [DOI] [PubMed] [Google Scholar]
- Lin H. J., Han C. Y., Lin B. K., Hardy S. Slow acetylator mutations in the human polymorphic N-acetyltransferase gene in 786 Asians, blacks, Hispanics, and whites: application to metabolic epidemiology. Am J Hum Genet. 1993 Apr;52(4):827–834. [PMC free article] [PubMed] [Google Scholar]
- Lloyd D. C., Purrott R. J., Reeder E. J. The incidence of unstable chromosome aberrations in peripheral blood lymphocytes from unirradiated and occupationally exposed people. Mutat Res. 1980 Aug;72(3):523–532. doi: 10.1016/0027-5107(80)90123-2. [DOI] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., McKinnon R. A. Cytochrome P450: evolution and functional diversity. Prog Liver Dis. 1994;12:63–97. [PubMed] [Google Scholar]
- Neumann H. G. Analysis of hemoglobin as a dose monitor for alkylating and arylating agents. Arch Toxicol. 1984 Nov;56(1):1–6. doi: 10.1007/BF00316343. [DOI] [PubMed] [Google Scholar]
- Nicolaides N. C., Papadopoulos N., Liu B., Wei Y. F., Carter K. C., Ruben S. M., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994 Sep 1;371(6492):75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- Nicotera T. M. Molecular and biochemical aspects of Bloom's syndrome. Cancer Genet Cytogenet. 1991 May;53(1):1–13. doi: 10.1016/0165-4608(91)90109-8. [DOI] [PubMed] [Google Scholar]
- Norppa H., Luomahaara S., Heikanen H., Roth S., Sorsa M., Renzi L., Lindholm C. Micronucleus assay in lymphocytes as a tool to biomonitor human exposure to aneuploidogens and clastogens. Environ Health Perspect. 1993 Oct;101 (Suppl 3):139–143. doi: 10.1289/ehp.93101s3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris P. G., Limb G. A., Hamblin A. S., Lehmann A. R., Arlett C. F., Cole J., Waugh A. P., Hawk J. L. Immune function, mutant frequency, and cancer risk in the DNA repair defective genodermatoses xeroderma pigmentosum, Cockayne's syndrome, and trichothiodystrophy. J Invest Dermatol. 1990 Jan;94(1):94–100. doi: 10.1111/1523-1747.ep12873952. [DOI] [PubMed] [Google Scholar]
- O'Neill J. P., McGinniss M. J., Berman J. K., Sullivan L. M., Nicklas J. A., Albertini R. J. Refinement of a T-lymphocyte cloning assay to quantify the in vivo thioguanine-resistant mutant frequency in humans. Mutagenesis. 1987 Mar;2(2):87–94. doi: 10.1093/mutage/2.2.87. [DOI] [PubMed] [Google Scholar]
- Osterman-Golkar S., Christakopoulos A., Zorcec V., Svensson K. Dosimetry of styrene 7,8-oxide in styrene- and styrene oxide-exposed mice and rats by quantification of haemoglobin adducts. Chem Biol Interact. 1995 Mar 30;95(1-2):79–87. doi: 10.1016/0009-2797(94)03348-x. [DOI] [PubMed] [Google Scholar]
- Osterman-Golkar S., Ehrenberg L., Segerbäck D., Hällström I. Evaluation of genetic risks of alkylating agents. II. Haemoglobin as a dose monitor. Mutat Res. 1976 Jan;34(1):1–10. doi: 10.1016/0027-5107(76)90256-6. [DOI] [PubMed] [Google Scholar]
- Ostling O., Johanson K. J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun. 1984 Aug 30;123(1):291–298. doi: 10.1016/0006-291x(84)90411-x. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N., Nicolaides N. C., Wei Y. F., Ruben S. M., Carter K. C., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M., Adams M. D. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994 Mar 18;263(5153):1625–1629. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- Parsons R., Li G. M., Longley M., Modrich P., Liu B., Berk T., Hamilton S. R., Kinzler K. W., Vogelstein B. Mismatch repair deficiency in phenotypically normal human cells. Science. 1995 May 5;268(5211):738–740. doi: 10.1126/science.7632227. [DOI] [PubMed] [Google Scholar]
- Perera F. P. The significance of DNA and protein adducts in human biomonitoring studies. Mutat Res. 1988 May-Aug;205(1-4):255–269. doi: 10.1016/0165-1218(88)90021-3. [DOI] [PubMed] [Google Scholar]
- Perry P., Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974 Sep 13;251(5471):156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Ponz de Leon M. Cancer-prone hereditary diseases associated with abnormalities of DNA repair. Recent Results Cancer Res. 1994;136:322–331. doi: 10.1007/978-3-642-85076-9_21. [DOI] [PubMed] [Google Scholar]
- Rainville I. R., Albertini R. J., Nicklas J. A. Breakpoints and junctional regions of intragenic deletions in the HPRT gene in human T-Cells. Somat Cell Mol Genet. 1995 Sep;21(5):309–326. doi: 10.1007/BF02257466. [DOI] [PubMed] [Google Scholar]
- Richeldi L., Sorrentino R., Saltini C. HLA-DPB1 glutamate 69: a genetic marker of beryllium disease. Science. 1993 Oct 8;262(5131):242–244. doi: 10.1126/science.8105536. [DOI] [PubMed] [Google Scholar]
- Santella R. M. Application of new techniques for the detection of carcinogen adducts to human population monitoring. Mutat Res. 1988 May-Aug;205(1-4):271–282. doi: 10.1016/0165-1218(88)90022-5. [DOI] [PubMed] [Google Scholar]
- Savitsky K., Bar-Shira A., Gilad S., Rotman G., Ziv Y., Vanagaite L., Tagle D. A., Smith S., Uziel T., Sfez S. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995 Jun 23;268(5218):1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- Segerbäck D., Calleman C. J., Ehrenberg L., Löfroth G., Osterman-Golkar S. Evaluation of genetic risks of alkylating agents IV. Quantitative determination of alkylated amino acids in haemoglobin as a measure of the dose after-treatment of mice with methyl methanesulfonate. Mutat Res. 1978 Jan;49(1):71–82. doi: 10.1016/0027-5107(78)90079-9. [DOI] [PubMed] [Google Scholar]
- Swift M., Morrell D., Massey R. B., Chase C. L. Incidence of cancer in 161 families affected by ataxia-telangiectasia. N Engl J Med. 1991 Dec 26;325(26):1831–1836. doi: 10.1056/NEJM199112263252602. [DOI] [PubMed] [Google Scholar]
- Swift M., Reitnauer P. J., Morrell D., Chase C. L. Breast and other cancers in families with ataxia-telangiectasia. N Engl J Med. 1987 May 21;316(21):1289–1294. doi: 10.1056/NEJM198705213162101. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., McConville C. M., Byrd P. J. Cancer and DNA processing disorders. Br Med Bull. 1994 Jul;50(3):708–717. doi: 10.1093/oxfordjournals.bmb.a072919. [DOI] [PubMed] [Google Scholar]
- Tucker J. D., Auletta A., Cimino M. C., Dearfield K. L., Jacobson-Kram D., Tice R. R., Carrano A. V. Sister-chromatid exchange: second report of the Gene-Tox Program. Mutat Res. 1993 Sep;297(2):101–180. doi: 10.1016/0165-1110(93)90001-4. [DOI] [PubMed] [Google Scholar]
- Turner D. R., Grist S. A., Janatipour M., Morley A. A. Mutations in human lymphocytes commonly involve gene duplication and resemble those seen in cancer cels. Proc Natl Acad Sci U S A. 1988 May;85(9):3189–3192. doi: 10.1073/pnas.85.9.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vineis P., Bartsch H., Caporaso N., Harrington A. M., Kadlubar F. F., Landi M. T., Malaveille C., Shields P. G., Skipper P., Talaska G. Genetically based N-acetyltransferase metabolic polymorphism and low-level environmental exposure to carcinogens. Nature. 1994 May 12;369(6476):154–156. doi: 10.1038/369154a0. [DOI] [PubMed] [Google Scholar]
- Wolf C. R., Smith C. A., Forman D. Metabolic polymorphisms in carcinogen metabolising enzymes and cancer susceptibility. Br Med Bull. 1994 Jul;50(3):718–731. doi: 10.1093/oxfordjournals.bmb.a072920. [DOI] [PubMed] [Google Scholar]
- Yu M. C., Skipper P. L., Taghizadeh K., Tannenbaum S. R., Chan K. K., Henderson B. E., Ross R. K. Acetylator phenotype, aminobiphenyl-hemoglobin adduct levels, and bladder cancer risk in white, black, and Asian men in Los Angeles, California. J Natl Cancer Inst. 1994 May 4;86(9):712–716. doi: 10.1093/jnci/86.9.712. [DOI] [PubMed] [Google Scholar]



