Abstract
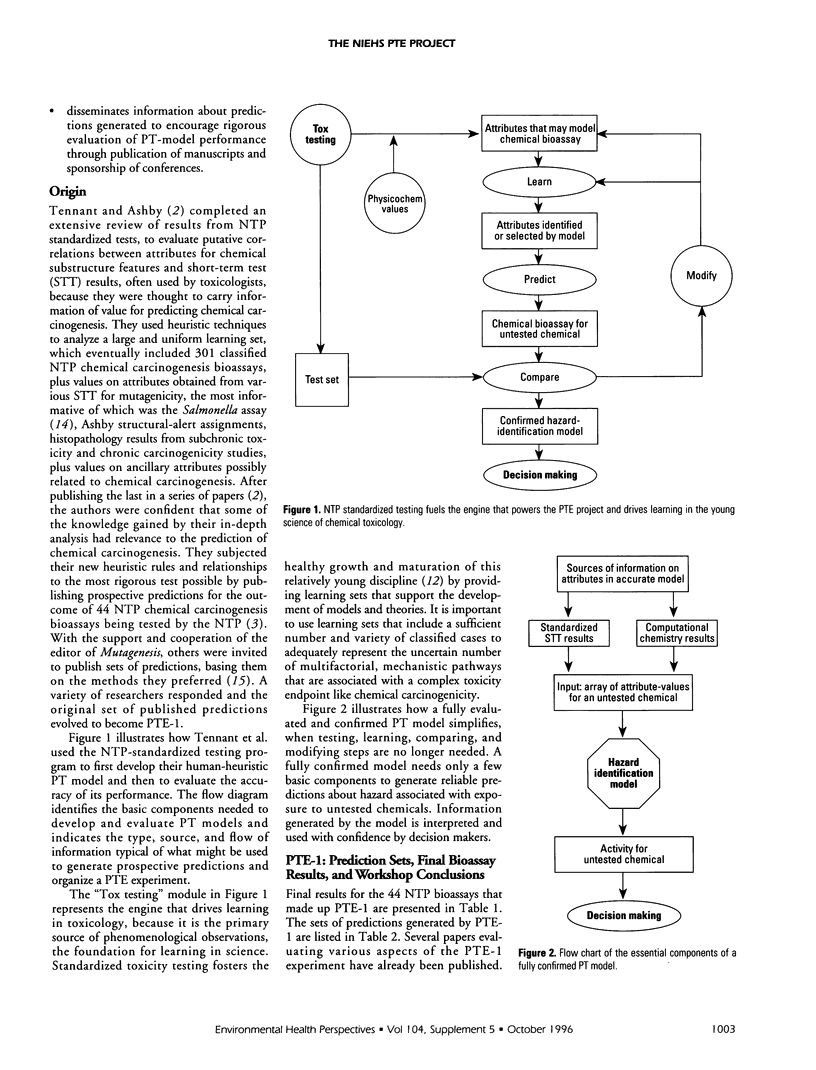
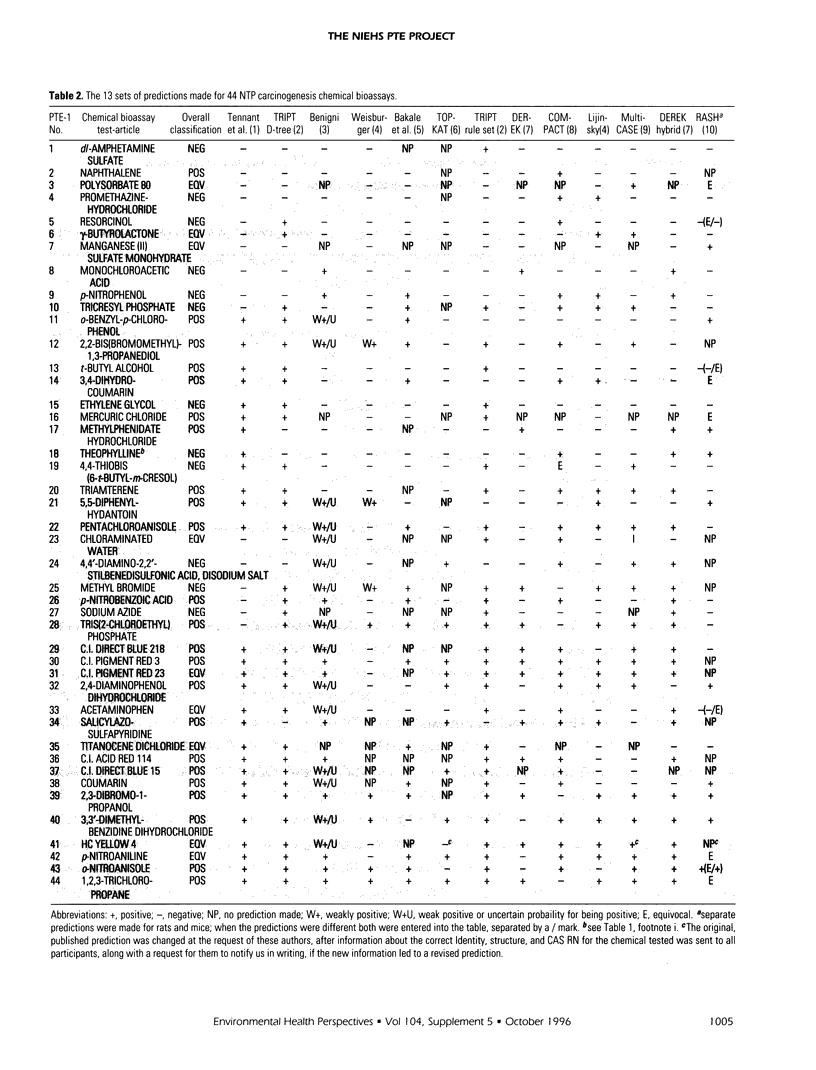
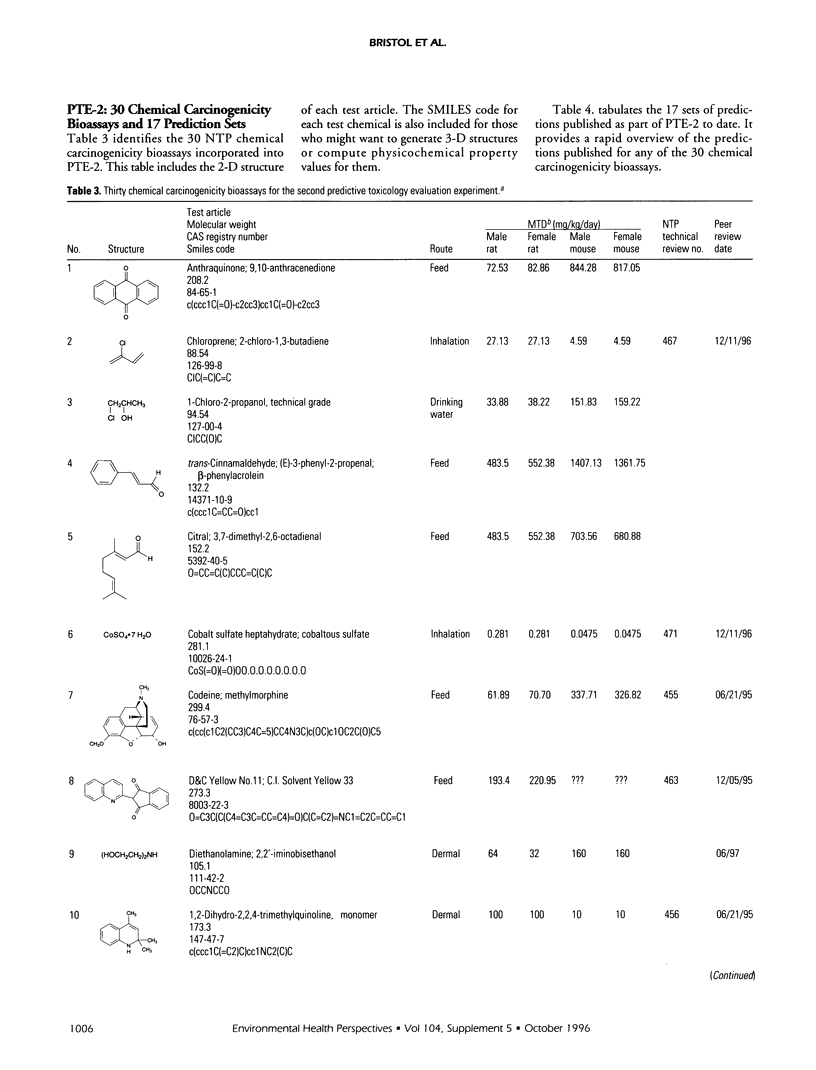
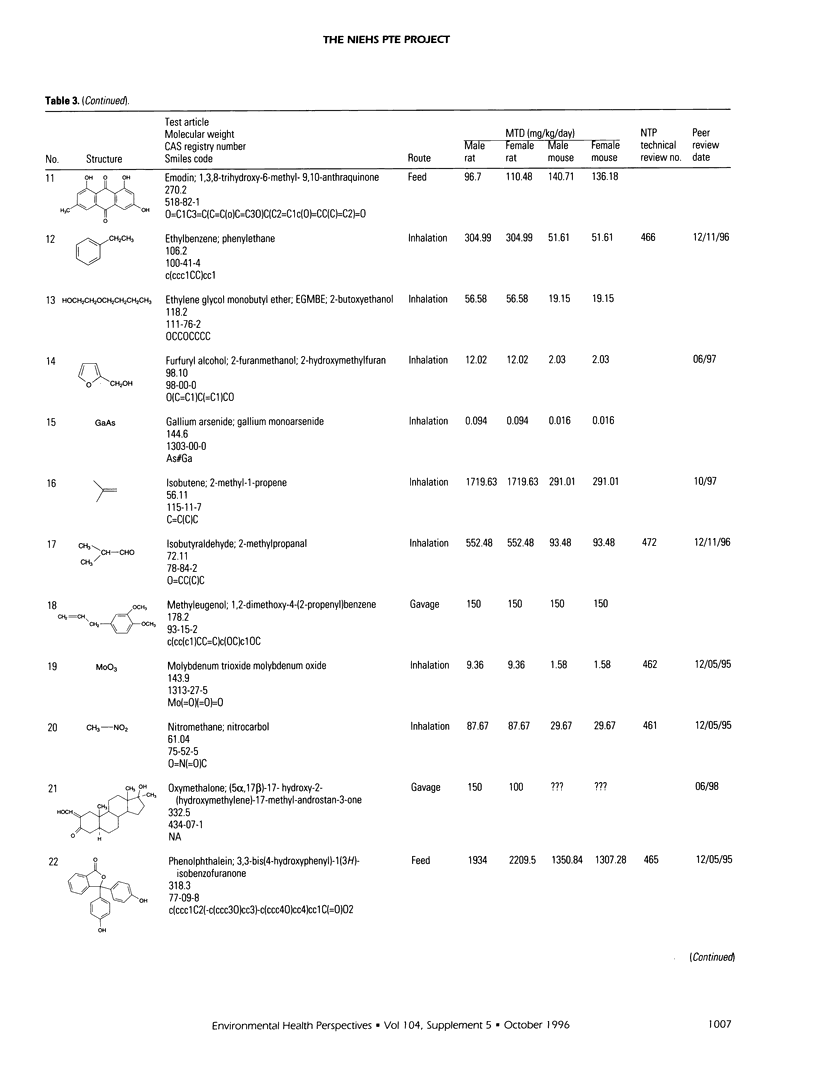
The Predictive-Toxicology Evaluation (PTE) project conducts collaborative experiments that subject the performance of predictive-toxicology (PT) methods to rigorous, objective evaluation in a uniquely informative manner. Sponsored by the National Institute of Environmental Health Sciences, it takes advantage of the ongoing testing conducted by the U.S. National Toxicology Program (NTP) to estimate the true error of models that have been applied to make prospective predictions on previously untested, noncongeneric-chemical substances. The PTE project first identifies a group of standardized NTP chemical bioassays either scheduled to be conducted or are ongoing, but not yet complete. The project then announces and advertises the evaluation experiment, disseminates information about the chemical bioassays, and encourages researchers from a wide variety of disciplines to publish their predictions in peer-reviewed journals, using whatever approaches and methods they feel are best. A collection of such papers is published in this Environmental Health Perspectives Supplement, providing readers the opportunity to compare and contrast PT approaches and models, within the context of their prospective application to an actual-use situation. This introduction to this collection of papers on predictive toxicology summarizes the predictions made and the final results obtained for the 44 chemical carcinogenesis bioassays of the first PTE experiment (PTE-1) and presents information that identifies the 30 chemical carcinogenesis bioassays of PTE-2, along with a table of prediction sets that have been published to date. It also provides background about the origin and goals of the PTE project, outlines the special challenge associated with estimating the true error of models that aspire to predict open-system behavior, and summarizes what has been learned to date.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby J. Prediction of rodent carcinogenicity for 30 chemicals. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1101–1104. doi: 10.1289/ehp.96104s51101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby J., Tennant R. W. Definitive relationships among chemical structure, carcinogenicity and mutagenicity for 301 chemicals tested by the U.S. NTP. Mutat Res. 1991 May;257(3):229–306. doi: 10.1016/0165-1110(91)90003-e. [DOI] [PubMed] [Google Scholar]
- Benigni R., Andreoli C., Zito R. Prediction of rodent carcinogenicity of further 30 chemicals bioassayed by the U.S. National Toxicology Program. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1041–1044. doi: 10.1289/ehp.96104s51041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman J. Speculations on the rodent carcinogenicity of 30 chemicals currently under evaluation in rat and mouse bioassays organised by the U.S. National Toxicology Program. Environ Mol Mutagen. 1996;27(3):237–243. doi: 10.1002/(SICI)1098-2280(1996)27:3<237::AID-EM9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Bristol D. W. Summary and recommendations for session B: activity classification and structure-activity relationship modeling for human health risk assessment of toxic substances. Toxicol Lett. 1995 Sep;79(1-3):265–280. doi: 10.1016/0378-4274(95)03377-w. [DOI] [PubMed] [Google Scholar]
- Huff J., Haseman J., Rall D. Scientific concepts, value, and significance of chemical carcinogenesis studies. Annu Rev Pharmacol Toxicol. 1991;31:621–652. doi: 10.1146/annurev.pa.31.040191.003201. [DOI] [PubMed] [Google Scholar]
- Huff J., Weisburger E., Fung V. A. Multicomponent criteria for predicting carcinogenicity: dataset of 30 NTP chemicals. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1105–1112. doi: 10.1289/ehp.96104s51105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. D., Easterly C. E. A RASH analysis of National Toxicology Program data: predictions for 30 compounds to be tested in rodent carcinogenesis experiments. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1017–1030. doi: 10.1289/ehp.96104s51017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawchak D. A., Zhao H., Scanlin T. F., Tomezsko J. L., Cnaan A., Stallings V. A. Longitudinal, prospective analysis of dietary intake in children with cystic fibrosis. J Pediatr. 1996 Jul;129(1):119–129. doi: 10.1016/s0022-3476(96)70198-1. [DOI] [PubMed] [Google Scholar]
- Kerckaert G. A., Brauninger R., LeBoeuf R. A., Isfort R. J. Use of the Syrian hamster embryo cell transformation assay for carcinogenicity prediction of chemicals currently being tested by the National Toxicology Program in rodent bioassays. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1075–1084. doi: 10.1289/ehp.96104s51075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. D., Srinivasan A. Prediction of rodent carcinogenicity bioassays from molecular structure using inductive logic programming. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1031–1040. doi: 10.1289/ehp.96104s51031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Buchanan B. G., Rosenkranz H. S. Carcinogenicity predictions for a group of 30 chemicals undergoing rodent cancer bioassays based on rules derived from subchronic organ toxicities. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1059–1063. doi: 10.1289/ehp.96104s51059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. F., Ioannides C., Parke D. V. COMPACT and molecular structure in toxicity assessment: a prospective evaluation of 30 chemicals currently being tested for rodent carcinogenicity by the NCI/NTP. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1011–1016. doi: 10.1289/ehp.96104s51011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant C. A. Prediction of rodent carcinogenicity using the DEREK system for 30 chemicals currently being tested by the National Toxicology Program. The DEREK Collaborative Group. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1065–1073. doi: 10.1289/ehp.96104s51065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi I., Hirano H., Hirono S. Prediction of the rodent carcinogenicity of organic compounds from their chemical structures using the FALS method. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1051–1058. doi: 10.1289/ehp.96104s51051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreskes N., Shrader-Frechette K., Belitz K. Verification, validation, and confirmation of numerical models in the Earth sciences. Science. 1994 Feb 4;263(5147):641–646. doi: 10.1126/science.263.5147.641. [DOI] [PubMed] [Google Scholar]
- Purdy R. A mechanism-mediated model for carcinogenicity: model content and prediction of the outcome of rodent carcinogenicity bioassays currently being conducted on 25 organic chemicals. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1085–1094. doi: 10.1289/ehp.96104s51085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Spalding J. Predictions for the outcome of rodent carcinogenicity bioassays: identification of trans-species carcinogens and noncarcinogens. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1095–1100. doi: 10.1289/ehp.96104s51095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Spalding J., Stasiewicz S., Ashby J. Prediction of the outcome of rodent carcinogenicity bioassays currently being conducted on 44 chemicals by the National Toxicology Program. Mutagenesis. 1990 Jan;5(1):3–14. doi: 10.1093/mutage/5.1.3. [DOI] [PubMed] [Google Scholar]
- Wachsman J. T., Bristol D. W., Spalding J., Shelby M., Tennant R. W. Predicting chemical carcinogenesis in rodents. Environ Health Perspect. 1993 Oct;101(5):444–445. doi: 10.1289/ehp.93101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. P., Sussman N., Macina O. T., Rosenkranz H. S., Klopman G. Prediction of the carcinogenicity of a second group of organic chemicals undergoing carcinogenicity testing. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1045–1050. doi: 10.1289/ehp.96104s51045. [DOI] [PMC free article] [PubMed] [Google Scholar]


