Abstract
Fluorescence in situ hybridization with chromosome-specific composite DNA probes (chromosome painting) is a reliable and efficient method for detecting structural chromosome aberrations. Painting is now being used to quantify chromosome damage in many human populations. In one such study we evaluated 91 unexposed people ranging in age from birth (cord bloods) to 79. We established a baseline frequency of stable aberrations that showed a highly significant curvilinear increase with age (p < 0.00001) that accounted for 70% of the variance among donors. The magnitude of this effect illustrates the importance of understanding the cytogenetic changes that occur with age, which is particularly important for quantifying the effects of prior adverse environmental, occupational, or accidental exposure. In this paper we use the data obtained in our previous study to characterize the distribution of stable aberrations by age and pack-years of cigarette smoking. We also provide estimates of the number of cell equivalents that need to be scored to detect a given increase in aberrations above the background level surveyed in this population.
Full text
PDF
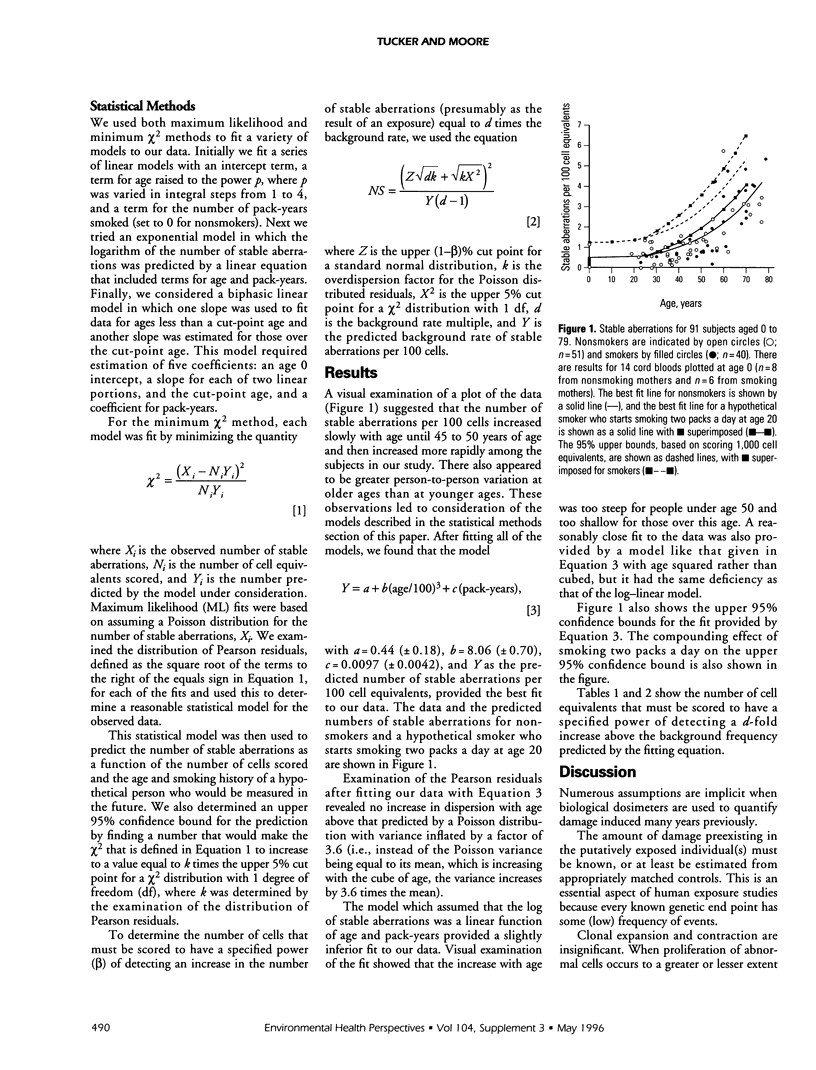

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awa A. A., Nakano M., Ohtaki K., Kodama Y., Lucas J., Gray J. Factors that determine the in vivo dose-response relationship for stable chromosome aberrations in A-bomb survivors. J Radiat Res. 1992 Mar;33 (Suppl):206–214. doi: 10.1269/jrr.33.supplement_206. [DOI] [PubMed] [Google Scholar]
- Bauchinger M., Schmid E., Zitzelsberger H., Braselmann H., Nahrstedt U. Radiation-induced chromosome aberrations analysed by two-colour fluorescence in situ hybridization with composite whole chromosome-specific DNA probes and a pancentromeric DNA probe. Int J Radiat Biol. 1993 Aug;64(2):179–184. doi: 10.1080/09553009314551271. [DOI] [PubMed] [Google Scholar]
- Boei J. J., Balajee A. S., de Boer P., Rens W., Aten J. A., Mullenders L. H., Natarajan A. T. Construction of mouse chromosome-specific DNA libraries and their use for the detection of X-ray-induced aberrations. Int J Radiat Biol. 1994 May;65(5):583–590. doi: 10.1080/09553009414550671. [DOI] [PubMed] [Google Scholar]
- Breneman J. W., Ramsey M. J., Lee D. A., Eveleth G. G., Minkler J. L., Tucker J. D. The development of chromosome-specific composite DNA probes for the mouse and their application to chromosome painting. Chromosoma. 1993 Nov;102(9):591–598. doi: 10.1007/BF00352306. [DOI] [PubMed] [Google Scholar]
- Breneman J. W., Swiger R. R., Ramsey M. J., Minkler J. L., Eveleth J. G., Langlois R. A., Tucker J. D. The development of painting probes for dual-color and multiple chromosome analysis in the mouse. Cytogenet Cell Genet. 1995;68(3-4):197–202. doi: 10.1159/000133913. [DOI] [PubMed] [Google Scholar]
- Ellard S., Parry E. M., Parry J. M. Use of multicolour chromosome painting to identify chromosomal rearrangements in human lymphocytes exposed to bleomycin: a comparison with conventional cytogenetic analysis of Giemsa-stained chromosomes. Environ Mol Mutagen. 1995;26(1):44–54. doi: 10.1002/em.2850260107. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Gelboin H. V. Role of human cytochrome P-450s in risk assessment and susceptibility to environmentally based disease. J Toxicol Environ Health. 1993 Oct-Nov;40(2-3):289–308. doi: 10.1080/15287399309531795. [DOI] [PubMed] [Google Scholar]
- Lucas J. N., Awa A., Straume T., Poggensee M., Kodama Y., Nakano M., Ohtaki K., Weier H. U., Pinkel D., Gray J. Rapid translocation frequency analysis in humans decades after exposure to ionizing radiation. Int J Radiat Biol. 1992 Jul;62(1):53–63. doi: 10.1080/09553009214551821. [DOI] [PubMed] [Google Scholar]
- Matsuoka A., Tucker J. D., Hayashi M., Yamazaki N., Sofuni T. Chromosome painting analysis of X-ray-induced aberrations in human lymphocytes in vitro. Mutagenesis. 1994 Mar;9(2):151–155. doi: 10.1093/mutage/9.2.151. [DOI] [PubMed] [Google Scholar]
- Natarajan A. T., Vyas R. C., Darroudi F., Vermeulen S. Frequencies of X-ray-induced chromosome translocations in human peripheral lymphocytes as detected by in situ hybridization using chromosome-specific DNA libraries. Int J Radiat Biol. 1992 Feb;61(2):199–203. doi: 10.1080/09553009214550821. [DOI] [PubMed] [Google Scholar]
- Rabbitts P., Impey H., Heppell-Parton A., Langford C., Tease C., Lowe N., Bailey D., Ferguson-Smith M., Carter N. Chromosome specific paints from a high resolution flow karyotype of the mouse. Nat Genet. 1995 Apr;9(4):369–375. doi: 10.1038/ng0495-369. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H. Chromosomal translocations in human cancer. Nature. 1994 Nov 10;372(6502):143–149. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- Ramsey M. J., Moore D. H., 2nd, Briner J. F., Lee D. A., Olsen L. a., Senft J. R., Tucker J. D. The effects of age and lifestyle factors on the accumulation of cytogenetic damage as measured by chromosome painting. Mutat Res. 1995 Oct;338(1-6):95–106. doi: 10.1016/0921-8734(95)00015-x. [DOI] [PubMed] [Google Scholar]
- Straume T., Lucas J. N. A comparison of the yields of translocations and dicentrics measured using fluorescence in situ hybridization. Int J Radiat Biol. 1993 Aug;64(2):185–187. doi: 10.1080/09553009314551281. [DOI] [PubMed] [Google Scholar]
- Straume T., Lucas J. N., Tucker J. D., Bigbee W. L., Langlois R. G. Biodosimetry for a radiation worker using multiple assays. Health Phys. 1992 Feb;62(2):122–130. doi: 10.1097/00004032-199202000-00001. [DOI] [PubMed] [Google Scholar]
- Tucker J. D., Ashworth L. K., Johnston G. R., Allen N. A., Carrano A. V. Variation in the human lymphocyte sister-chromatid exchange frequency: results of a long-term longitudinal study. Mutat Res. 1988 Mar;204(3):435–444. doi: 10.1016/0165-1218(88)90039-0. [DOI] [PubMed] [Google Scholar]
- Tucker J. D., Lee D. A., Moore D. H., 2nd Validation of chromosome painting. II. A detailed analysis of aberrations following high doses of ionizing radiation in vitro. Int J Radiat Biol. 1995 Jan;67(1):19–28. doi: 10.1080/09553009514550031. [DOI] [PubMed] [Google Scholar]
- Tucker J. D., Lee D. A., Ramsey M. J., Briner J., Olsen L., Moore D. H., 2nd On the frequency of chromosome exchanges in a control population measured by chromosome painting. Mutat Res. 1994 Oct-Dec;313(2-3):193–202. doi: 10.1016/0165-1161(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Tucker J. D., Morgan W. F., Awa A. A., Bauchinger M., Blakey D., Cornforth M. N., Littlefield L. G., Natarajan A. T., Shasserre C. A proposed system for scoring structural aberrations detected by chromosome painting. Cytogenet Cell Genet. 1995;68(3-4):211–221. doi: 10.1159/000133916. [DOI] [PubMed] [Google Scholar]
- Tucker J. D., Ramsey M. J., Lee D. A., Minkler J. L. Validation of chromosome painting as a biodosimeter in human peripheral lymphocytes following acute exposure to ionizing radiation in vitro. Int J Radiat Biol. 1993 Jul;64(1):27–37. doi: 10.1080/09553009314551081. [DOI] [PubMed] [Google Scholar]


